GLP-1RA Essentials in Gastroenterology: Side Effect Management, Precautions for Endoscopy and Applications for Gastrointestinal Disease Treatment
Abstract
1. Introduction
2. Background
3. Exploring the Multifaceted Impact of GLP-1RAs: Beyond Glycemic Control
3.1. Weight Reduction
3.2. Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis
| Year & Study Author | Participants & Condition | Type of Study | Drug & Outcomes |
|---|---|---|---|
| 2009; Jendle et al. [25] | 314 T2DM patients, 18–80 years, BMI ≤ 40 kg/m2 (LEAD-2), ≤45 kg/m2 (LEAD-3), HbA1c 7.0–11.0% | Randomized, double-blind, and parallel-group trials (LEAD-2 and LEAD-3), where the reduction in fat mass and hepatic steatosis was a primary outcome. | Liraglutide 1.8 mg/metformin: Significant increase in liver-to-spleen attenuation ratio, indicating reduced hepatic steatosis. |
| 2015; Tang et al. [26] | 35 T2DM patients inadequately controlled on metformin monotherapy or combination | Randomized study; insulin vs. liraglutide effects on liver fat were the primary outcome. | Insulin: Improved glycated hemoglobin (7.9% to 7.2%, p = 0.005), decreased liver MRI-PDFF (13.8% to 10.6%, p = 0.005), liver volume, and total liver fat index (304.4 vs. 209.3%·mL, p = 0.01). Liraglutide: Improved glycated hemoglobin (7.6% to 6.7%, p < 0.001), no significant change in liver MRI-PDFF, liver volume, or liver fat index. |
| 2015; Eguchi et al. [27] | 27 subjects with NASH and glucose intolerance, post lifestyle modification intervention. | Prospective, uncontrolled study; the impact on histological findings in NASH was a primary outcome. | After 24 weeks of liraglutide treatment at 0.9 mg/body per day, 19 subjects showed significant improvements in body mass index, visceral fat accumulation, aminotransferases, and glucose abnormalities. Six subjects who continued liraglutide for 96 weeks showed a decrease in histological inflammation as determined by NASH activity score and stage as determined by Brunt classification without significant adverse events. |
| 2016; Armstrong et al. [28] | 52 overweight patients with clinical evidence of non-alcoholic steatohepatitis. | Multicentre, double-blinded, randomized, placebo-controlled phase 2 trial; resolution of non-alcoholic steatohepatitis without worsening in fibrosis was a primary outcome. | 1.8 mg daily of liraglutide led to a resolution of definite non-alcoholic steatohepatitis in 39% of patients, compared with 9% in the placebo group (relative risk 4.3 [95% CI 1.0–17.7]; p = 0.019). A total of 2 (9%) of 23 patients in the liraglutide group versus 8 (36%) of 22 patients in the placebo group had fibrosis progression. Adverse events were mostly mild to moderate, with gastrointestinal disorders being more common in the liraglutide group. |
| 2016; Dutour et al. [29] | 44 obese subjects with T2DM uncontrolled on oral antidiabetic drugs. | Prospective randomized clinical trial; hepatic and epicardial fat reduction was a primary outcome. | Exenatide treatment resulted in significant weight loss (−5.3 ± 0.4 kg; p = 0.001 for the difference between groups) and a decrease in epicardial adipose tissue (EAT) (−8.8 ± 2.1%) and hepatic triglyceride content (HTGC) (−23.8 ± 9.5%), compared with the reference treatment (EAT: +1.2 ± 1.6%; HTGC: +12.5 ± 9.6%; p = 0.003 and p = 0.007, respectively). No significant change in myocardial triglyceride content (MTGC) was observed. |
| 2016; Armstrong et al. [21] | 14 NASH patients | Double-blind, randomized, placebo-controlled trial; the effect on insulin sensitivity, hepatic lipid handling, and adipose dysfunction was a primary outcome. | Liraglutide treatment led to a reduction in BMI (−1.9 vs. +0.04 kg/m2; p < 0.001), HbA1c (−0.3 vs. +0.3%; p < 0.01), LDL cholesterol (−0.7 vs. +0.05 mmol/L; p < 0.01), and ALT (−54 vs. −4.0 U/L; p < 0.01). It also increased hepatic insulin sensitivity and decreased endogenous glucose production (p < 0.05), increased adipose tissue insulin sensitivity (p < 0.05), and inhibited lipolysis and de novo lipogenesis (both p < 0.05) in vivo and in primary human hepatocytes. |
| 2017; Seko et al. [30] | 15 biopsy-proven NAFLD patients with T2DM refractory to diet intervention. | Retrospective study; the effectiveness of dulaglutide in NAFLD patients with T2DM was the main focus, implying it was a primary outcome. | Dulaglutide (0.75 mg for 12 weeks) significantly reduced body weight, hemoglobin A1c, transaminase activities, total body fat mass, and liver stiffness. |
