Folate Decorated Nanomicelles Loaded with a Potent Curcumin Analogue for Targeting Retinoblastoma
Abstract
:1. Introduction
2. Methods
2.1. Materials
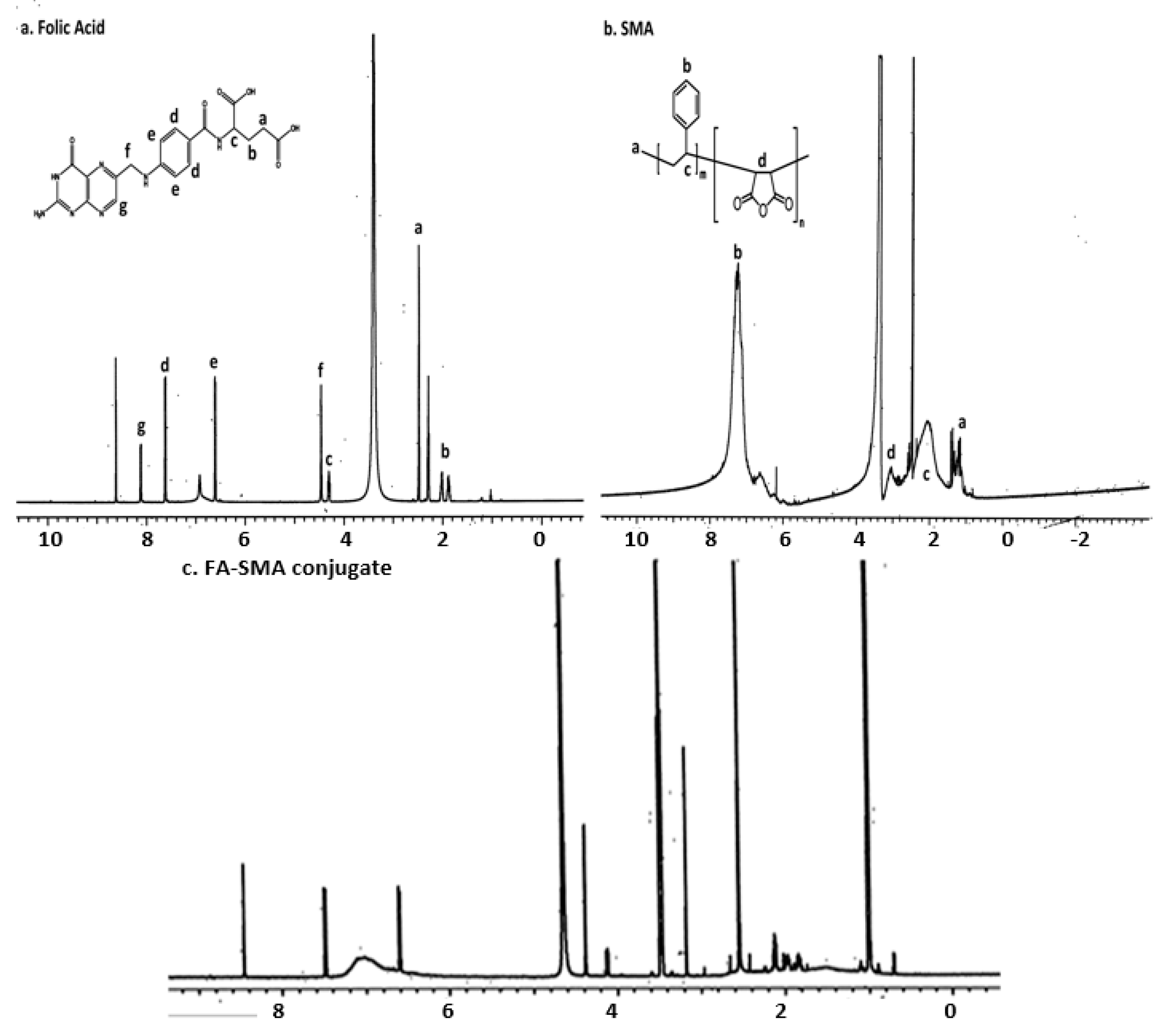
2.2. Synthesis of Folate-Conjugated SMA Copolymer
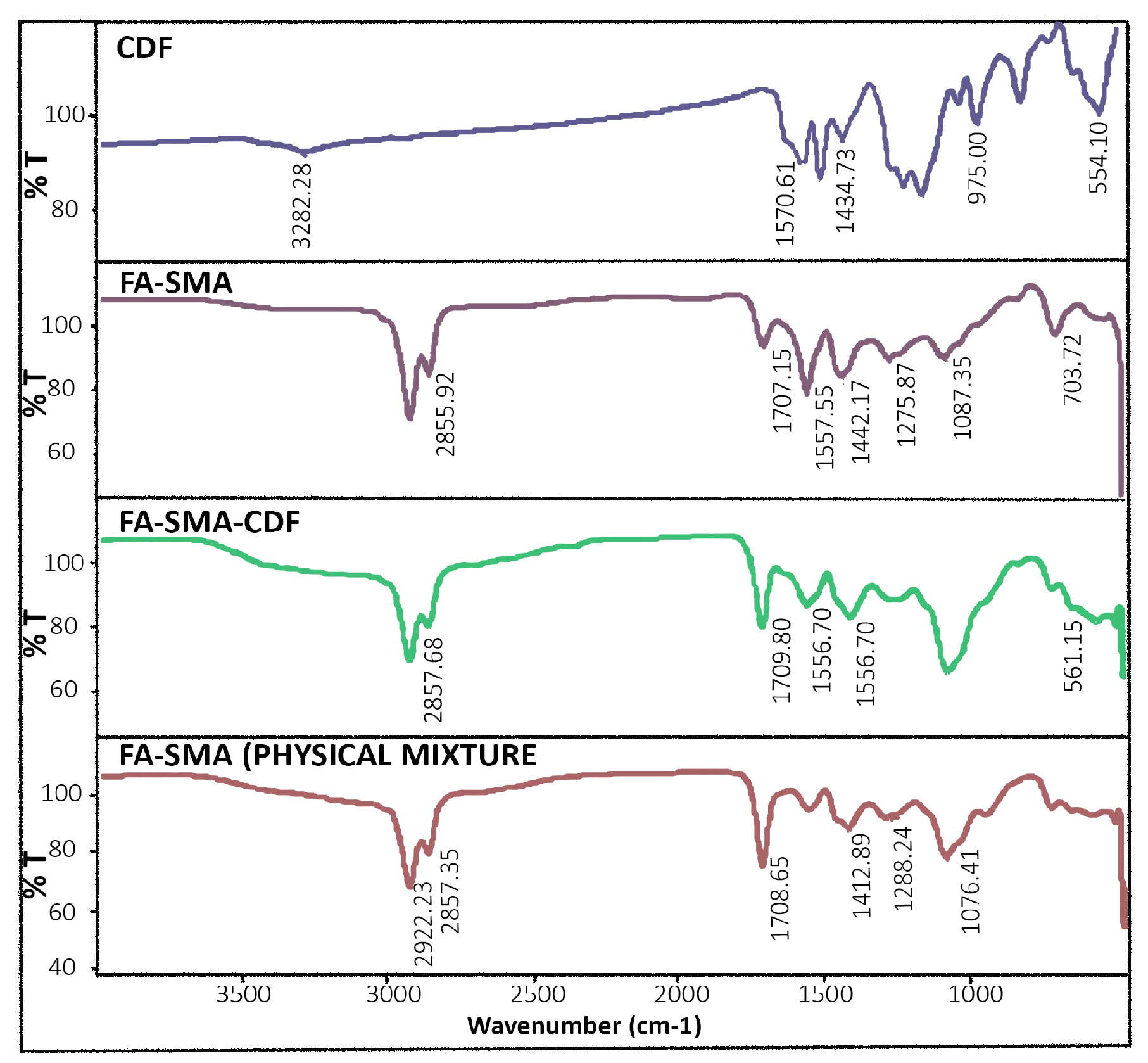
2.3. Fabrication FA-SMA-CDF Nanomicelles
2.4. Characterization of FA-SMACDF Nanomicelles
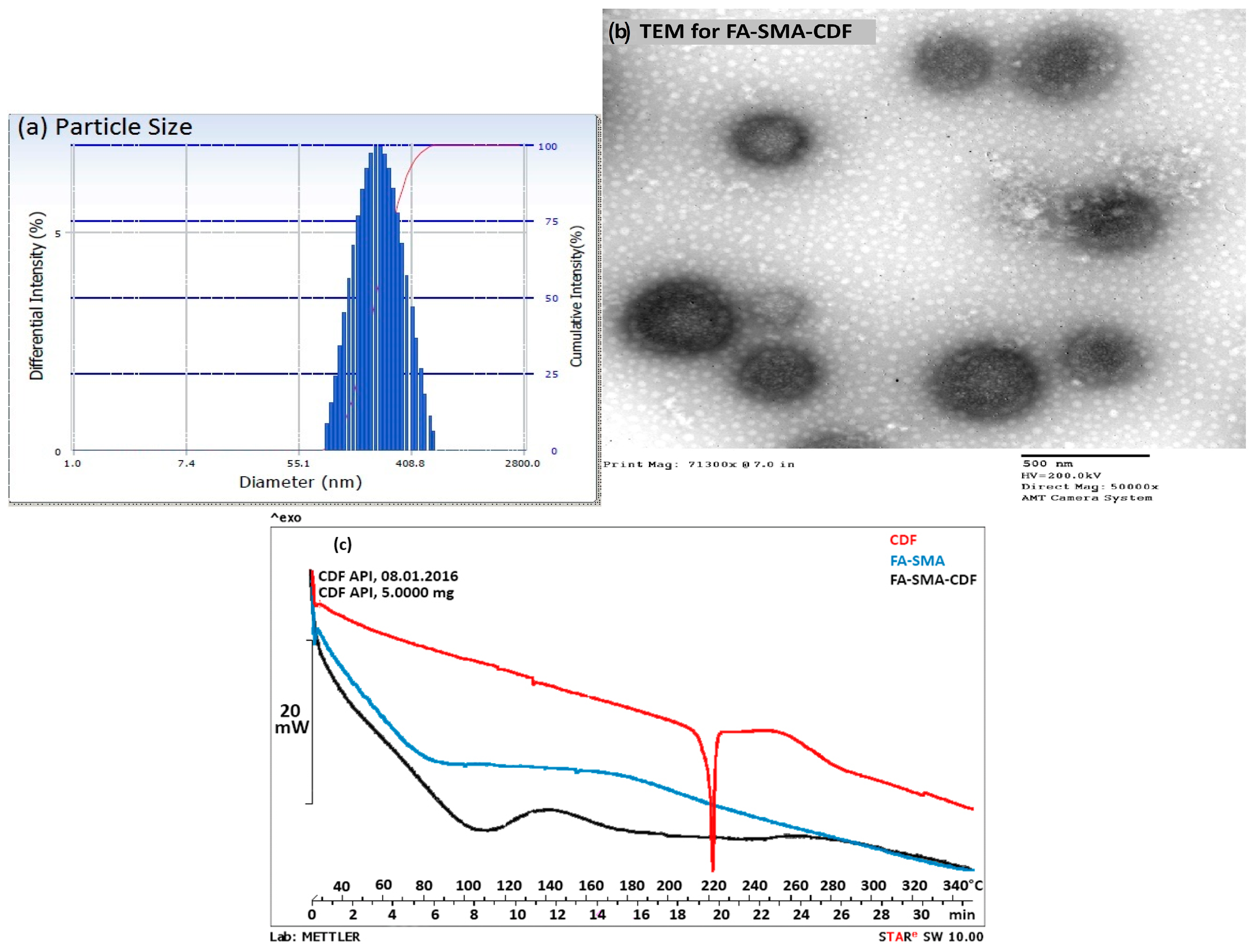
2.5. Differential Scanning Calorimetry
2.6. Particle Size and Morphology
2.7. HPLC Chromatographic Conditions
2.8. Drug Encapsulation Efficiency
2.9. Stability Indication Assay
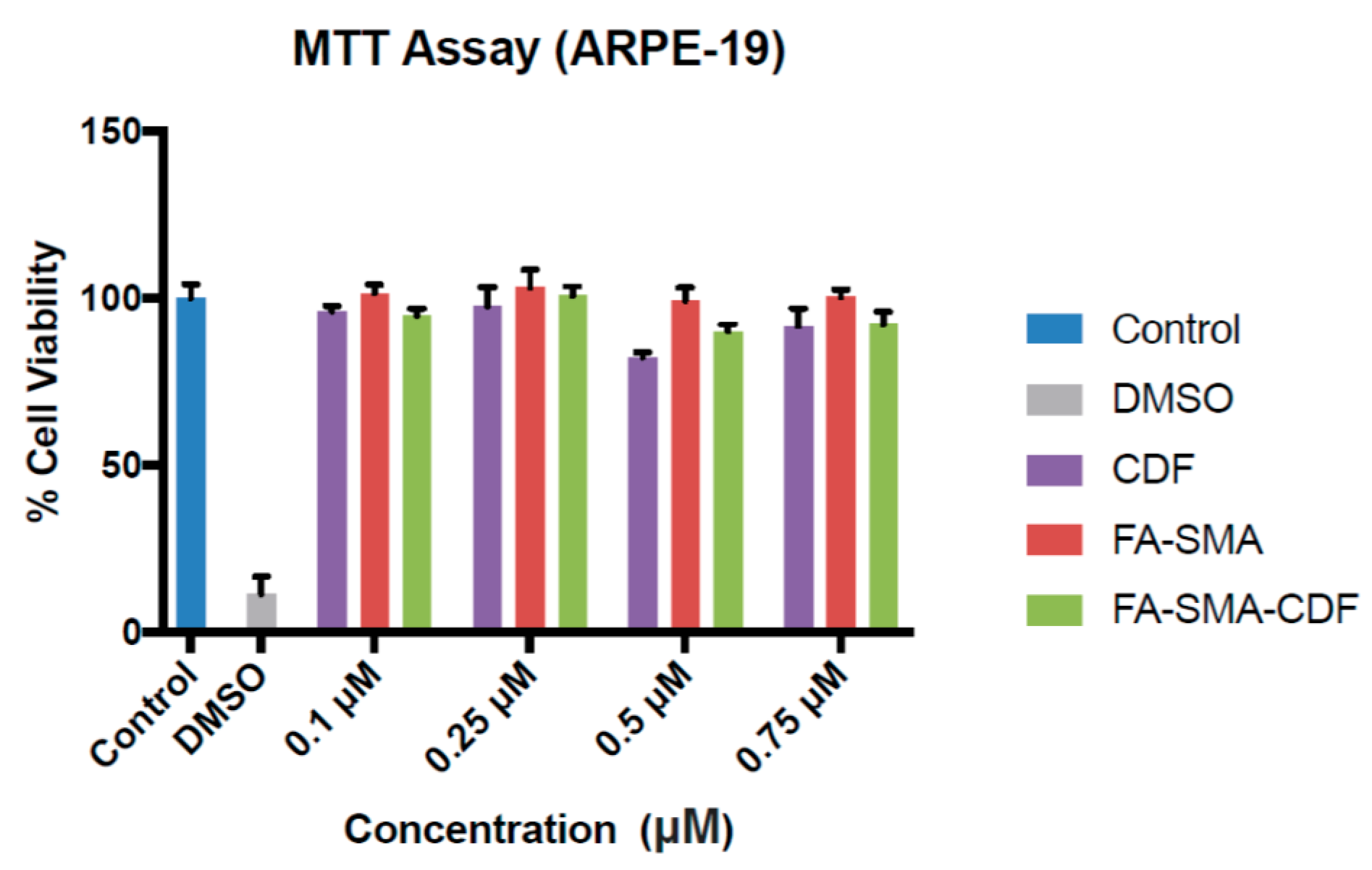
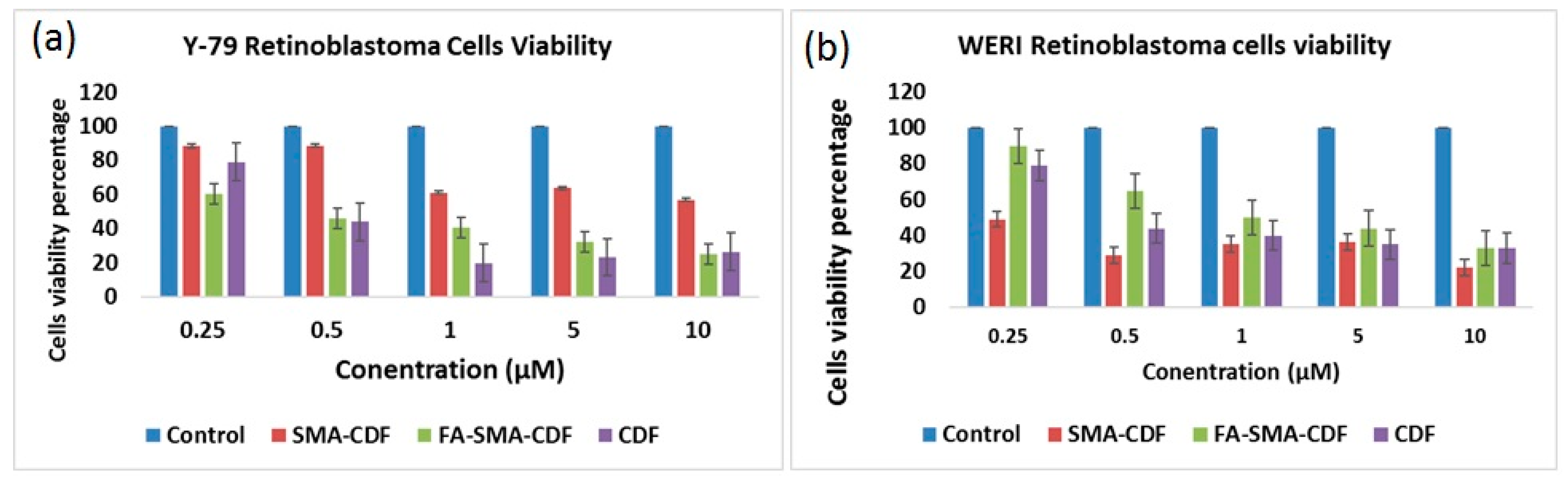
2.10. Cell Viability Study on ARPE-19, Y-79 and WERI-RB1
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sreenivasan, S.; Thirumalai, K.; Danda, R.; Krishnakumar, S. Effect of curcumin on miRNA expression in human Y79 retinoblastoma cells. Curr. Eye Res. 2012, 37, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, R.; Drago-Ferrante, R.; D’Anneo, A.; Augello, G.; Carlisi, D.; De Blasio, A.; Giuliano, M.; Tesoriere, G.; Vento, R. In human retinoblastoma Y79 cells okadaic acid-parthenolide co-treatment induces synergistic apoptotic effects, with