Comparative Clinical Effectiveness and Cost-Effectiveness of the Cochlear Osia System and Baha Attract System in Patients with Conductive or Mixed Hearing Loss or Single-Sided Deafness
Abstract
1. Introduction
- (i)
- assessed as being, at least, non-inferior in terms of clinical effectiveness; and
- (ii)
- the cost of the product is relative to its clinical effectiveness” [9].
- conduct a systematic literature review to inform a robust indirect treatment comparison (ITC) of the clinical effectiveness of the Osia System with the Australian comparator BCHI, the passive transcutaneous Baha Attract System and
- construct a Markov cost-utility model to inform the cost-effectiveness of the Osia System versus the Baha Attract System, the transcutaneous BCHI most likely replaced in an Australian healthcare setting.
2. Materials and Methods
2.1. Systematic Literature Review
2.1.1. Study Eligibility Criteria
- (1)
- a comparative clinical study and
- (2)
- a prospective arm of the Osia System and/or the Baha Attract System in the study and
- (3)
- sample size was representative, i.e., ≥20 patients with CHL, MHL and/or SSD for Osia System and Baha Attract System and
- (4)
- adequate information for review in English.
2.1.2. Data Extraction
2.2. Meta-Analysis of Study Endpoints and Indirect Treatment Comparison
Audiological Outcomes
2.3. Economic Evaluation
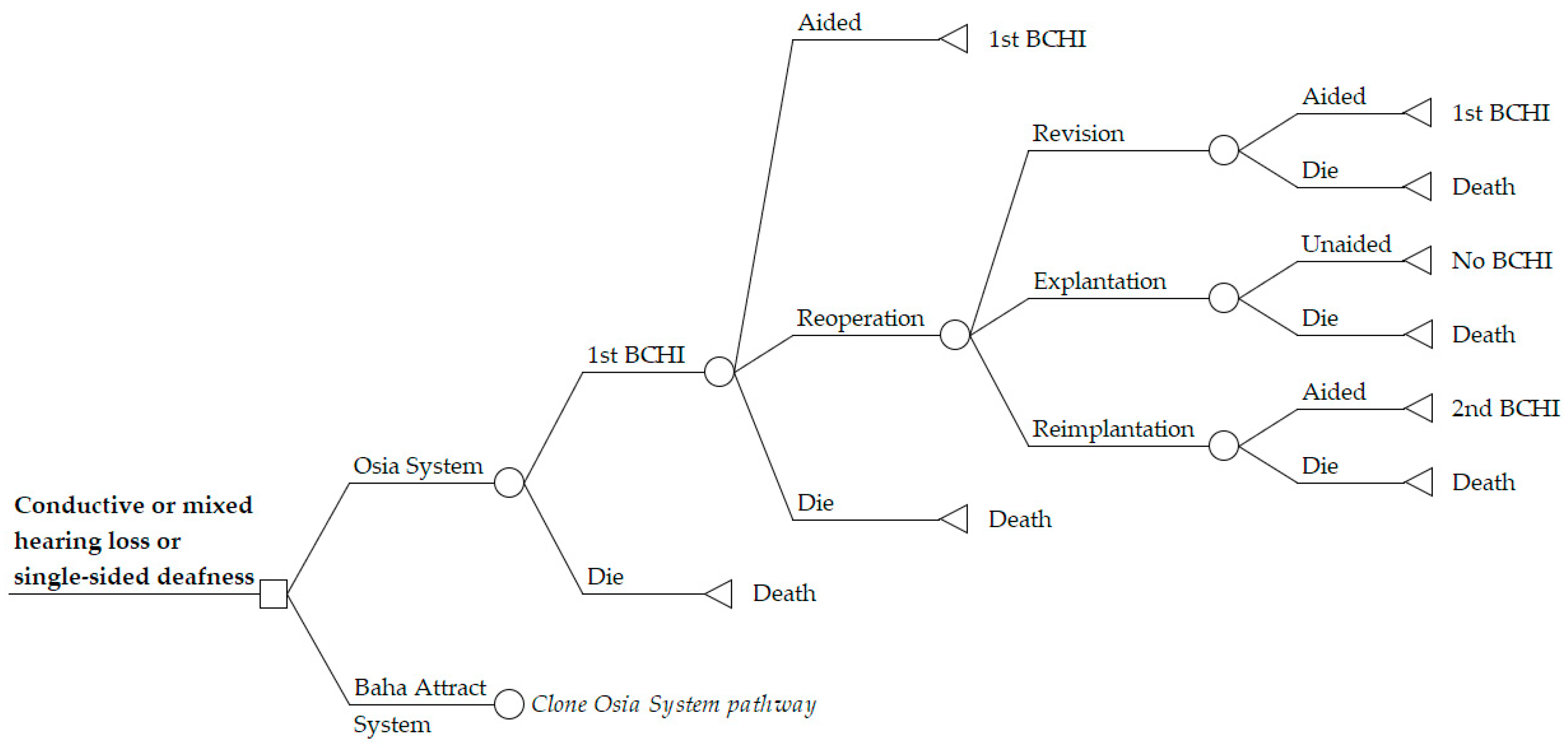
2.3.1. Model Perspective and Structure
2.3.2. Time Horizon
2.3.3. Choice of Health Outcomes
2.3.4. Transition Probabilities and Adverse Event Rates
2.3.5. Cost Inputs
2.3.6. Parameter Analysis
2.3.7. Probabilistic Sensitivity Analysis
3. Results
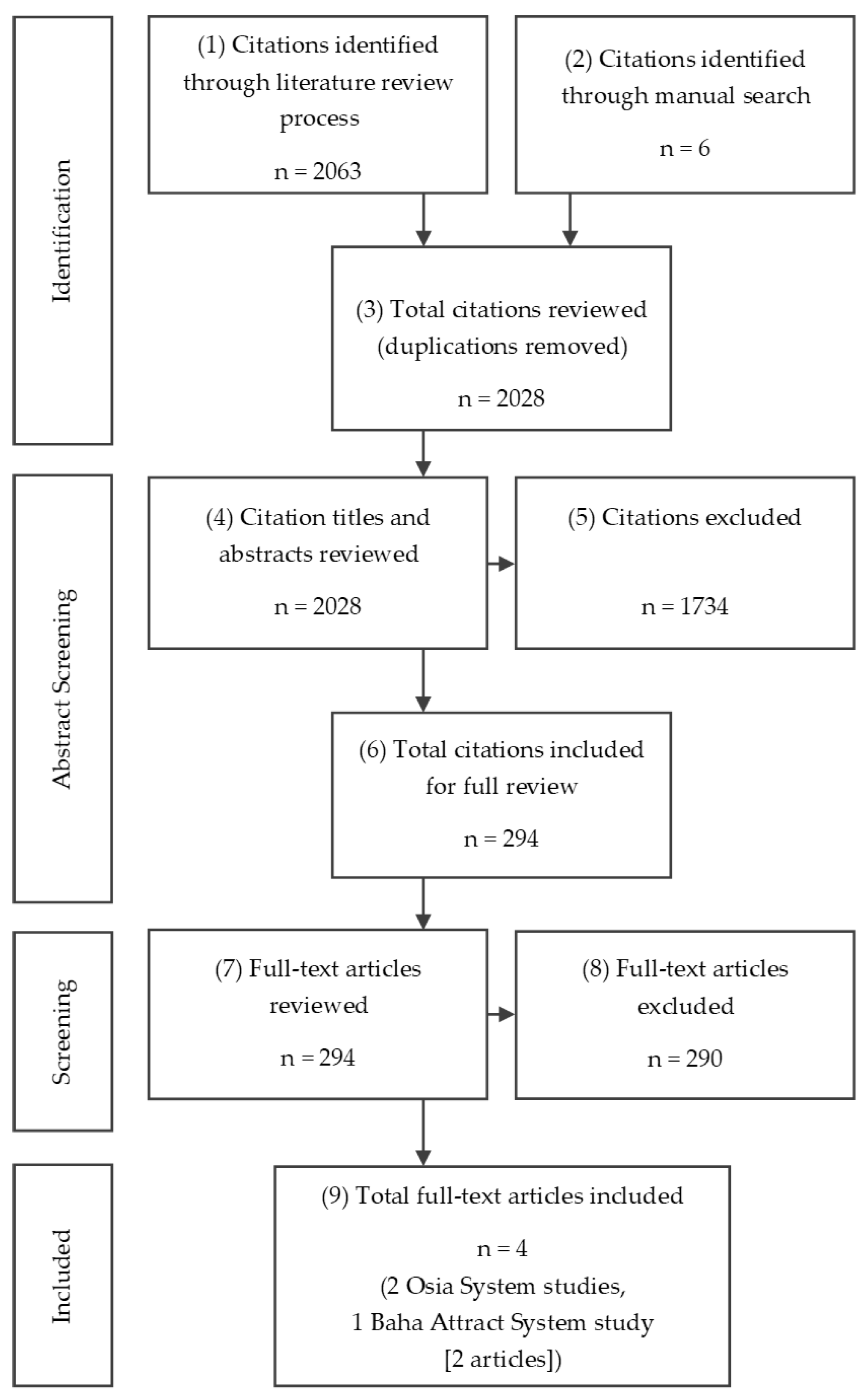
3.1. Search Results and Study Selection
Identified Studies
- objective audiological outcomes: threshold audiometry, speech performance in quiet and speech performance in noise,
- surgical and other AEs,
- patient-reported outcomes (PROs), and
- the HUI3, a measure considered to be most relevant to hearing.
