Spatial Responses of Net Ecosystem Productivity of the Yellow River Basin under Diurnal Asymmetric Warming
Abstract
:1. Introduction
2. Overview of Study Area and Data Sources
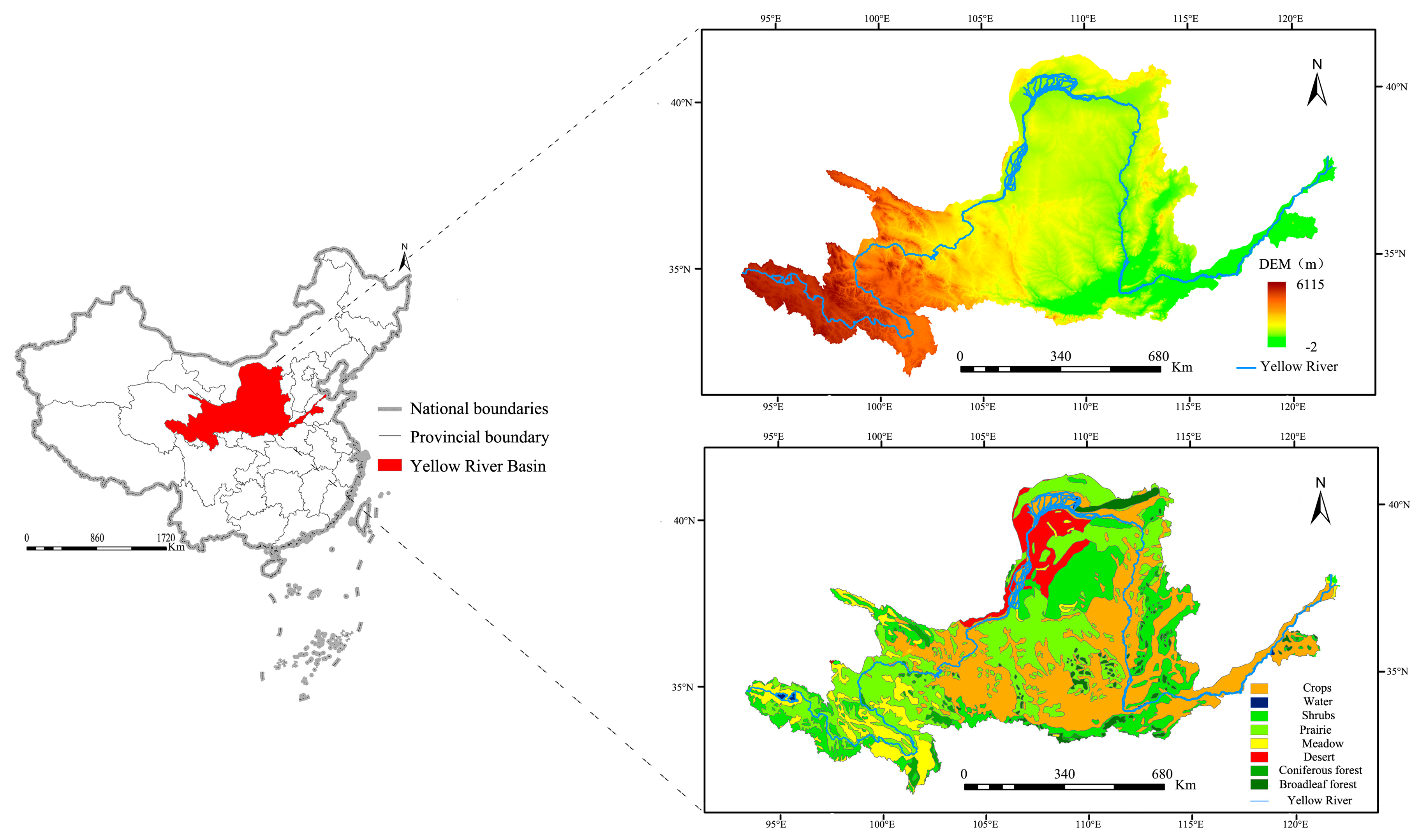
2.1. Overview of Study Area
2.2. Data Sources
2.2.1. Remote Sensing Data of the Normalized Difference Vegetation Index (NDVI)
2.2.2. Meteorological Data
2.2.3. Vegetation Data
3. Research Method and Models
3.1. The modified CASA Model for Calculating NPP
3.2. Calculation of NEP
3.3. Analysis of NEP Changes