| 2017; Khoo et al. [23] | Non-diabetic Asian adults with NAFLD; BMI ≥ 30 kg/m2, mean weight 96.0 ± 16.3 kg | Randomized study; comparing liraglutide and lifestyle intervention on NAFLD was a primary outcome. | Both the liraglutide group (3 mg daily) and the diet/exercise group saw similar and significant weight reductions (−3.5 ± 3.3 kg vs. −3.5 ± 2.1 kg, respectively, p = 0.72) and liver fat fraction decreases (−8.9 ± 13.4% vs. −7.2% ± 7.1%, p = 0.70). Changes in serum alanine aminotransferase (−42 ± 46 vs. −34 ± 27 U/L, p = 0.52) and aspartate aminotransferase (−23 ± 24 vs. −18 ± 15 U/L, p = 0.53) were not statistically significant. |
| 2019; Newsome et al. [31] | Subjects with obesity and/or T2DM at risk of NAFLD. | Data from a 104-week cardiovascular outcomes trial and a 52-week weight management trial; effect on alanine aminotransferase (ALT) and high-sensitivity C-reactive protein (hsCRP) as primary outcomes. | In the weight management trial of patients with elevated baseline ALT, semaglutide led to end-of-treatment ALT reductions of 6–21% (p < 0.05 for doses ≥0.2 mg/day) and hsCRP reductions of 25–43% vs. placebo (p < 0.05 for 0.2 and 0.4 mg/day). Normalization of elevated baseline ALT occurred in 25–46% of weight management trial subjects vs. 18% on placebo. In the cardiovascular outcomes trial, no significant ALT reduction was noted at 0.5 mg/week. A reduction was observed at this dose at week 30 but was not sustained to week 56, while a 9% reduction vs. placebo was seen at 1.0 mg/week (p = 0.0024). |
| 2020; Teshome et al. [32] | 590 participants with non-alcoholic fatty liver disease (NAFLD). | Systematic review; the study compiled data from randomized controlled trials, single-arm trials, and cohorts. | GLP-1 analogs led to decreased serum transaminases, improved liver histology and insulin resistance, reduced body weight, and normalized liver enzymes. Specifically, ALT, AST, and GGT decreased by 5.5%, 59.5%, 52.8%, and 44.8%, respectively, and there was a reduction in proinflammatory cytokines and an enhancement of protective adipokines noted in some studies. |
3.3. Neurodegenerative Applications of GLP-1RAs
3.4. Cardiovascular Implications of GLP-1RAs
4. GLP-1RA-Associated Side Effects and Potential Concerns
4.1. Gastrointestinal Impact of GLP-1 Receptor Agonists
4.2. Pancreatic Concerns
4.3. Concern for Thyroid Neoplasms
4.4. Cardiovascular Implications
4.5. Endocrinological and Glycemic Considerations
4.6. Allergenic and Immune Responses
4.7. Musculoskeletal Implications
4.8. Dermatological Implications
4.9. Renal Concerns
4.10. Facial Implications
4.11. Implications of Overdose
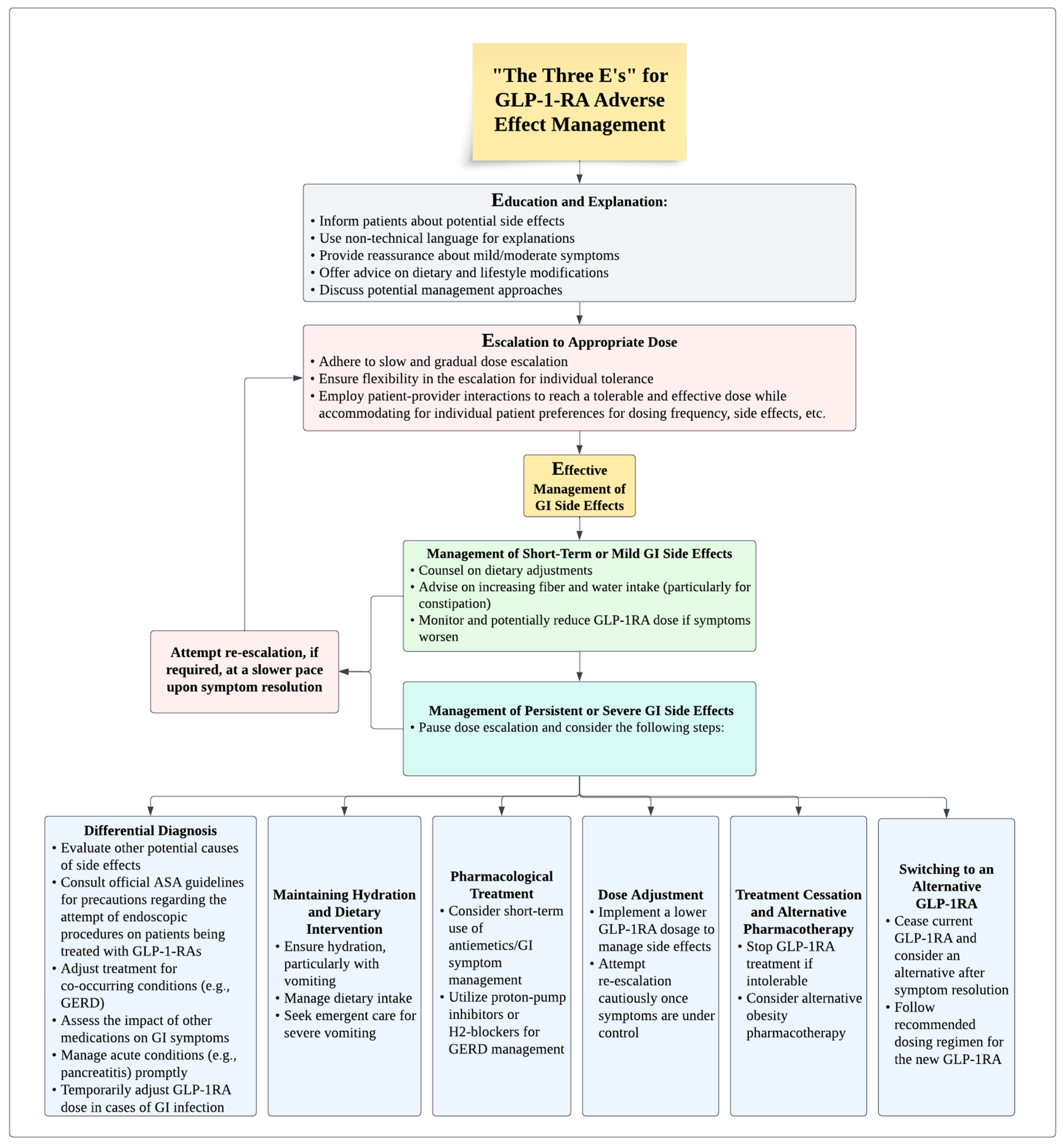
5. Management of Gastrointestinal Adverse Events: “The Three E’s”
6. Safety and Tolerability of GLP-1RAs
Official Statements Regarding Endoscopic Procedure Precautions
7. Alternative Non-GLP-1-RA Approaches to Weight Loss
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Barbalho, S.M.; Guiguer, E.L.; da Silva Soares de Souza, M.; de Souza, G.A.; Fidalgo, T.M.; Araújo, A.C.; de Souza Gonzaga, H.F.; de Bortoli Teixeira, D.; de Oliveira Silva Ullmann, T.; et al. GLP-1a: Going beyond Traditional Use. Int. J. Mol. Sci. 2022, 23, 739. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lv, Y.; Yu, M.; Mei, M.; Xiang, L.; Zhao, S.; Li, R. GLP-1 receptor agonist-associated tumor adverse events: A real-world study from 2004 to 2021 based on FAERS. Front. Pharmacol. 2022, 13, 925377. [Google Scholar] [CrossRef] [PubMed]
- Shaefer, C.F.; Kushner, P.; Aguilar, R. User’s guide to mechanism of action and clinical use of GLP-1 receptor agonists. Postgrad. Med. 2015, 127, 818–826. [Google Scholar] [CrossRef]
- Drucker, D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 2022, 57, 101351. [Google Scholar] [CrossRef]
- Fala, L. Tanzeum (Albiglutide): A Once-Weekly GLP-1 Receptor Agonist Subcutaneous Injection Approved for the Treatment of Patients with Type 2 Diabetes. Am. Health Drug Benefits 2015, 8, 126–130. [Google Scholar]
- GlaxoSmithKline UK Ltd. GlaxoSmithKline Safety Advisory: Reminder Letter Regarding the Discontinuation of Eperzan (Albiglutide). 2018. Available online: https://assets.publishing.service.gov.uk/media/5b4c89c5ed915d436ea7ea8b/Eperzan-25062018.pdf (accessed on 13 February 2024).