3.2. Results of the Indirect Treatment Comparison
3.2.1. Audiological Outcomes
3.2.2. Utilities
3.2.3. Patient Reported Outcomes
3.2.4. Adverse Events
3.3. Base Case Results of the Economic Evaluation
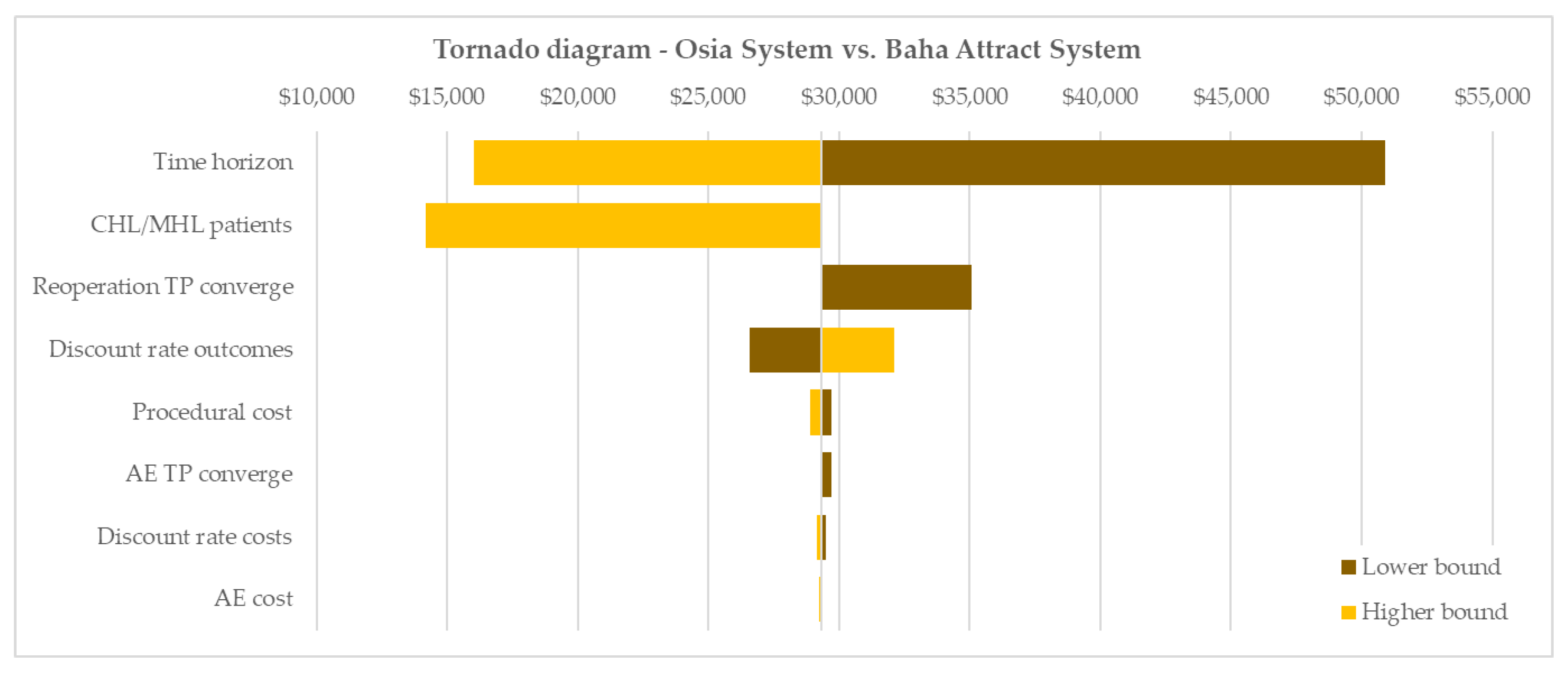
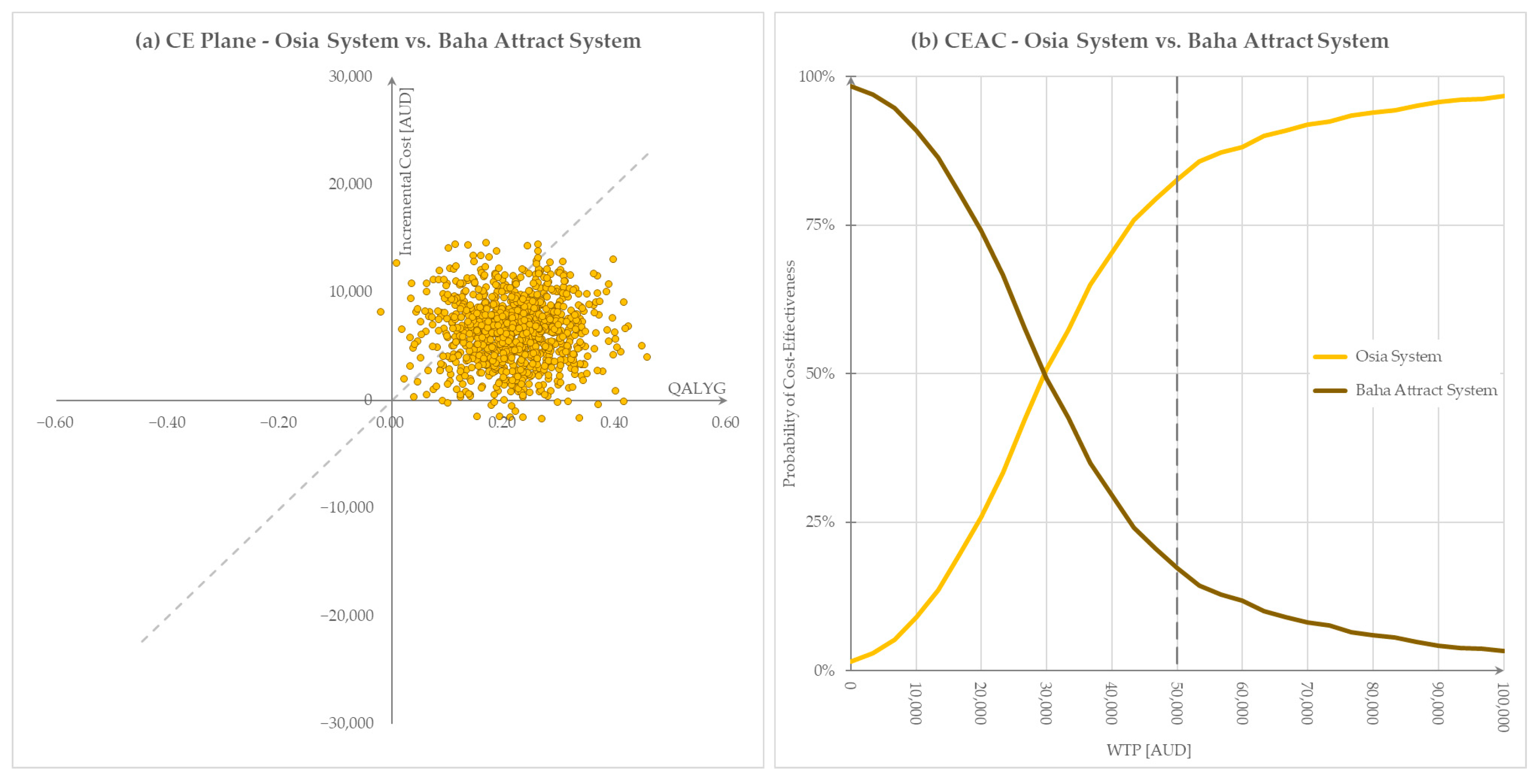
3.4. Results of Sensitivity Analyses
3.4.1. Parameter Analysis Results
3.4.2. Probabilistic Sensitivity Analysis Results
4. Discussion
4.1. Clinical Effectiveness
4.2. Cost-Effectiveness
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mylanus, E.A.M.; Hua, H.; Wigren, S.; Arndt, S.; Skarzynski, P.H.; Telian, S.A.; Briggs, R.J.S. Multicenter Clinical Investigation of a New Active Osseointegrated Steady-State Implant System. Otol. Neurotol. 2020, 41, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; Birman, C.S.; Baulderstone, N.; Lewis, A.T.; Ng, I.H.; Östblom, A.; Rousset, A.; Tari, S.; Tong, M.C.; Cowan, R. Clinical Performance, Safety, and Patient-Reported Outcomes of an Active Osseointegrated Steady-State Implant System. Otol. Neurotol. 2022, 43, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Kirkby-Strachan, G.; Que-Hee, C. Implantable hearing devices—An update. Aust. J. Gen. Pract. 2016, 45, 370–373. [Google Scholar]
- Ueda, C.H.Y.; Soares, R.M.; Jardim, I.; Bento, R.F. Assessment Protocol for Candidates for Bone-Anchored Hearing Devices. Int. Arch. Otorhinolaryngol. 2022, 26, e718–e724. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-C.; Wallace, C.G.; Ho, V.W.-Y.; Wu, C.-M.; Chen, H.-Y.; Chen, Z.-C. Simultaneous auricular reconstruction and transcutaneous bone conduction device implantation in patients with microtia. J. Formos. Med. Assoc. 