3.3.1. Model for Slope Analysis
3.3.2. Model for Stability Analysis (CV)
3.3.3. Hurst Exponent Model Based on R/S Analysis
3.4. Partial Correlation Analysis between the Nep and Climatic Factors
4. Results and Discussion
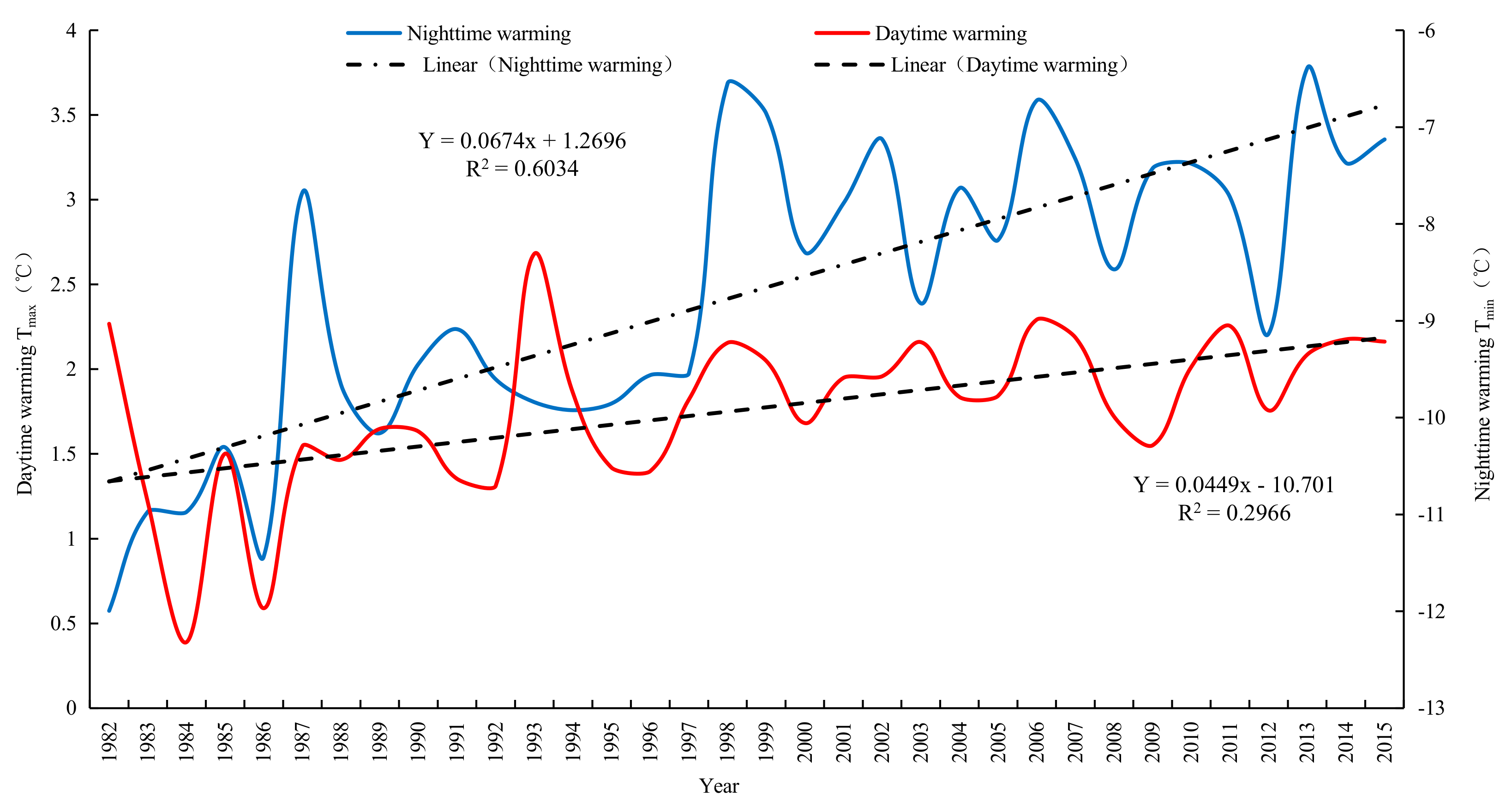
4.1. Comparative Analysis of the YRB’s Diurnal Asymmetric Warming and NPP
4.2. Response of the YRB’s NEP to Diurnal Asymmetric Warming
5. Conclusions
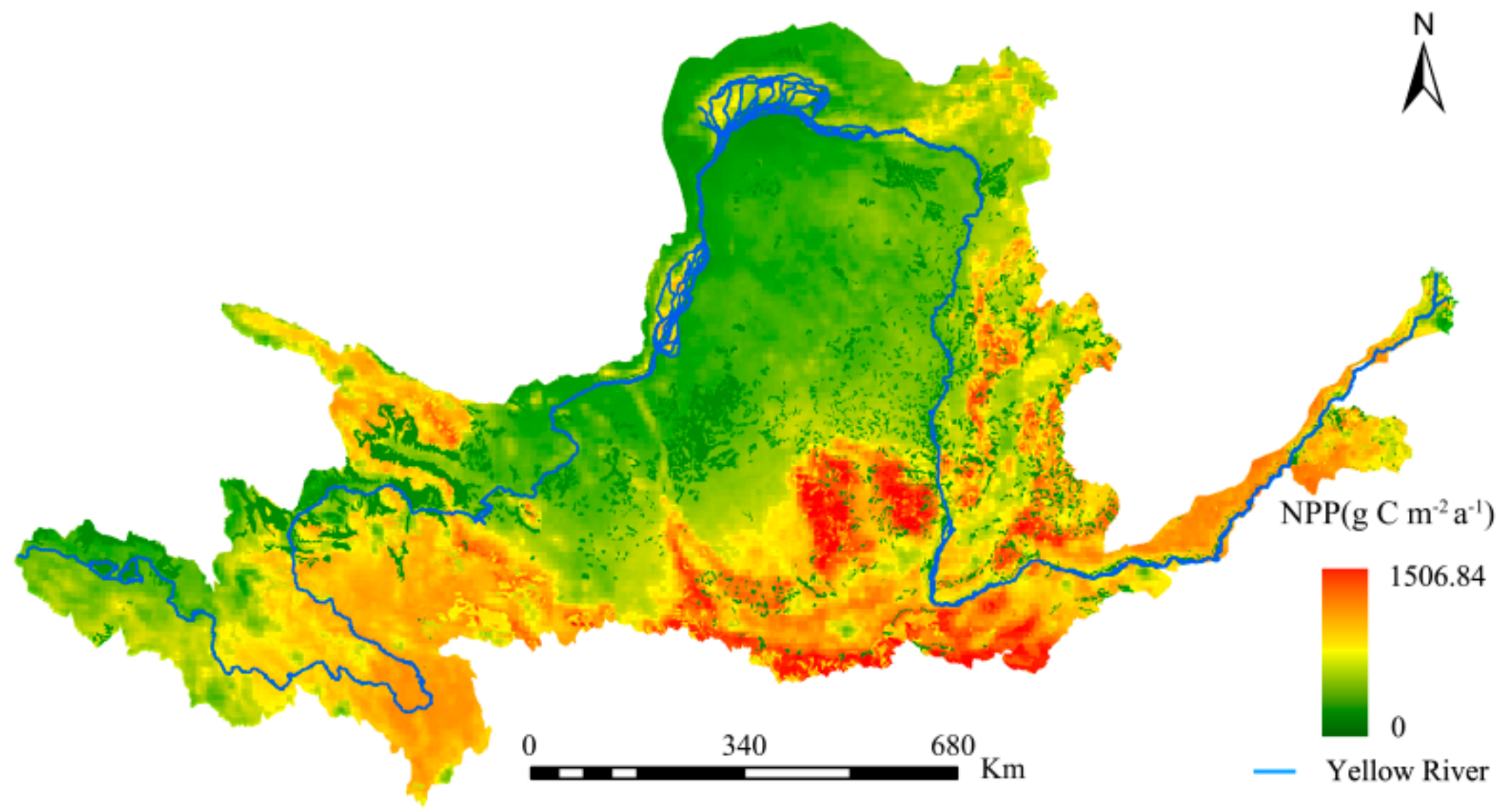