- Smith, L.L.; Mosley, J.F.; Parke, C.; Brown, J.; Barris, L.S.; Phan, L.D. Dulaglutide (Trulicity): The Third Once-Weekly GLP-1 Agonist. Phys. Ther. 2016, 41, 357–360. [Google Scholar]
- Bridges, A.; Bistas, K.G.; Jacobs, T.F. Exenatide; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yoshida, Y.; Joshi, P.; Barri, S.; Wang, J.; Corder, A.L.; O’Connell, S.S.; Fonseca, V.A. Progression of retinopathy with glucagon-like peptide-1 receptor agonists with cardiovascular benefits in type 2 diabetes—A systematic review and meta-analysis. J. Diabetes Complicat. 2022, 36, 108255. [Google Scholar] [CrossRef]
- Jackson, S.H.; Martin, T.S.; Jones, J.D.; Seal, D.; Emanuel, F. Liraglutide (victoza): The first once-daily incretin mimetic injection for type-2 diabetes. Phys. Ther. 2010, 35, 498–529. [Google Scholar]
- Leon, N.; LaCoursiere, R.; Yarosh, D.; Patel, R.S. Lixisenatide (Adlyxin): A Once-Daily Incretin Mimetic Injection for Type-2 Diabetes. Phys. Ther. 2017, 42, 676–711. [Google Scholar]
- Chao, A.M.; Tronieri, J.S.; Amaro, A.; Wadden, T.A. Clinical Insight on Semaglutide for Chronic Weight Management in Adults: Patient Selection and Special Considerations. Drug Des. Devel Ther. 2022, 16, 4449–4461. [Google Scholar] [CrossRef] [PubMed]
- van Can, J.; Sloth, B.; Jensen, C.B.; Flint, A.; Blaak, E.E.; Saris, W.H.M. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 2014, 38, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Kadouh, H.; Chedid, V.; Halawi, H.; Burton, D.D.; Clark, M.M.; Khemani, D.; Vella, A.; Acosta, A.; Camilleri, M. GLP-1 Analog Modulates Appetite, Taste Preference, Gut Hormones, and Regional Body Fat Stores in Adults with Obesity. J. Clin. Endocrinol. Metab. 2020, 105, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, M.; Breitschaft, A.; Tadayon, S.; Wizert, A.; Skovgaard, D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes. Metab. 2021, 23, 754–762. [Google Scholar] [CrossRef]
- Pratley, R.E.; Kang, J.; Trautmann, M.E.; Hompesch, M.; Han, O.; Stewart, J.; Sorli, C.H.; Jacob, S.; Yoon, K.H. Body weight management and safety with efpeglenatide in adults without diabetes: A phase II randomized study. Diabetes Obes. Metab. 2019, 21, 2429–2439. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; Le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Hull, D.; Guo, K.; Barton, D.; Hazlehurst, J.M.; Gathercole, L.L.; Nasiri, M.; Yu, J.; Gough, S.C.; Newsome, P.N.; et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 2016, 64, 399–408. [Google Scholar] [CrossRef]
- Nevola, R.; Epifani, R.; Imbriani, S.; Tortorella, G.; Aprea, C.; Galiero, R.; Rinaldi, L.; Marfella, R.; Sasso, F.C. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 1703. [Google Scholar] [CrossRef]
- Khoo, J.; Hsiang, J.; Taneja, R.; Law, N.; Ang, T. Comparative effects of liraglutide 3 mg vs structured lifestyle modification on body weight, liver fat and liver function in obese patients with non-alcoholic fatty liver disease: A pilot randomized trial. Diabetes Obes. Metab. 2017, 19, 1814–1817. [Google Scholar] [CrossRef]
- Desjardins, E.M.; Wu, J.; Lavoie, D.C.T.; Ahmadi, E.; Townsend, L.K.; Morrow, M.R.; Wang, D.; Tsakiridis, E.E.; Batchuluun, B.; Fayyazi, R.; et al. Combination of an ACLY inhibitor with a GLP-1R agonist exerts additive benefits on nonalcoholic steatohepatitis and hepatic fibrosis in mice. Cell Rep. Med. 2023, 4, 101193. [Google Scholar] [CrossRef]
- Jendle, J.; Nauck, M.A.; Matthews, D.R.; Frid, A.; Hermansen, K.; Düring, M.; Zdravkovic, M.; Strauss, B.J.; Garber, A.J. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes. Metab. 2009, 11, 1163–1172. [Google Scholar] [CrossRef]
- Tang, A.; Rabasa-Lhoret, R.; Castel, H.; Wartelle-Bladou, C.; Gilbert, G.; Massicotte-Tisluck, K.; Chartrand, G.; Olivié, D.; Julien, A.-S.; de Guise, J.; et al. Effects of Insulin Glargine and Liraglutide Therapy on Liver Fat as Measured by Magnetic Resonance in Patients with Type 2 Diabetes: A Randomized Trial. Diabetes Care 2015, 38, 1339–1346. [Google Scholar] [CrossRef]
- Eguchi, Y.; Kitajima, Y.; Hyogo, H.; Takahashi, H.; Kojima, M.; Ono, M.; Araki, N.; Tanaka, K.; Yamaguchi, M.; Matsuda, Y.; et al. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol. Res. 2015, 45, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Dutour, A.; Abdesselam, I.; Ancel, P.; Kober, F.; Mrad, G.; Darmon, P.; Ronsin, O.; Pradel, V.; Lesavre, N.; Martin, J.C.; et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: A prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes. Metab. 2016, 18, 882–891. [Google Scholar] [CrossRef]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Umemura, A.; Nishikawa, T.; et al. Effect of 12-week dulaglutide therapy in Japanese patients with biopsy-proven non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol. Res. 2017, 47, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.; Francque, S.; Harrison, S.; Ratziu, V.; Van Gaal, L.; Calanna, S.; Hansen, M.; Linder, M.; Sanyal, A. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment. Pharmacol. Ther. 2019, 50, 193–203. [Google Scholar] [CrossRef]