PTEN as a key player. Cancer Biol. Ther. 2013, 14, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Aerts, I.; Pacquement, H.; Doz, F.; Mosseri, V.; Desjardins, L.; Sastre, X.; Michon, J.; Rodriguez, J.; Schlienger, P.; Zucker, J.M.; et al. Outcome of second malignancies after retinoblastoma: A retrospective analysis of 25 patients treated at the Institut Curie. Eur. J. Cancer 2004, 40, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Boice, J.D.; Abramson, D.H. Cancer incidence after retinoblastoma: Radiation dose and sarcoma risk. Surv. Ophthalmol. 1998, 43, 288–289. [Google Scholar]
- Zhang, J.; Benavente, C.A.; McEvoy, J.; Flores-Otero, J.; Ding, L.; Chen, X.; Ulyanov, A.; Wu, G.; Wilson, M.; Wang, J.; et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 2012, 481, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Kesharwani, P.; Killinger, B.A.; Moszczynska, A.; Sarkar, F.H.; Padhye, S.; Rishi, A.K.; Iyer, A.K. Solubility enhancement and targeted delivery of a potent anticancer flavonoid analogue to cancer cells using ligand decorated dendrimer nano-architectures. J. Colloid Interface Sci. 2016, 484, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.; Newman, R. Bioavailability of