- The YRB experienced obvious diurnal asymmetric warming from 1982 to 2015, with the rate of increase for Tmin being 1.50 times that of Tmax. The total NPP showed an overall linear upward trend with large inter-annual fluctuations. In terms of spatial distribution, the YRB’s NPP exhibited a pattern of high spatial differentiation, with low values in the northern region and high values in western and southeastern regions.
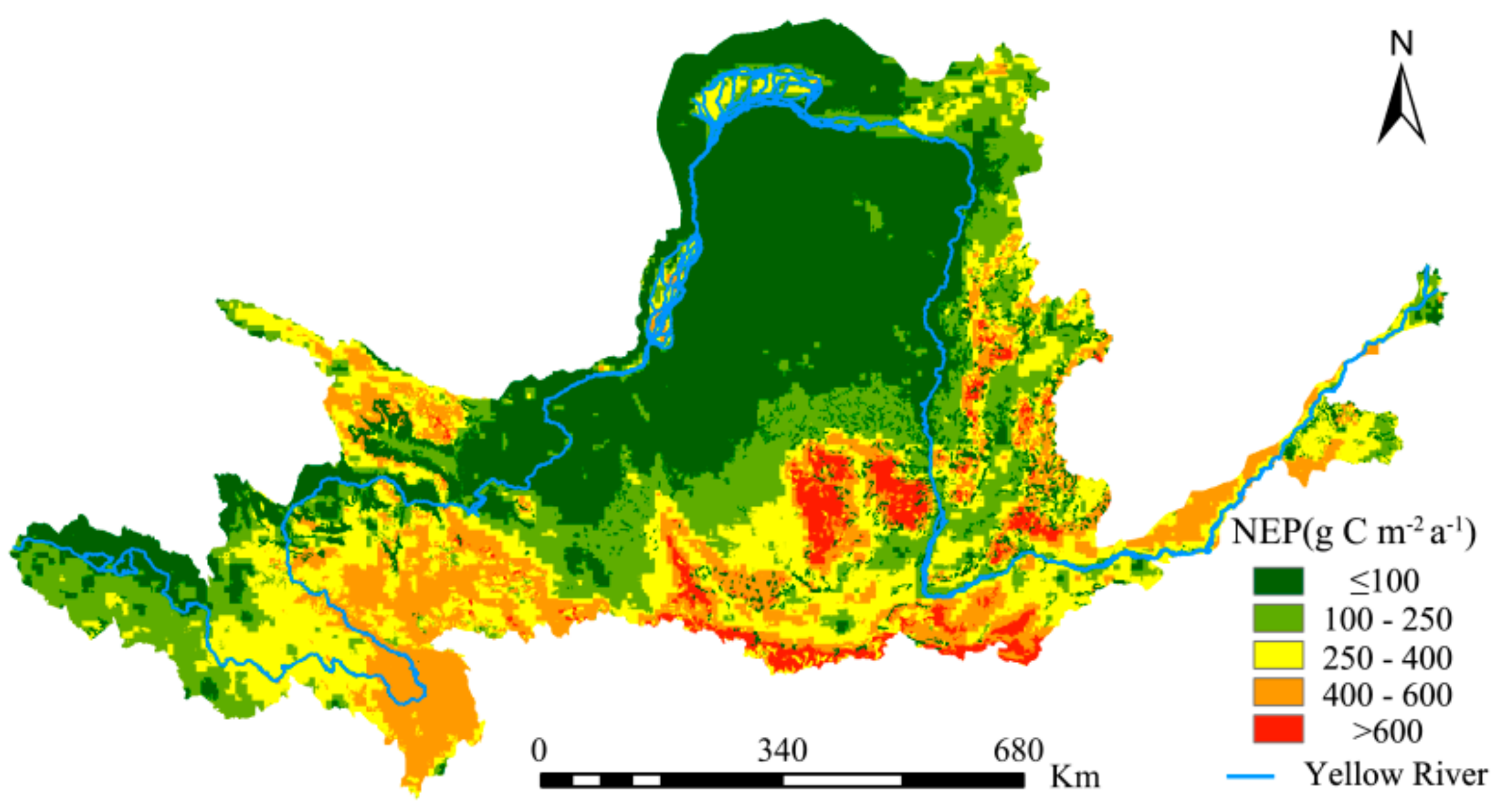
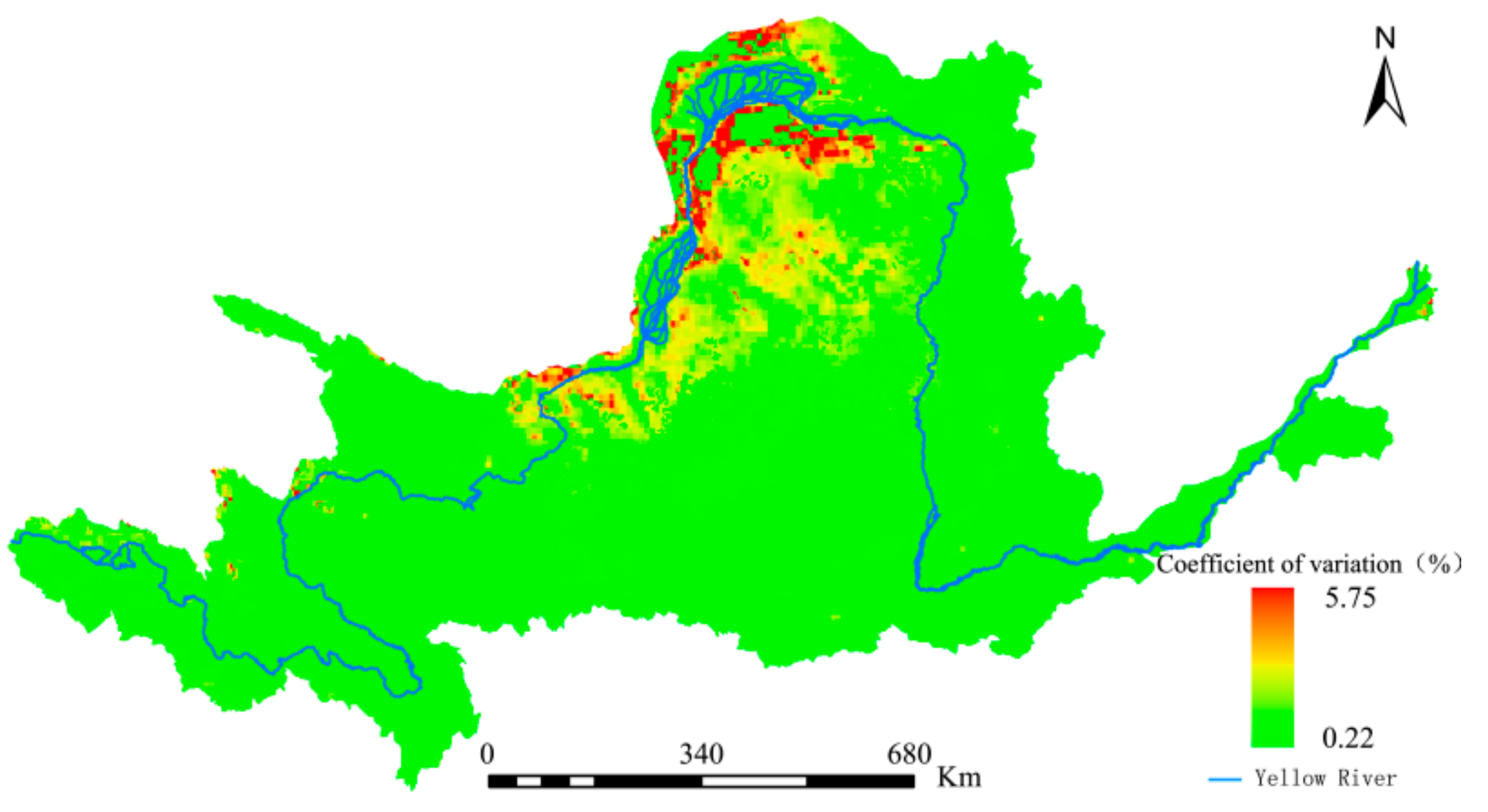
- Temporal variations of the YRB’s NEP were characterized by upward fluctuations. There were substantial variations in NEP between the various vegetation types in the following order: broadleaf forests > coniferous forests and meadows and marshes > shrubs and coppice forests > agricultural crops > grasslands and savannah bushes > deserts. For temporal fluctuations of NEP arising from different vegetation types, the characteristics were similar. Spatially, NEP values were highest in western and southeastern regions of the YRB, and lowest in the northern region. Overall, the NEP within the basin were relatively stable.