- Teshome, G.; Ambachew, S.; Fasil, A.; Abebe, M. Efficacy of Glucagon-Like Peptide-1 Analogs in Nonalcoholic Fatty Liver Disease: A Systematic Review. Hepat. Med. 2020, 12, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.N.; Stein, L.M.; Fortin, S.M.; Hayes, M.R. The role of glia in the physiology and pharmacology of glucagon-like peptide-1: Implications for obesity, diabetes, neurodegeneration and glaucoma. Br. J. Pharmacol. 2022, 179, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.X.; Gao, H.; Guo, Y.X.; Wang, B.Y.; Hua, R.X.; Gao, L.; Shang, H.W.; Lu, X.; Xu, J.D. GLP-1 and Underlying Beneficial Actions in Alzheimer’s Disease, Hypertension, and NASH. Front. Endocrinol. 2021, 12, 721198. [Google Scholar] [CrossRef] [PubMed]
- Candeias, E.M. Gut-brain connection: The neuroprotective effects of the anti-diabetic drug liraglutide. World J. Diabetes 2015, 6, 807. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Glotfelty, E.J.; Karlsson, T.; Fortuno, L.V.; Harvey, B.K.; Greig, N.H. The metabolite GLP-1 (9-36) is neuroprotective and anti-inflammatory in cellular models of neurodegeneration. J. Neurochem. 2021, 159, 867–886. [Google Scholar] [CrossRef]
- Athauda, D.; Foltynie, T. Protective effects of the GLP-1 mimetic exendin-4 in Parkinson’s disease. Neuropharmacology 2018, 136, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Foltynie, T.; Athauda, D. Repurposing anti-diabetic drugs for the treatment of Parkinson’s disease: Rationale and clinical experience. Prog. Brain Res. 2020, 252, 493–523. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.; Hua, P.; Dunaief, J.L.; Cui, Q.N.; VanderBeek, B.L. Glucagon-like peptide 1 receptor agonist use is associated with reduced risk for glaucoma. Br. J. Ophthalmol. 2023, 107, 215–220. [Google Scholar] [CrossRef]
- Glotfelty, E.J.; Delgado, T.E.; Tovar-y-Romo, L.B.; Luo, Y.; Hoffer, B.J.; Olson, L.; Karlsson, T.E.; Mattson, M.P.; Harvey, B.K.; Tweedie, D.; et al. Incretin Mimetics as Rational Candidates for the Treatment of Traumatic Brain Injury. ACS Pharmacol. Transl. Sci. 2019, 2, 66–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Chai, S.; Yu, K.; Quan, X.; Yang, Z.; Wu, S.; Zhang, Y.; Ji, L.; Wang, J.; Shi, L. Gastrointestinal Adverse Events of Glucagon-Like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Diabetes Technol. Ther. 2015, 17, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Frid, A.; Hermansen, K.; Shah, N.S.; Tankova, T.; Mitha, I.H.; Zdravkovic, M.; Düring, M.; Matthews, D.R. Efficacy and Safety Comparison of Liraglutide, Glimepiride, and Placebo, All in Combination with Metformin, in Type 2 Diabetes. Diabetes Care 2009, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Nauck, M.; Forst, T.; Sheu, W.H.-H.; Shenouda, S.K.; Heilmann, C.R.; Hoogwerf, B.J.; Gao, A.; Boardman, M.K.; Fineman, M.; et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): A randomised, open-label study. Lancet 2013, 381, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Russell-Jones, D.; Cuddihy, R.M.; Hanefeld, M.; Kumar, A.; González, J.G.; Chan, M.; Wolka, A.M.; Boardman, M.K. Efficacy and Safety of Exenatide Once Weekly Versus Metformin, Pioglitazone, and Sitagliptin Used as Monotherapy in Drug-Naive Patients with Type 2 Diabetes (DURATION-4). Diabetes Care 2012, 35, 252–258. [Google Scholar] [CrossRef]
- Ratner, R.E.; Maggs, D.; Nielsen, L.L.; Stonehouse, A.H.; Poon, T.; Zhang, B.; Bicsak, T.A.; Brodows, R.G.; Kim, D.D. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2006, 8, 419–428. [Google Scholar] [CrossRef]
- Buse, J.B.; Klonoff, D.C.; Nielsen, L.L.; Guan, X.; Bowlus, C.L.; Holcombe, J.H.; Maggs, D.G.; Wintle, M.E. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: An interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin. Ther. 2007, 29, 139–153. [Google Scholar] [CrossRef]
- Wharton, S.; Davies, M.; Dicker, D.; Lingvay, I.; Mosenzon, O.; Rubino, D.M.; Pedersen, S.D. Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: Recommendations for clinical practice. Postgrad. Med. 2022, 134, 14–19. [Google Scholar] [CrossRef]