curcumin: Problems and promises. Pharmaceutics 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Banerjee, S.; Chavan, D.; Pandye, S. Fluorocurcumins as cyclooxygenase-2 inhibitor: Molecular docking, pharmacokinetics and tissue distribution in mice. Pharmaceutical 2009, 26, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Yang, H.; Jamadar, A.; Cui, Q. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharmaceutical 2009, 26, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.S.; Yu, Y.; Nautiyal, J.; Patel, B.B.; Padhye, S.; Sarkar, F.H.; Majumdar, A.P. Difluorinated-curcumin (CDF): A novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm. Res 2011, 28, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Allison, E.; Rizzuti, B.A.; Ira, J.; Dunkel, M.D.H.A. The adverse events of chemotherapy for retinoblastoma. Arch. Ophthalmol. 2008, 126, 862–865. [Google Scholar]
- Boddu, S.H.S.; Jwala, J.; Chowdhury, M.R.; Mitra, A.K. In vitro evaluation of a targeted and sustained release system for retinoblastoma cells using Doxorubicin as a model drug. J. Ocul. Pharmacol. Ther. 2010, 26, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Engi, H.; Viveiros, M.; Molnar, J. Comparison of multidrug resistant efflux pumps of cancer and bacterial cells with respect to the same inhibitory agents. In Vivo 2007, 21, 237–244. [Google Scholar] [PubMed]