2019, 118, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Motlagh Zadeh, L.; Silbert, N.H.; Sternasty, K.; Swanepoel, W.; Hunter, L.L.; Moore, D.R. Extended high-frequency hearing enhances speech perception in noise. Proc. Natl. Acad. Sci. USA 2019, 116, 23753–23759. [Google Scholar] [CrossRef] [PubMed]
- Magele, A.; Schoerg, P.; Stanek, B.; Gradl, B.; Sprinzl, G.M. Active transcutaneous bone conduction hearing implants: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0221484. [Google Scholar] [CrossRef]
- Plontke, S.K.; Götze, G.; Wenzel, C.; Rahne, T.; Mlynski, R. Implantation of a new active bone conduction hearing device with optimized geometry. HNO 2020, 68 (Suppl. S2), 106–115. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Aged Care. Prosthesis List Guide 2020; Commonwealth of Australia: Canberra, Australia, 2020.
- Gawęcki, W.; Gibasiewicz, R.; Marszał, J.; Błaszczyk, M.; Gawłowska, M.; Wierzbicka, M. The evaluation of a surgery and the short-term benefits of a new active bone conduction hearing implant—The Osia®. Braz. J. Otorhinolaryngol. 2022, 88, 289–295. [Google Scholar] [CrossRef] [PubMed]
- PBAC. Guidelines for Preparing a Submission to the Pharmaceutical Benefits Advisory Committee; Australian Government Department of Health and Aged Care: Canberra, Australia, 2016.
- MSAC. Guidelines for Preparing Assessments for the Medical Services Advisory Committee; Australian Government Department of Health and Aged Care: Canberra, Australia, 2021.
- WHO. Australian Life Tables; WHO: Geneva, Switzerland, 2019.