- Nauck, M.A.; Kemmeries, G.; Holst, J.J.; Meier, J.J. Rapid Tachyphylaxis of the Glucagon-Like Peptide 1–Induced Deceleration of Gastric Emptying in Humans. Diabetes 2011, 60, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Nauck, M.A.; Barnett, A.H.; Feinglos, M.N.; Ovalle, F.; Harman-Boehm, I.; Ye, J.; Scott, R.; Johnson, S.; Stewart, M.; et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): A randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014, 2, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Sorli, C.; Harashima S ichi Tsoukas, G.M.; Unger, J.; Karsbøl, J.D.; Hansen, T.; Bain, S.C. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, H.W.; Lingvay, I.; Reed, J.; de la Rosa, R.; Rose, L.; Sugimoto, D.; Araki, E.; Chu, P.-L.; Wijayasinghe, N.; Norwood, P. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2291–2301. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Bhosekar, V.; Busch, R.; Holst, I.; Ludvik, B.; Thielke, D.; Thrasher, J.; Woo, V.; Philis-Tsimikas, A. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): A randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. New Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Aroda, V.R.; Rosenstock, J.; Terauchi, Y.; Altuntas, Y.; Lalic, N.M.; Morales Villegas, E.C.; Jeppesen, O.K.; Christiansen, E.; Hertz, C.L.; Haluzík, M.; et al. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison with Placebo in Patients with Type 2 Diabetes. Diabetes Care 2019, 42, 1724–1732. [Google Scholar] [CrossRef]
- Pratley, R.; Amod, A.; Hoff, S.T.; Kadowaki, T.; Lingvay, I.; Nauck, M.; Pedersen, K.B.; Saugstrup, T.; Meier, J.J. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): A randomised, double-blind, phase 3a trial. Lancet 2019, 394, 39–50. [Google Scholar] [CrossRef]
- Zinman, B.; Aroda, V.R.; Buse, J.B.; Cariou, B.; Harris, S.B.; Hoff, S.T.; Pedersen, K.B.; Tarp-Johansen, M.J.; Araki, E.; Zinman, B.; et al. Efficacy, Safety, and Tolerability of Oral Semaglutide Versus Placebo Added to Insulin with or without Metformin in Patients with Type 2 Diabetes: The PIONEER 8 Trial. Diabetes Care 2019, 42, 2262–2271. [Google Scholar] [CrossRef]
- Davies, M.; Pieber, T.R.; Hartoft-Nielsen, M.L.; Hansen, O.K.H.; Jabbour, S.; Rosenstock, J. Effect of Oral Semaglutide Compared with Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients with Type 2 Diabetes. JAMA 2017, 318, 1460. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar] [CrossRef]
- Sun, F.; Yu, K.; Yang, Z.; Wu, S.; Zhang, Y.; Shi, L.; Ji, L.; Zhan, S. Impact of GLP-1 Receptor Agonists on Major Gastrointestinal Disorders for Type 2 Diabetes Mellitus: A Mixed Treatment Comparison Meta-Analysis. Exp. Diabetes Res. 2012, 2012, 230624. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Bonora, E.; Nevarez Ruiz, L.; Li, Y.G.; Yu, Z.; Milicevic, Z.; Malik, R.; Bethel, M.A.; Cox, D.A. Efficacy and Safety of Dulaglutide 3.0 mg and 4.5 mg Versus Dulaglutide 1.5 mg in Metformin-Treated Patients with Type 2 Diabetes in a Randomized Controlled Trial (AWARD-11). Diabetes Care 2021, 44, 765–773. [Google Scholar] [CrossRef]
- Van, J.; Frias, J.P.; Bonora, E.; Raha, S.; Meyer, J.; Jung, H.; Cox, D.; Konig, M.; Peleshok, J.; Bethel, M.A. Gastrointestinal Tolerability of Once-Weekly Dulaglutide 3.0 mg and 4.5 mg: A Post Hoc Analysis of the Incidence and Prevalence of Nausea, Vomiting, and Diarrhea in AWARD-11. Diabetes Ther. 2021, 12, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Shomali, M. Optimizing the Care of Patients with Type 2 Diabetes Using Incretin-Based Therapy: Focus on GLP-1 Receptor Agonists. Clin. Diabetes 2014, 32, 32–43. [Google Scholar] [CrossRef]
- Borner, T.; Workinger, J.L.; Tinsley, I.C.; Fortin, S.M.; Stein, L.M.; Chepurny, O.G.; Holz, G.G.; Wierzba, A.J.; Gryko, D.; Nexø, E.; et al. Corrination of a GLP-1 Receptor Agonist for Glycemic Control without Emesis. Cell Rep. 2020, 31, 107768. [Google Scholar] [CrossRef]
- Gutzwiller, J.P.; Hruz, P.; Huber, A.R.; Hamel, C.; Zehnder, C.; Drewe, J.; Gutmann, H.; Stanga, Z.; Vogel, D.; Beglinger, C. Glucagon-Like Peptide-1 Is Involved in Sodium and Water Homeostasis in Humans. Digestion. 2006, 73, 142–150. [Google Scholar] [CrossRef]
- Xiao, C.; Bandsma, R.H.J.; Dash, S.; Szeto, L.; Lewis, G.F. Exenatide, a Glucagon-like Peptide-1 Receptor Agonist, Acutely Inhibits Intestinal Lipoprotein Production in Healthy Humans. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1513–1519. [Google Scholar] [CrossRef]
- Langmach Wegeberg, A.; Hansen, C.S.; Farmer, A.D.; Karmisholt, J.S.; Drewes, A.M.; Jakobsen, P.E.; Brock, B.; Brock, C. Liraglutide accelerates colonic transit in people with type 1 diabetes and polyneuropathy: A randomised, double-blind, placebo-controlled trial. United Eur. Gastroenterol. J. 2020, 8, 695–704. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Carraro, R.; Finer, N.; Hartvig, H.; Lindegaard, M.L.; Rössner, S.; Van Gaal, L.; Astrup, A. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int. J. Obes. 2014, 38, 689–697. [Google Scholar] [CrossRef]
- Ahrén, B.; Atkin, S.L.; Charpentier, G.; Warren, M.L.; Wilding JP, H.; Birch, S.; Holst, A.G.; Leiter, L.A. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes. Metab. 2018, 20, 2210–2219. [Google Scholar] [CrossRef]
- Lingvay, I.; Hansen, T.; Macura, S.; Marre, M.; Nauck, M.A.; de la Rosa, R.; Woo, V.; Yildirim, E.; Wilding, J. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res. Care 2020, 8, e001706. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, W.A.; Kumar, A.A.; Naguib, H.S.; Taylor, H.C. Exenatide-Induced Acute Pancreatitis. Endocr. Pract. 2010, 16, 80–83. [Google Scholar] [CrossRef]