- Yoshihiko, E. RB protein status and chemosensitivity in non-small cell lung cancers. Oncology 1997, 5, 447–451. [Google Scholar]
- Jwala, J.; Vadlapatla, R.K.; Vadlapudi, A.D.; Boddu, S.H.S.; Pal, D.; Mitra, A.K. Differential expression of folate receptor-alpha, sodium-dependent multivitamin transporter, and amino acid transporter (B (0, +)) in human retinoblastoma (Y-79) and retinal pigment epithelial (ARPE-19) cell lines. J. Ocul. Pharmacol. Ther. 2012, 28, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; Pal, D.; Jain, R. Identification and functional characterization of riboflavin transporter in human-derived retinoblastoma cell line (Y-79): Mechanisms of cellular uptake and translocation. J. Ocul. 2005, 21, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Park, T.G. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J. Control Release 2004, 96, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, T.; Hattori, Y.; Kawano, K.; Ohguchi, Y. Effect of polyethylene glycol linker chain length of folate-linked microemulsions loading aclacinomycin A on targeting ability and antitumor effect in vitro and in vivo. Clin. Cancer 2005, 11, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; Paturi, D.; Luo, S.; Gaudana, R. Folic acid transport via high affinity carrier mediated system in human retinoblastoma cells. Int. J. 2008, 355, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Parenterally administrable nano-micelles of 3, 4-difluorobenzylidene curcumin for treating pancreatic cancers. Colloid Surf. B Biointer. 2015, 132, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Hyaluronic acid engineered nanomicelles loaded with 3,4-difluorobenzylidene curcumin for targeted killing of CD44+ stem-like pancreatic cancer cells. Biomacromolecules 2015, 16, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Harmonisation, I.C. Validation of analytical procedures: Text and methodology. In ICH Harmonised Tripartite Guideline, Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, San Diego, CA, USA, 2 June 2014. [Google Scholar]
- Yoo, H.S.; Park, T.G. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugate. J. Control. Release 2004, 100, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Sau, S.; Kesharwani, P.; Iyer, A.K. Polyvalent folate-dendrimer-coated iron oxide