- Wiley, T.L.; Chappell, R.; Carmichael, L.; Nondahl, D.M.; Cruickshanks, K.J. Changes in hearing thresholds over 10 years in older adults. J. Am. Acad. Audiol. 2008, 19, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Australian Government Department of Health and Aged Care. Prostheses List; Commonwealth of Australia: Canberra, Australia, 2022.
- Australian Government Department of Health and Aged Care. Medicare Benefits Schedule; Commonwealth of Australia: Canberra, Australia, 2022.
- Independent Health and Aged Care Pricing Authority (IHACPA). National Hospital Cost Data Collection (NHCDC) Public Hospitals Report—Round 24 (Financial Year 2019–20) Appendix AR-DRG Version 10.0; Independent Health and Aged Care Pricing Authority: Sydney, Australia, 2020.
- den Besten, C.A.; Monksfield, P.; Bosman, A.; Skarzynski, P.H.; Green, K.; Runge, C.; Wigren, S.; Blechert, J.I.; Flynn, M.C.; Mylanus, E.A.M.; et al. Audiological and clinical outcomes of a transcutaneous bone conduction hearing implant: Six-month results from a multicentre study. Clin. Otolaryngol. 2019, 44, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Kruyt, I.J.; Monksfield, P.; Skarzynski, P.H.; Green, K.; Runge, C.; Bosman, A.; Blechert, J.I.; Wigren, S.; Mylanus, E.A.M.; Hol, M.K.S. Results of a 2-Year Prospective Multicenter Study Evaluating Long-term Audiological and Clinical Outcomes of a Transcutaneous Implant for Bone Conduction Hearing. Otol. Neurotol. 2020, 41, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). 2019. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf19/K191921.pdf (accessed on 14 February 2022).