- Denker, P.S.; Dimarco, P.E. Exenatide (Exendin-4)–Induced Pancreatitis. Diabetes Care 2006, 29, 471. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Sugitani, S.; Yamaji, S.; Otsu, S.; Higashi, Y.; Ohtomo, Y.; Inoue, G. Pancreatitis with Pancreatic Tail Swelling Associated with Incretin-based Therapies Detected Radiologically in Two Cases of Diabetic Patients with End-Stage Renal Disease. Intern. Med. 2012, 51, 3045–3049. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, H.; Huang, L.; Yang, Y.; Tian, B.; Yu, C. Exenatide-Induced Chronic Damage of Pancreatic Tissue in Rats. Pancreas 2012, 41, 1235–1240. [Google Scholar] [CrossRef]
- Gier, B.; Matveyenko, A.V.; Kirakossian, D.; Dawson, D.; Dry, S.M.; Butler, P.C. Chronic GLP-1 Receptor Activation by Exendin-4 Induces Expansion of Pancreatic Duct Glands in Rats and Accelerates Formation of Dysplastic Lesions and Chronic Pancreatitis in the KrasG12D Mouse Model. Diabetes 2012, 61, 1250–1262. [Google Scholar] [CrossRef]
- Lando, H.M.; Alattar, M.; Dua, A.P. Elevated Amylase and Lipase Levels in Patients Using Glucagonlike Peptide-1 Receptor Agonists or Dipeptidyl-Peptidase-4 Inhibitors in the Outpatient Setting. Endocr. Pract. 2012, 18, 472–477. [Google Scholar] [CrossRef]
- Dore, D.D.; Seeger, J.D.; Chan, K.A. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr. Med. Res. Opin. 2009, 25, 1019–1027. [Google Scholar] [CrossRef]
- Funch, D.; Gydesen, H.; Tornøe, K.; Major-Pedersen, A.; Chan, K.A. A prospective, claims-based assessment of the risk of pancreatitis and pancreatic cancer with liraglutide compared to other antidiabetic drugs. Diabetes Obes. Metab. 2014, 16, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Giorda, C.B.; Nada, E.; Tartaglino, B.; Marafetti, L.; Gnavi, R. A systematic review of acute pancreatitis as an adverse event of type 2 diabetes drugs: From hard facts to a balanced position. Diabetes Obes. Metab. 2014, 16, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.G.; Blind, E.; Dunder, K.; de Graeff, P.A.; Hummer, B.T.; Bourcier, T.; Rosebraugh, C. Pancreatic Safety of Incretin-Based Drugs—FDA and EMA Assessment. New Engl. J. Med. 2014, 370, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Best, J.H.; Hoogwerf, B.J.; Herman, W.H.; Pelletier, E.M.; Smith, D.B.; Wenten, M.; Hussein, M.A. Risk of Cardiovascular Disease Events in Patients with Type 2 Diabetes Prescribed the Glucagon-Like Peptide 1 (GLP-1) Receptor Agonist Exenatide Twice Daily or Other Glucose-Lowering Therapies. Diabetes Care 2011, 34, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.J.; Habacher, W.; Augustin, T.; Krahulec, E.; Semlitsch, T. A systematic review and meta-analysis of the efficacy of lixisenatide in the treatment of patients with type 2 diabetes. Diabetes Obes. Metab. 2014, 16, 769–779. [Google Scholar] [CrossRef]
- Katout, M.; Zhu, H.; Rutsky, J.; Shah, P.; Brook, R.D.; Zhong, J.; Rajagopalan, S. Effect of GLP-1 Mimetics on Blood Pressure and Relationship to Weight Loss and Glycemia Lowering: Results of a Systematic Meta-Analysis and Meta-Regression. Am. J. Hypertens. 2014, 27, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.E.; Holt, T.A.; Rees, K.; Randeva, H.S.; O’Hare, J.P. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: Systematic review and meta-analysis. BMJ Open 2013, 3, e001986. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Reil, J.C.; Deedwania, P.; Kim, J.B.; Borer, J.S. Resting Heart Rate: Risk Indicator and Emerging Risk Factor in Cardiovascular Disease. Am. J. Med. 2015, 128, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.J.; Khutoryansky, N.; Zdravkovic, M.; Sprenger, C.R.; Litwin, J.S. Absence of QTc Prolongation in a Thorough QT Study with Subcutaneous Liraglutide, a Once-Daily Human GLP-1 Analog for Treatment of Type 2 Diabetes. J. Clin. Pharmacol. 2009, 49, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ratner, R.E.; Han, J.; Kim, D.D.; Fineman, M.S.; Baron, A.D. Effects of Exenatide (Exendin-4) on Glycemic Control and Weight Over 30 Weeks in Metformin-Treated Patients with Type 2 Diabetes. Diabetes Care 2005, 28, 1092–1100. [Google Scholar] [CrossRef]
- Zinman, B.; Hoogwerf, B.J.; Durán García, S.; Milton, D.R.; Giaconia, J.M.; Kim, D.D.; Trautmann, M.E.; Brodows, R.G. The Effect of Adding Exenatide to a Thiazolidinedione in Suboptimally Controlled Type 2 Diabetes. Ann. Intern. Med. 2007, 146, 477. [Google Scholar] [CrossRef]
- Ratner, R.E.; Rosenstock, J.; Boka, G. Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with Type 2 diabetes inadequately controlled with metformin: A randomized, double-blind, placebo-controlled trial. Diabet. Med. 2010, 27, 1024–1032. [Google Scholar] [CrossRef]
- Pencek, R.; Brunell, S.C.; Li, Y.; Hoogwerf, B.J.; Malone, J. Exenatide Once Weekly for the Treatment of Type 2 Diabetes Mellitus: Clinical Results in Subgroups of Patients Using Different Concomitant Medications. Postgrad. Med. 2012, 124, 33–40. [Google Scholar] [CrossRef]