theranostic nanoparticles for simultaneous magnetic resonance imaging and precise cancer cell targeting. Biomacromolecules 2017, 18, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Gawde, K.A.; Kesharwani, P.; Sau, S.; Sarkar, F.H.; Padhye, S.; Kashaw, S.K.; Iyer, A.K. Synthesis and characterization of folate decorated albumin bio-conjugate nanoparticles loaded with a synthetic curcumin difluorinated analogue. J. Colloid Interface Sci. 2017, 496, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Kesharwani, P.; Banerjee, S.; Gupta, U.; Mohd Amin, M.C.I.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater. Today 2015, 18, 565–572. [Google Scholar] [CrossRef]
- Kesharwani, P.; Xie, L.; Banerjee, S.; Mao, G.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloid. Surf. B. Biointer. 2015, 136, 413–423. [Google Scholar] [CrossRef] [PubMed]

| Sample | Hydrodynamic size (nm) | PDI | Zeta potential (mV) | EE (%) |
|---|---|---|---|---|
| FA-SMA-CDF | 193.6 ± 20 nm | 0.175 ± 0.05 | –7.12 ± 4 | 75.98 ± 12 |
| SMA-CDF | 183 ± 31 nm | 0.183 ± 0.07 | –34 ± 5 | 70.21 ± 9 |
| Formulation | Temperature | ||
|---|---|---|---|
| 4 °C | 25 °C | 35 °C | |
| FA-SMA-CDF (% drug content recovery) | 100.73 ± 2.10 | 96.9 ± 3.2 | 89.76 ± 2.76 |