- Food and Drug Administration (FDA). Implantable Middle Ear Hearing Device—Guidance for Industry and FDA Staff; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2003.
- Drummond, M. Introducing economic and quality of life measurements into clinical studies. Ann. Med. 2001, 33, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gum, D.; Merlin, T. Comparing the ICERs in Medicine Reimbursement Submissions to NICE and PBAC-Does the Presence of an Explicit Threshold Affect the ICER Proposed? Value Health 2018, 21, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E. CRD’s Guidance for Undertaking Reviews in Health Care. Lancet Infect. Dis. 2010, 10, 226. [Google Scholar] [CrossRef]
- Crowson, M.G.; Tucci, D.L. Mini Review of the Cost-Effectiveness of Unilateral Osseointegrated Implants in Adults: Possibly Cost-Effective for the Correct Indication. Audiol. Neurotol. 2016, 21, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yeung, M.; Falk, L.; Ali, A.; Walter, M. Implantable Devices for Single-Sided Deafness and Conductive or Mixed Hearing Loss: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2020, 20, 1–165. [Google Scholar]
- Amin, N.; Soulby, A.J.; Borsetto, D.; Pai, I. Longitudinal economic analysis of Bonebridge 601 versus percutaneous bone-anchored hearing devices over a 5-year follow-up period. Clin. Otolaryngol. 2021, 46, 263–272. [Google Scholar] [CrossRef] [PubMed]
| Participants |
|
| Intervention | Osia System |
| Comparison | Baha Attract System at fitting range equivalent to the Osia System (up to 55 dB HL), as this is the transcutaneous BCHI most likely to be replaced by the Osia System in the Australian healthcare setting. |
| Outcomes |
|
| Study Design | Prospective arm of either the Osia System or Baha Attract System. Non-systematic reviews, studies with retrospective arms of either BCHI system, case reports, letters, editorials, animal/invitro studies and prospective clinical studies with n < 20 CHL and/or MHL and/or SSD patients were excluded. |
| Cost Item (AUD) | Osia System | Baha Attract System | Source |
|---|---|---|---|
| BCHI system | $15,125 | $8571 | Prostheses List Benefit; Osia System (CO089); Baha Attract System: BI300 (CO051) + BIM400 (CO068) + Baha 6 Max (CO087) [15] |
| Replacement sound processor | $7166 | $6484 | Prostheses List Benefit; Osia System: Assumption (Benefit of SAMBA 2 Audio Processor (US029)); Baha: Baha 6 Max (CO087) [15] |
| Surgery | $1002 | $1002 | MBS; Items 41,603 (osseo-integration of fixture) + 41,604 (fixation of transcutaneous implant) + 20,120 (initiation of anaesthesia) + 23,085 (anaesthesia perfusion time 1:46–2:00 h) [16] |
| Hospitalisation | $5415 | $5415 | Approximation from public AR-DRG; D12B (Other Ear, Nose, Mouth and Throat Interventions, Minor Complexity, minus Prosthesis component) [17] |
| AE (soft tissue complication) | $138 | $138 | MBS; Items 104 (initial specialist visit) + 105 (subsequent specialist visit) [16] |
| Study Characteristics | Osia System | Baha Attract System | |
|---|---|---|---|
| Reference | Mylanus et al. [1] | Briggs et al. [2] | den Besten et al. [18], Kruyt et al. [19] |
| Intervention | Osia System a | Osia System a | Baha Attract System |
| Primary comparator | Pre-operative unaided hearing condition | ||
| Key inclusion criteria | Adults (18 years or older) | ||
| CHL or MHL with BC threshold of PTA4 ≤ 55 dB or SSD with AC threshold of PTA4 ≤20 dB in the contralateral ear | CHL or MHL with BC threshold of PTA4 < 30 dB or SSD with PTA4 ≤ 30 dB (≤ 20 dB for USA) in the contralateral ear | ||