- Gao, Y.; Yoon, K.H.; Chuang, L.M.; Mohan, V.; Ning, G.; Shah, S.; Jang, H.C.; Wu, T.-J.; Johns, D.; Northrup, J.; et al. Efficacy and safety of exenatide in patients of Asian descent with type 2 diabetes inadequately controlled with metformin or metformin and a sulphonylurea. Diabetes Res. Clin. Pract. 2009, 83, 69–76. [Google Scholar] [CrossRef]
- Levin, P.A.; Mersey, J.H.; Zhou, S.; Bromberger, L.A. Clinical Outcomes Using Long-Term Combination Therapy with Insulin Glargine and Exenatide in Patients with Type 2 Diabetes Mellitus. Endocr. Pract. 2012, 18, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.J.; Zhang, Q.M.; Lv, L.; Chen, R.; Lv, C.; Yu, P.; Yu, D. Efficacy and safety comparison between liraglutide as add-on therapy to insulin and insulin dose-increase in Chinese subjects with poorly controlled type 2 diabetes and abdominal obesity. Cardiovasc. Diabetol. 2012, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Exenatide SPC. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf (accessed on 15 October 2023).
- Dulaglutide-EMA Assessment Report. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002825/WC500179473.pdf (accessed on 15 October 2023).
- Lixisenatide SPC. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf (accessed on 15 October 2023).
- Liraglutide SPC. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf (accessed on 15 October 2023).
- Albiglutide SPC. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002735/WC500165117.pdf (accessed on 15 October 2023).
- Faludi, P.; Brodows, R.; Burger, J.; Ivanyi, T.; Braun, D.K. The effect of exenatide re-exposure on safety and efficacy. Peptides 2009, 30, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Sheng, H.; Zhang, M.; Bu, L.; Yang, P.; Li, L.; Li, F.; Sheng, C.; Han, Y.; Qu, S.; et al. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists’ treatment: A meta-analysis of randomized controlled trials. Endocrine 2015, 48, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Exenatide Once Weekly SPC. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002020/WC500108241.pdf (accessed on 17 October 2023).
- Boysen, N.C.; Stone, M.S. Eosinophil-rich granulomatous panniculitis caused by exenatide injection. J. Cutan. Pathol. 2014, 41, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Elisaf, M.S. Effects of glucagon-like peptide-1 receptor agonists on renal function. World J. Diabetes 2013, 4, 190. [Google Scholar] [CrossRef]
- Johansen, O.E.; Whitfield, R. Exenatide may aggravate moderate diabetic renal impairment: A case report. Br. J. Clin. Pharmacol. 2008, 66, 568–569. [Google Scholar] [CrossRef]
- Weise, W.J.; Sivanandy, M.S.; Block, C.A.; Comi, R.J. Exenatide-Associated Ischemic Renal Failure. Diabetes Care 2009, 32, e22–e23. [Google Scholar] [CrossRef]
- Gutzwiller, J.P.; Tschopp, S.; Bock, A.; Zehnder, C.E.; Huber, A.R.; Kreyenbuehl, M.; Gutmann, H.; Drewe, J.; Henzen, C.; Goeke, B.; et al. Glucagon-Like Peptide 1 Induces Natriuresis in Healthy Subjects and in Insulin-Resistant Obese Men. J. Clin. Endocrinol. Metab. 2004, 89, 3055–3061. [Google Scholar] [CrossRef]
- Gurney, K.; MacConell, L.; Brown, C.; Han, J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: Integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab Syndr Obes. 2012, 5, 29–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pendergrass, M.; Fenton, C.; Haffner, S.M.; Chen, W. Exenatide and sitagliptin are not associated with increased risk of acute renal failure: A retrospective claims analysis. Diabetes Obes. Metab. 2012, 14, 596–600. [Google Scholar] [CrossRef]
- Suran, M. As Ozempic’s Popularity Soars, Here’s What to Know About Semaglutide and Weight Loss. JAMA 2023, 329, 1627. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.A.; Waltzman, J.T.; Tadisina, K.K.; Rueda, S.; Richards, B.G.; Schleicher, W.F.; Marten, E.; Larson, J.D.; Rotemberg, S.C.; Zins, J.E. Objective Assessment of Facial Rejuvenation After Massive Weight Loss. Aesthetic Plast. Surg. 2015, 39, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.A.; Charafeddine, A.H.; Zins, J.E. Facelift in Patients with Massive Weight Loss. Clin. Plast. Surg. 2019, 46, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Cohen, V.; Teperikidis, E.; Jellinek, S.P.; Rose, J. Acute exenatide (Byetta®) poisoning was not associated with significant hypoglycemia. Clin. Toxicol. 2008, 46, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, L.; Dhatariya, K.; Gerontitis, D. No clinical harm from a massive exenatide overdose—A short report. Clin. Toxicol. 2013, 51, 61. [Google Scholar] [CrossRef] [PubMed]
- Bode, S.F.N.; Egg, M.; Wallesch, C.; Hermanns-Clausen, M. 10-Fold Liraglutide Overdose Over 7 Months Resulted Only in Minor Side-Effects. J. Clin. Pharmacol. 2013, 53, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Saxenda Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206321s007lbl.pdf (accessed on 15 October 2023).