- There were significant spatial differences in terms of the impacts of diurnal warming on the YRB’s vegetation carbon sequestration capacity, which were enhanced by daytime warming but significantly inhibited by nighttime warming. The numbers of areas with nighttime warming that passed significance testing were slightly higher than those with daytime warming.
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability; Cambridge University Press (IPCC Secretariat): Cambridge, UK, 2014. [Google Scholar]
- Schuur, E.A.G.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Solow, A.R. Global warming: A call for peace on climate and conflict. Nature 2013, 497, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Hoegh, G.O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Oreskes, N.; Doran, P.T.; Anderegg, W.R.L.; Verheggen, B.; Maibach, E.W.; Carlton, J.S.; Lewandowsky, S.; Skuce, A.G.; Green, S.A. Consensus on consensus: A synthesis of consensus estimates on human-caused global warming. Environ. Res. Lett. 2016, 11, 048002. [Google Scholar] [CrossRef]
- Ang, B.W.; Su, B. Carbon emission intensity in electricity production: A global analysis. Energy Policy 2016, 94, 56–63. [Google Scholar] [CrossRef]
- Liu, Z.; Guan, D.; Wei, W.; Davis, S.J.; Ciais, P.; Bai, J.; Peng, S.S.; Zhang, Q.; Hubacek, K.; Marland, G.; et al. Reduced carbon emission estimates from fossil fuel combustion and cement production in China. Nature 2015, 524, 335–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, M.; Dooley, J.; Luckow, P.; Calvin, K.; Kyle, P. Agriculture, land use, energy and carbon emission impacts of global biofuel mandates to mid-century. Appl. Energy 2014, 114, 763–773. [Google Scholar] [CrossRef]
- Shuai, C.; Shen, L.; Jiao, L.; Wu, Y.; Tan, Y.T. Identifying key impact factors on carbon emission: Evidences from panel and time-series data of 125 countries from 1990 to 2011. Appl. Energy 2017, 187, 310–325. [Google Scholar] [CrossRef]
- Schimel, D.; Pavlick, R.; Fisher, J.B. Observing terrestrial ecosystems and the carbon cycle from space. Glob. Chang. Biol. 2015, 21, 1762–1776. [Google Scholar] [CrossRef] [PubMed]
- Schimel, D.; Stephens, B.B.; Fisher, J.B. Effect of increasing CO2 on the terrestrial carbon cycle. Proc. Natl. Acad. Sci. USA 2015, 112, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Friend, A.D.; Lucht, W.; Rademacher, T.T.; Asner, G.P.; Saatchi, S.; Townsend, P.; Miller, C.; Frankenberg, C.; Hibbard, K.; Cox, P. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2. Proc. Natl. Acad. Sci. USA 2014, 111, 3280–3285. [Google Scholar] [CrossRef] [PubMed]
- Reyer, C.; Lasch-Born, P.; Suckow, F.; Gutsch, M.; Murawski, A.; Pilz, T. Projections of regional changes in forest net primary productivity for different tree species in Europe driven by climate change and carbon dioxide. Ann. For. Sci. 2014, 71, 211–225. [Google Scholar] [CrossRef]
- Seaquist, J.W.; Olsson, L.; Ouml, J.A. A remote sensing-based primary production model for grassland biomes. Ecol. Model. 2003, 169, 131–155. [Google Scholar] [CrossRef]
- Lieth, H.; Whittaker, R.H. (Eds.) Primary Productivity of the Biosphere; Springer: New York, NY, USA, 1975; p. 274. [Google Scholar]
- Melillo, J.M.; McGuire, A.D.; Kicklighter, D.W.; Moore, B.; Vorosmarty, C.J.; Schloss, A.L. Global climate change and terrestrial net primary production. Nature 1993, 363, 234. [Google Scholar] [CrossRef]
- Nemani, R.R.; Keeling, C.D.; Hashimoto, H.; Jolly, W.M. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 2003, 300, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Running, S.W.; Nemani, R.R.; Heinsch, F.A.; Zhao, M.; Reeves, M.; Hashimoto, H. A Continuous Satellite-Derived Measure of Global Terrestrial Primary Production. BioScience 2004, 54, 547–560. [Google Scholar] [CrossRef]
- Reeves, M.C.; Moreno, A.L.; Bagne, K.E.; Running, S.W. Estimating climate change effects on net primary production of rangelands in the United States. Clim. Chang. 2014, 126, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, E.M. The Conservation programme of the international biological programme (IBP). Polar Record 1969, 14, 760–764. [Google Scholar] [CrossRef]