| Study design | Open, single cohort, before-and-after, prospective, multicentre clinical investigation | ||
| Countries | Australia, Germany, the Netherlands, Poland, USA | Australia, Hong Kong | The Netherlands, Poland, United Kingdom, USA |
| Reporting period | Primary: efficacy 3 months, safety 6 months; final: 12 months | Primary: efficacy and safety 3 months; final: 6 months | Primary: efficacy and safety 6 months; final: 24 months |
| Key clinical endpoints | Investigational device vs. unaided hearing (primary comparison) and Baha on Softband (secondary comparison for audiological outcomes) | ||
| Threshold audiometry, free field (PTA4, mean of 0.5, 1, 2 and 4 kHz) | |||
| Speech in quiet (50 dB, 65 dB and 80 dB SPL) | |||
| Adaptive speech recognition in noise with S0N180 (50% SNR) | Adaptive speech recognition in noise with S0N0 (50% SNR) | Adaptive speech recognition in noise with S0N180 (50% SNR) | |
| Self-reported assessments: Profile of Hearing Aid Benefit (APHAB), Health Utilities Index (HUI3), Speech, Spatial and Qualities of Hearing Scale (SSQ) | |||
| Baseline Characteristics | Osia System | Baha Attract System | |
|---|---|---|---|
| Reference | Mylanus et al. [1] | Briggs et al. [2] | den Besten et al. [18], Kruyt et al. [19] |
| Number enrolled | 51 | 29 | 54 |
| Age (years), mean (SD) | 47.4 (14.7) | 46.7 (19.7) | 42.1 (13.6) |
| Gender, n (%) | Male: 27 (52.9%) Female: 24 (47.1%) | Male: 13 (44.8%) Female: 16 (55.2%) | Male: 21 (38.9%) Female: 33 (61.1%) |
| Type of hearing loss, n (%) | CHL/MHL: 37 (72.6%) SSD: 14 (27.5%) | CHL/MHL: 24 (82.8%) SSD: 5 (17.2%) | CHL/MHL: 39 (72.2%) SSD: 15 (27.8%) |
| Pre-operative PTA4 unaided hearing (dB), mean (SD) | 53.9 (11.6) a | 53.6 (11.3) a | 51.9 (10.5) b |
| Audiological Outcomes Mean (SD), N; 95% CI | Osia System | Baha Attract System | Osia System versus Baha Attract System | ||
| Reference | Mylanus et al. [1] | Briggs et al. [2] | Meta-analysis | den Besten et al. [18], Kruyt et al. [19] | |
| PTA4 (dB) | |||||
| Pre-operative | 53.90 (11.60), 51 a | 53.60 (11.30), 29 a | 53.79; 51.27, 56.31 | 51.94 (10.46), 54; 49.15, 54.73 b | |
| Change at 6 months | 27.90 (9.10), 49 a | 28.40 (9.60), 28 a | 28.07; 26.00, 30.14 | 21.02 (10.41), 54; 18.25, 23.80 b | 7.05; 3.58, 10.51 |
| Speech discrimination in quiet (65 dB) (%) | |||||
| Pre-operative | 25.50 (25.40), 51 a | 37.80 (30.30), 29 a | 30.88; 18.92, 42.84 | 46.76 (32.62), 54; 38.06, 55.46 b | |
| Change at 6 months | 61.50 (27.70), 48 a | 54.00 (29.80), 28 a | 58.80; 51.74, 65.86 | 43.44 (31.47), 54; 35.05, 51.84 b | 15.35; 4.39, 26.32 |
| Speech discrimination in noise (presented from behind) (dB) | |||||
| Pre-operative | 4.98 (7.76), 51; 2.85, 7.11 a | NR | NA | 8.57 (6.26), 36; 6.52, 10.61 b | |
| Change at 6 months | 13.70 (8.10), 48; 11.41, 15.99 a | NR | NA | 4.26 (5.66), 36; 2.41, 6.11 b | 9.44 (6.5, 12.39) |
| HUI3 Mean (SD), N; 95% CI | Osia System | Baha Attract System | ||
|---|---|---|---|---|
| Reference | Mylanus et al. [1] | Briggs et al. [2] | Meta-analysis | den Besten et al. [18], Kruyt et al. [19] |
| All patients | ||||
| Pre-operative | 0.65 (0.22), 46; 0.59, 0.71 a | 0.69 (0.23), 29; 0.61, 0.77 a | 0.67; 0.61, 0.72 | 0.66 (0.24), 52; 0.60, 0.73 |
| Change at 3 months | 0.08 (0.23), 42; 0.01, 0.15 a | 0.10 (0.17), 27; 0.03, 0.16 a | 0.09; 0.04, 0.14 | NR |
| Change at 6 months | NR | 0.09 (0.17), 27; 0.03 a, 0.15 a | NA | 0.06 (0.25), 47; −0.01, 0.13 |