- Gough, S.C.L.; Bode, B.; Woo, V.; Rodbard, H.W.; Linjawi, S.; Poulsen, P.; Damgaard, L.H.; Buse, J.B. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: Results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014, 2, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, P.; Chepurny, O.G.; Holz, G.G. Regulation of Glucose Homeostasis by GLP-1. Prog. Mol. Biol. Transl. Sci. 2014, 121, 23–65. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Waldrop, G.; Zhong, J.; Peters, M.; Goud, A.; Chen, Y.-H.; Davis, S.N.; Mukherjee, B.; Rajagopalan, S. Incretin-based therapy in type 2 diabetes: An evidence based systematic review and meta-analysis. J. Diabetes Complicat. 2018, 32, 113–122. [Google Scholar] [CrossRef]
- Garvey, W.T.; Birkenfeld, A.L.; Dicker, D.; Mingrone, G.; Pedersen, S.D.; Satylganova, A.; Skovgaard, D.; Sugimoto, D.; Jensen, C.; Mosenzon, O. Efficacy and Safety of Liraglutide 3.0 mg in Individuals with Overweight or Obesity and Type 2 Diabetes Treated with Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes Care 2020, 43, 1085–1093. [Google Scholar] [CrossRef]
- Wadden, T.A.; Hollander, P.; Klein, S.; Niswender, K.; Woo, V.; Hale, P.M.; Aronne, L. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: The SCALE Maintenance randomized study. Int. J. Obes. 2013, 37, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Carraro, R.; Finer, N.; Harper, A.; Kunesova, M.; Lean, M.E.J.; Niskanen, L.; Rasmussen, M.F.; Rissanen, A.; Rössner, S.; et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. 2012, 36, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes. Metab. 2022, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Kramer, C.K.; Zinman, B.; Choi, H.; Retnakaran, R. Effect of chronic liraglutide therapy and its withdrawal on time to postchallenge peak glucose in type 2 diabetes. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E287–E295. [Google Scholar] [CrossRef] [PubMed]
- Kobori, T.; Onishi, Y.; Yoshida, Y.; Tahara, T.; Kikuchi, T.; Kubota, T.; Iwamoto, M.; Sawada, T.; Kobayashi, R.; Fujiwara, H.; et al. Association of glucagon-like peptide-1 receptor agonist treatment with gastric residue in an esophagogastroduodenoscopy. J. Diabetes Investig. 2023, 14, 767–773. [Google Scholar] [CrossRef]
- GI Multi-Society Statement Regarding GLP-1 Agonists and Endoscopy. Available online: https://www.aasld.org/news/gi-multi-society-statement-regarding-glp-1-agonists-and-endoscopy (accessed on 29 October 2023).
- Joshi, G.P.; Abdelmalak, B.B.; Weigel, W.A.; Soriano, S.G.; Harbell, M.W.; Kuo, C.I.; Stricker, P.A.; Domino, K.B. American Society of Anesthesiologists Consensus-Based Guidance on Preoperative Management of Patients (Adults and Children) on Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists; American Society of Anesthesiologists: Schaumburg, IL, USA, 2023. [Google Scholar]
- Giruzzi, N. Plenity (Oral Superabsorbent Hydrogel). Clin. Diabetes 2020, 38, 313–314. [Google Scholar] [CrossRef]

| Drug Name | Approval Date & Use(s) | Dosing and Administration | Key Precautions and Side Effects | Clinical and Post-Marketing | Use in Populations and Conclusion |
|---|---|---|---|---|---|
| Albiglutide (Tanzeum/Eperzan) [7,8] |
|
|
|
|
|
| Dulaglutide (Trulicity) [9] |
|
|
|
|
|
| Exenatide (Byetta/Bydureon) [10] |
|
|
|
|
|
| Liraglutide (Victoza, Saxenda) [11,12] |
|
|
|
|
|
|
|
|
|
| |
| Lixisenatide (Adlyxin) [13,14] |
|
|
|
|
|
| Semaglutide (Ozempic/Rybelsus/Wegovy) [14] |
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, J.; Ferrari, C.; Tadros, M. GLP-1RA Essentials in Gastroenterology: Side Effect Management, Precautions for Endoscopy and Applications for Gastrointestinal Disease Treatment. Gastroenterol. Insights 2024, 15, 191-212. https://doi.org/10.3390/gastroent15010014
Wan J, Ferrari C, Tadros M. GLP-1RA Essentials in Gastroenterology: Side Effect Management, Precautions for Endoscopy and Applications for Gastrointestinal Disease Treatment. Gastroenterology Insights. 2024; 15(1):191-212. https://doi.org/10.3390/gastroent15010014
Chicago/Turabian StyleWan, Justin, Caesar Ferrari, and Micheal Tadros. 2024. "GLP-1RA Essentials in Gastroenterology: Side Effect Management, Precautions for Endoscopy and Applications for Gastrointestinal Disease Treatment" Gastroenterology Insights 15, no. 1: 191-212. https://doi.org/10.3390/gastroent15010014
APA StyleWan, J., Ferrari, C., & Tadros, M. (2024). GLP-1RA Essentials in Gastroenterology: Side Effect Management, Precautions for Endoscopy and Applications for Gastrointestinal Disease Treatment. Gastroenterology Insights, 15(1), 191-212. https://doi.org/10.3390/gastroent15010014