- An, N.; Price, K.; Blair, J. Estimating above-ground net primary productivity of the tallgrass prairie ecosystem of the Central Great Plains using AVHRR NDVI. Int. J. Remote Sens. 2013, 34, 3717–3735. [Google Scholar] [CrossRef]
- Potter, C.S.; Randerson, J.T.; Field, C.B.; Matson, P.A.; Vitousek, P.M.; Mooney, H.A.; Klooster, S.A. Terrestrial ecosystem production: a process model based on global satellite and surface data. Glob. Biogeochem. Cycles 1993, 7, 811–841. [Google Scholar] [CrossRef]
- Hicke, J.A.; Asner, G.P.; Randerson, J.T. Satellite-derived increases in net primary productivity across North America, 1982–1998. Geophys. Res. Lett. 2002, 29, 1–4. [Google Scholar] [CrossRef]
- Nayak, R.K.; Patel, N.R.; Dadhwal, V.K. Estimation and analysis of terrestrial net primary productivity over India by remote-sensing-driven terrestrial biosphere model. Environ. Monit. Assess. 2010, 170, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Zhang, R.; Liu, T.; Song, X.; Adams, M.A. Empirical and model-based estimates of spatial and temporal variations in net primary productivity in semi-arid grasslands of Northern China. PLoS ONE 2017, 12, e0187678. [Google Scholar] [CrossRef]
- Liu, Z.D.; Li, B.; Fang, X.; Tucker, C.; Los, S.; Birdsey, R.; Jenkins, J.C.; Field, C.; Holland, E. Dynamic characteristics of forest carbon storage and carbon density in the Hunan Province. Acta Ecol. Sin. 2016, 36, 6897–6908. (In Chinese) [Google Scholar] [CrossRef]
- Huang, C.D.; Zhang, J.; Yang, W.Q.; Tang, X.; Zhao, A.J. Dynamics on forest carbon stock in Sichuan and Chongqing city. Acta Ecol. Sin. 2008, 28, 966–975. (In Chinese) [Google Scholar] [CrossRef]
- Pan, J.H.; Huang, K.J.; Li, Z. Spatio-temporal variation in vegetation net primary productivity and its relationship with climatic factors in the Shule River basin from 2001 to 2010. Acta Ecol. Sin. 2017, 37, 1888–1899. [Google Scholar] [CrossRef]
- Mao, D.H.; Wang, Z.M.; Han, J.X.; Ren, C.Y. Spatio-temporal pattern of net primary productivity and its driven factors in Northeast China in 1982–2010. Sci. Geogr. Sin. 2012, 32, 1106–1111. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, Z.; Piao, S.L.; Myneni, R.B.; Huang, M.T.; Zeng, Z.Z.; Canadell, J.G.; Ciais, P.; Sitch, S.; Friedlingstein, P.; Arneth, A.; et al. Greening of the Earth and its drivers. Nat. Clim. Chang. 2016, 6, 791–795. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Liu, X.J.; Du, Z.Q.; Wu, Z.T.; Xu, X.M. Effects of the asymmetric diurnal-warming on vegetation dynamics in Xinjiang. China Environ. Sci. 2017, 37, 2316–2321. (In Chinese) [Google Scholar]
- Bates, D.; Lindstrom, M.; Wahba, G. Gcvpack-routines for generalized cross validation. Commun. Stat. Simul. Comput. 1987, 16, 263–297. [Google Scholar] [CrossRef]
- Kobayashi, H.; Dye, D.G. Atmospheric conditions for monitoring the long-term vegetation dynamics in the Amazon using normalized difference vegetation index. Remote. Sens. Environ. 2005, 97, 519–525. [Google Scholar] [CrossRef]
- Guo, N.; Zhu, Y.J.; Wang, J.M.; Deng, C.P. The relationship between NDVI changes and climatic factors in different types of vegetation in Northwest China over the past 22 years. Chin. J. Plant Ecol. 2008, 32, 319–327. (In Chinese) [Google Scholar] [CrossRef]
- China Meteorological Administration. Standard for Ground Meteorological Observation; GBT352212017; Meteorology Press: Beijing, China, 2003. (In Chinese)
- China Meterological Administration. People’s Republic of China Meteorological Industry Standard (QX/T 22-2004); Ground climate data for 30 years and its statistical methods; QXT222004; China Meterological Administration: Beijing, China, 2007. (In Chinese)
- Tan, J.B.; Li, A.N.; Lei, G.B. Contrast on Anusplin and Cokriging meteorological spatial interpolation in southeastern margin of Qinghai-Xizang Plateau. Plateau Meteorol. 2016, 35, 875–886. (In Chinese) [Google Scholar] [CrossRef]
- Ren, X.; Zheng, J.H.; Mu, C.; Yan, K.; Xu, Y.B. Reliability evaluation of different meteorological interpolation methods in the NPP estimation of grassland in Xinjiang. Pratac. Sci. 2017, 3, 439–448. (In Chinese) [Google Scholar] [CrossRef]
- China Academy of Sciences (Chinese Academy of Sciences) Editorial Board of Vegetation Map. 1:1000000 Vegetation Atlas of China; Science Press: Beijing, China, 2001. (In Chinese) [Google Scholar]