| CHL/MHL patients | ||||
| Pre-operative | 0.61 (0.22), 34; 0.53, 0.68 a | 0.65 (0.22), 24; 0.56, 0.73 a | 0.62; 0.56, 0.68 | 0.67 (0.21), 37; 0.60, 0.74 |
| Change at 3 months | 0.10 (0.24), 30; 0.02, 0.19 a | 0.12 (0.17), 23; 0.05, 0.19 a | 0.11; 0.06, 0.16 | NR |
| Change at 6 months | NR | 0.12 (0.15), 23; 0.06, 0.19 a | NA | 0.06 (0.23), 34; −0.01, 0.14 |
| Mean Improvement vs. Pre-Operative (SD) | Osia System | Baha Attract System | |
|---|---|---|---|
| Reference | Mylanus et al. [1] N = 48, reported at 12 months | Briggs et al. [2] N = 27, reported at 6 months | den Besten et al. [18] N = 53, reported at 6 months |
| Abbreviated Profile of Hearing Aid Benefit (APHAB) | |||
| Global Scale | 26.3 (18.5) | 25.9 (26.2) | 22.9 (18.1) |
| Speech, Spatial and Qualities of Hearing Scale (SSQ) | |||
| SSQ Speech Scale | 2.94 (1.94) | 2.68 (1.89) | 2.5 (1.7) |
| SSQ Spatial Scale | 2.95 (2.52) | 2.30 (2.42) | 1.9 (1.9) (N = 52) |
| SSQ Qualities Scale | 2.13 (2.30) | 2.41 (1.81) | 1.7 (1.4) |
| Adverse Events | Osia System | Baha Attract System | |
|---|---|---|---|
| Reference | Mylanus et al. [1] | Briggs et al. [2] | den Besten et al. [18], Kruyt et al. [19] |
| Follow-up period | 12 months | 6 months | 6 months, 24 months |
| Subjects, N | 51 | 29 | 54 |
| Soft tissue complications (classified as moderate severity AE) | 1 event in 1 subject | 2 events in 2 subjects | 4 events in 4 subjects [11] |
| Reoperation/Serious AE | 1 explantation due to 3 serious AEs | None | 2 explantations (magnet removal, 1 due to serious AE) 2 reimplantations (conversion to percutaneous BCHI) [12] |
| Deterministic Results | Osia System | Baha Attract System | Incremental Change | ICER |
|---|---|---|---|---|
| Undiscounted | ||||
| Total costs (AUD) | $35,084 | $28,630 | $6453 | $24,301 per QALY gained |
| Total QALYs | 7.50 | 7.24 | 0.27 | |
| Discounted | ||||
| Total costs (AUD) | $31,605 | $25,257 | $6348 | $29,301 per QALY gained |
| Total QALYs | 6.09 | 5.88 | 0.22 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunner, M.; Schou, M.; Briggs, R.J.; Kingsford Smith, D. Comparative Clinical Effectiveness and Cost-Effectiveness of the Cochlear Osia System and Baha Attract System in Patients with Conductive or Mixed Hearing Loss or Single-Sided Deafness. J. Mark. Access Health Policy 2024, 12, 5-20. https://doi.org/10.3390/jmahp12010003
Brunner M, Schou M, Briggs RJ, Kingsford Smith D. Comparative Clinical Effectiveness and Cost-Effectiveness of the Cochlear Osia System and Baha Attract System in Patients with Conductive or Mixed Hearing Loss or Single-Sided Deafness. Journal of Market Access & Health Policy. 2024; 12(1):5-20. https://doi.org/10.3390/jmahp12010003
Chicago/Turabian StyleBrunner, Matthias, Manjula Schou, Robert J. Briggs, and Dell Kingsford Smith. 2024. "Comparative Clinical Effectiveness and Cost-Effectiveness of the Cochlear Osia System and Baha Attract System in Patients with Conductive or Mixed Hearing Loss or Single-Sided Deafness" Journal of Market Access & Health Policy 12, no. 1: 5-20. https://doi.org/10.3390/jmahp12010003
APA StyleBrunner, M., Schou, M., Briggs, R. J., & Kingsford Smith, D. (2024). Comparative Clinical Effectiveness and Cost-Effectiveness of the Cochlear Osia System and Baha Attract System in Patients with Conductive or Mixed Hearing Loss or Single-Sided Deafness. Journal of Market Access & Health Policy, 12(1), 5-20. https://doi.org/10.3390/jmahp12010003