| SMA-CDF (% drug content recovery) | 99.41 ± 1.83 | 98.42 ± 1.81 | 90.91 ± 1.54 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaab, H.; Alzhrani, R.M.; Kesharwani, P.; Sau, S.; Boddu, S.H.; Iyer, A.K. Folate Decorated Nanomicelles Loaded with a Potent Curcumin Analogue for Targeting Retinoblastoma. Pharmaceutics 2017, 9, 15. https://doi.org/10.3390/pharmaceutics9020015
Alsaab H, Alzhrani RM, Kesharwani P, Sau S, Boddu SH, Iyer AK. Folate Decorated Nanomicelles Loaded with a Potent Curcumin Analogue for Targeting Retinoblastoma. Pharmaceutics. 2017; 9(2):15. https://doi.org/10.3390/pharmaceutics9020015
Chicago/Turabian StyleAlsaab, Hashem, Rami M. Alzhrani, Prashant Kesharwani, Samaresh Sau, Sai HS. Boddu, and Arun K. Iyer. 2017. "Folate Decorated Nanomicelles Loaded with a Potent Curcumin Analogue for Targeting Retinoblastoma" Pharmaceutics 9, no. 2: 15. https://doi.org/10.3390/pharmaceutics9020015
APA StyleAlsaab, H., Alzhrani, R. M., Kesharwani, P., Sau, S., Boddu, S. H., & Iyer, A. K. (2017). Folate Decorated Nanomicelles Loaded with a Potent Curcumin Analogue for Targeting Retinoblastoma. Pharmaceutics, 9(2), 15. https://doi.org/10.3390/pharmaceutics9020015