- Potter, C.S. Terrestrial biomass and the effects of deforestation on the global carbon cycle: Results from a model of primary production using satellite observations. Bioscience 1999, 49, 769–778. [Google Scholar] [CrossRef]
- Zhu, W.Q. Remote Sensing Estimation of Net Primary Productivity of Vegetation in China Land Ecosystem and Its Relationship with Climate Change. Ph.D. Thesis, Beijing Normal University, Beijing, China, 2005. (In Chinese). [Google Scholar]
- Zhu, W.Q.; Chen, Y.Y.; Xu, D.; Li, J. Advances in terrestrial net primary productivity (NPP) estimation models. Chin. J. Ecol. 2005, 24, 296–300. (In Chinese) [Google Scholar] [CrossRef]
- Piao, S.L.; Fang, J.Y.; Guo, Q.H. Using CASA model to estimate the net primary productivity of vegetation in China. Chin. J. Plant Ecol. 2001, 25, 603–608. (In Chinese) [Google Scholar] [CrossRef]
- Ruimy, A.; Saugier, B.; Dedieu, G. Methodology for the estimation of terrestrial net primary production from remotely sensed data. J. Geophys. Res.-A 1994, 99, 5263–5283. [Google Scholar] [CrossRef]
- Sellers, P.J. Canopy reflectance, photosynthesis, and transpiration, II. The role of biophysics in the linearity of their interdependence. Int. J. Remote Sens. 1992, 6, 1335–1372. [Google Scholar] [CrossRef]
- Huemmrich, K.F.; Goward, S.N. Spectral vegetation indexes and the remote sensing of biophysical parameters. IGARSS 1992, 2, 1017–1019. [Google Scholar] [CrossRef]
- Field, C.B.; Randerson, J.T.; Malmström, C.M. Global net primary production: Combining ecology and remote sensing. Remote Sens. Environ. 1995, 51, 74–88. [Google Scholar] [CrossRef] [Green Version]
- Los, S.O.; Justice, C.O.; Tucker, C.J. A global 1 by 1 NDVI data set for climate studies derived from the GIMMS continental NDVI data. Int. J. Remote Sens. 1994, 15, 3493–3518. [Google Scholar] [CrossRef]
- Sellers, P.J.; Randall, D.A.; Collat, G.J.; Berry, J.A.; Field, C.B. A revised land surface parameterization (SiB2) for atmospheric GCMS. Part I: Model formulation. J. Clim. 1996, 9, 706–737. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Pan, Y.Z.; He, H.; Yu, D.Y.; Hu, H.B. Simulation of maximum light utilization rate of typical vegetation in China. Chin. Sci. Bull. 2006, 51, 700–706. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Pan, Y.Z.; Zhang, J.S. Estimation of net primary productivity of land vegetation using remote sensing in China. Chin. J. Plant Ecol. 2007, 31, 413–424. (In Chinese) [Google Scholar] [CrossRef]
- Houghton, R.A. Terrestrial sources and sinks of carbon inferred from terrestrial data. Tellus B 1996, 48, 420–432. [Google Scholar] [CrossRef]
- Woodwell, G.M.; Whittaker, R.H.; Reiners, W.A.; Delwiche, C.C.; Botkin, D.B. The biota and the world carbon budget. Science 1978, 199, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Stow, D.; Daeschner, S.; Hope, A.; Douglas, D.; Petersen, A.; Mynemy, R.; Zhou, L.; Oechel, W. Variability of the seasonally integrated normalized difference vegetation index across the north slope of Alaska in the 1990s. Int. J. Remote Sens. 2003, 24, 1111–1117. [Google Scholar] [CrossRef]
- Pizzo, H.P.; Ettenger, R.B.; Gjertson, D.W.; Reed, E.F.; Zhang, J.H.; Gritsch, A.; Tsai, E.W. Sirolimus and tacrolimus coefficient of variation is associated with rejection, donor-specific antibodies, and nonadherence. Pediat. Nephrol. 2016, 31, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Hurst, H.E. Long term storage capacity of reservoirs. ASCE Trans. 1951, 116, 770–808. [Google Scholar] [CrossRef]
- Dasgupta, S.; Tyler, S.; Srinivasan, R.; Grossman, E.D. Functional Connectivity of Co-localized Brain Regions during Biological Motion, Face and Social Perception using Partial Correlation Analysis. J. Vis. 2014, 14, 1011. [Google Scholar] [CrossRef]
- Kenett, D.Y.; Tumminello, M.; Madi, A.; Gershgoren, G.G.; Mantegna, R.N.; Ben-Jacob, E. Dominating clasp of the financial sector revealed by partial correlation analysis of the stock market. PLoS ONE 2010, 5, 15032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press (IPCC Secretariat): Cambridge, UK, 2007. [Google Scholar]
- Yuan, W.P.; Liu, S.G.; Yu, G.R. Global estimates of evapotranspiration and gross primary production based on MODIS and global meteorology data. Remote Sens. Environ. 2010, 114, 1416–1431. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Chen, Y.H.; Wang, M.; Jiang, W.G.; Hou, P.; Li, Y. Change of vegetation net primary productivity in Yellow River watersheds from 2001 to 2010 and its climatic driving factors analysis. Chin. J. Appl. Ecol. 2014, 25, 2811–2818. (In Chinese) [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Y.; Qian, C.Y. Temporal and spatial variation of net ecosystem productivity in the white Longjiang basin of Gansu. Acta Ecol. Sin. 2017, 37, 5121–5128. (In Chinese) [Google Scholar] [CrossRef]
- Pang, R.; Gu, F.X.; Zhang, Y.D.; Hou, Z.Y.; Liu, S.R. Temporal and spatial dynamics of net ecosystem productivity in alpine region of Southwest China. Acta Ecol. Sin. 2012, 32, 7844–7856. [Google Scholar] [CrossRef]
- Hu, B.; Sun, R.; Chen, Y.J.; Feng, L.C.; Sun, L. Estimation of the Net Ecosystem Productivity in Huang-Huai Hai Region Combining with Biome-BGC Model and Remote Sensing Data. J. Nat. Resour. 2011, 26, 2061–2071. (In Chinese) [Google Scholar] [CrossRef]
- Lobell, D.B. Changes in diurnal temperature range and national cereal yields. Agric. For. Meteorol. 2007, 145, 229–238. [Google Scholar] [CrossRef]
- Zhang, Q. Specific Response of Water Use Efficiency to Asymmetric Day-Night Warming in Typical Grasslands of Northern China. Master’s Thesis, Henan University, Henan, China, 2013. (In Chinese). [Google Scholar]
- Dai, E.F.; Huang, Y.; Wu, Z.; Zhao, D.S. Temporal and spatial patterns of carbon source/sink in grassland ecosystems in Inner Mongolia and their relationship with climatic factors. Acta Geogr. Sin. 2016, 71, 21–34. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Y.Z.; Ma, Y.D.; Jiang, H.; Zhu, Q.A.; Liu, J.X.; Peng, C.H. Evaluating the carbon budget pattern of Chinese terrestrial ecosystem from 1960 to 2006 using Integrated Biosphere Simulator. Acta Ecol. Sin. 2016, 36, 3911–3922. (In Chinese) [Google Scholar] [CrossRef]
- Liang, C.L.; Yu, Q.Z.; Liu, Y.J.; Zhang, Z.L. Effect of day and night warming on NDVI of wetland vegetation in Nansi Lake. Trop. Geogr. 2015, 35, 422–436. (In Chinese) [Google Scholar] [CrossRef]
- Lovettdoust, J. Plant strategies, vegetation processes, and ecosystem properties. J. Veg. Sci. 2002, 13, 294–295. [Google Scholar] [CrossRef]
- Shi, F.S.; Wu, N.; Luo, P. Responses of plant community structure and biomass to subtropical alpine meadow in Northwest Sichuan. Acta Ecol. Sin. 2008, 28, 5286–5293. (In Chinese) [Google Scholar] [CrossRef]
- Wan, S.Q.; Xia, J.; Liu, W.; Niu, S. Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 2009, 90, 2700–2710. [Google Scholar] [CrossRef] [PubMed]










| Grade | Range of Hurst Exponent | Strength of Sustainability | Grade | Range of Hurst Exponent | Strength of Unsustainability |
|---|---|---|---|---|---|
| 1 | 0.50 < H ≤ 0.55 | Very weak | −1 | 0.45 < H ≤ 0.50 | Very weak |
| 2 | 0.55 < H ≤ 0.65 | Quite weak | −2 | 0.35 < H ≤ 0.45 | Quite weak |
| 3 | 0.65 < H ≤ 0.75 | Quite strong | −3 | 0.25 < H ≤ 0.35 | Quite strong |
| 4 | 0.75 < H ≤ 0.80 | Strong | −4 | 0.20 < H ≤ 0.25 | Strong |
| 5 | 0.80 < H ≤ 1.00 | Very strong | −5 | 0.00 < H ≤ 0.20 | Very strong |
| Vegetation Types | Tmax | Tmin |
|---|---|---|
| Broadleaf forests | 0.457 ** | –0.261 |
| Coniferous forests | 0.072 | 0.014 |
| Meadows and marshes | –0.005 | 0.011 |
| Shrubs and coppice forests | 0.381 * | 0.266 |
| Agricultural crops | 0.383 * | 0.163 |
| Grasslands and savannah Bushes | 0.195 * | 0.097 |
| Deserts | 0.323 | 0.101 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Zhang, P.; Jing, W.; Yan, Y. Spatial Responses of Net Ecosystem Productivity of the Yellow River Basin under Diurnal Asymmetric Warming. Sustainability 2018, 10, 3646. https://doi.org/10.3390/su10103646
He J, Zhang P, Jing W, Yan Y. Spatial Responses of Net Ecosystem Productivity of the Yellow River Basin under Diurnal Asymmetric Warming. Sustainability. 2018; 10(10):3646. https://doi.org/10.3390/su10103646
Chicago/Turabian StyleHe, Jianjian, Pengyan Zhang, Wenlong Jing, and Yuhang Yan. 2018. "Spatial Responses of Net Ecosystem Productivity of the Yellow River Basin under Diurnal Asymmetric Warming" Sustainability 10, no. 10: 3646. https://doi.org/10.3390/su10103646





