Evaluating the Fires and Oxygen Deficiency Risks Caused by Stored Agricultural Waste
Abstract
:1. Introduction
2. Experiments
2.1. Samples
2.2. Thermogravimetric Differential Thermal Analysis
2.3. Calvet Calorimeter
2.4. High-Sensitivity Isothermal Calorimeter
2.5. Gas Chromatography
3. Results
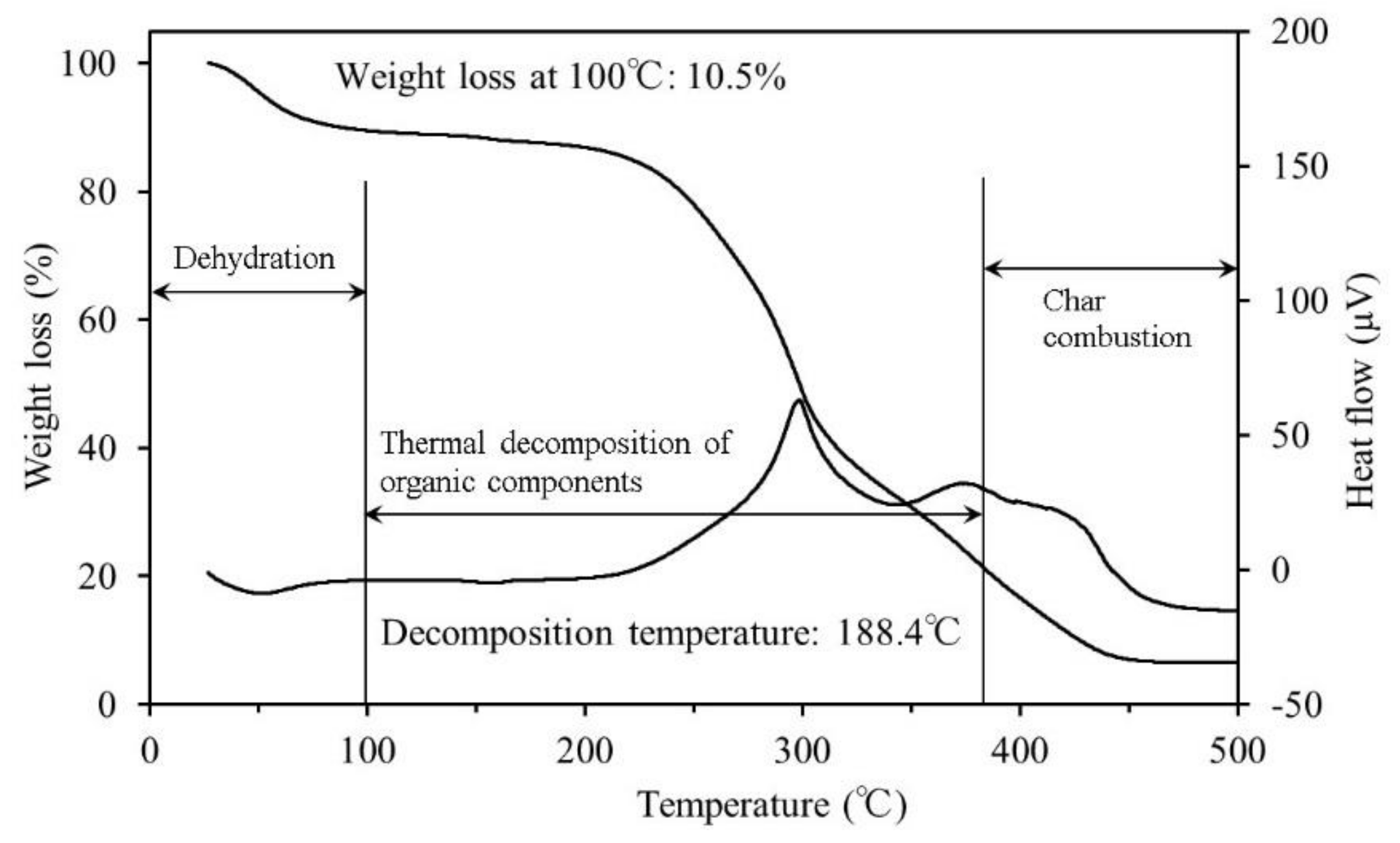
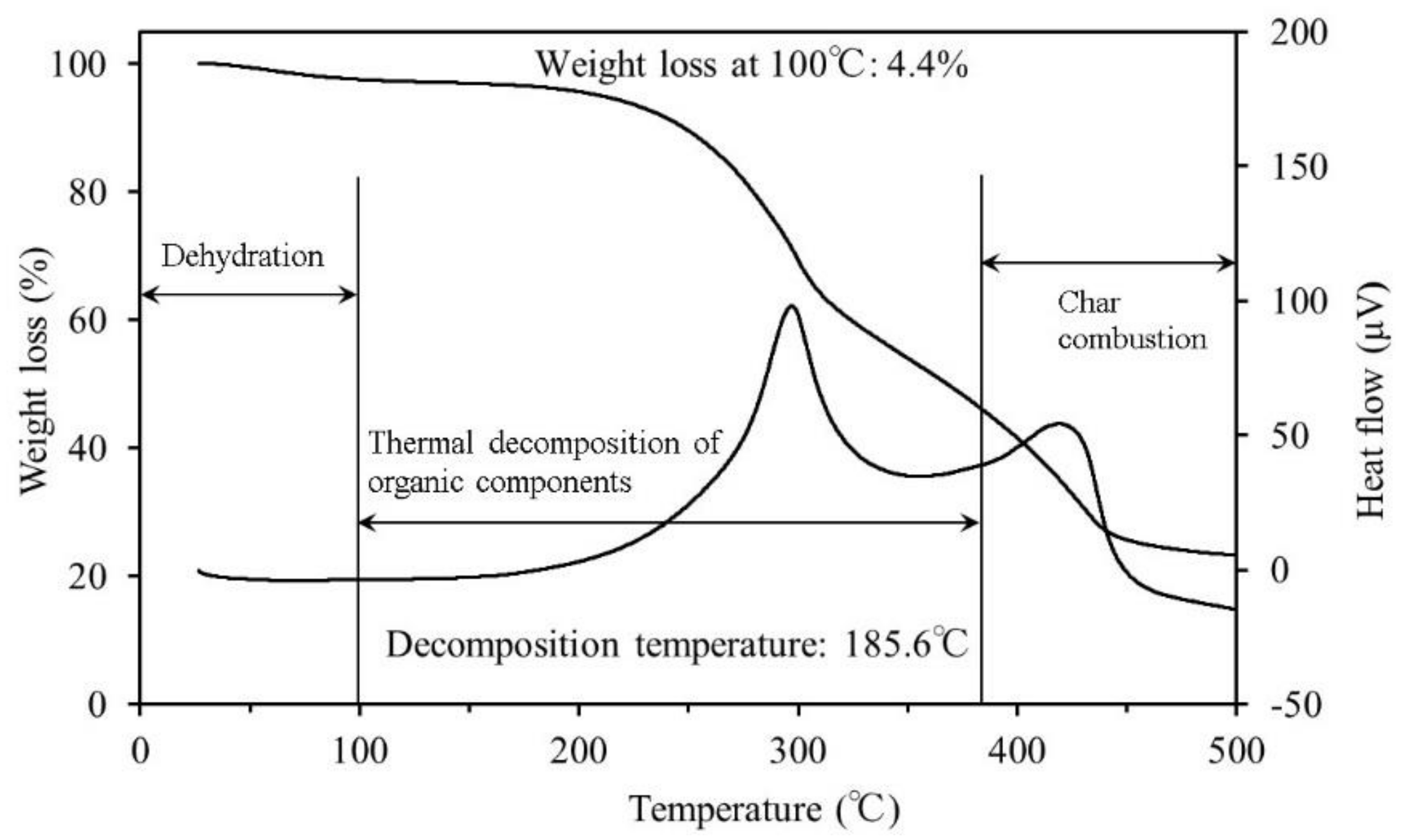
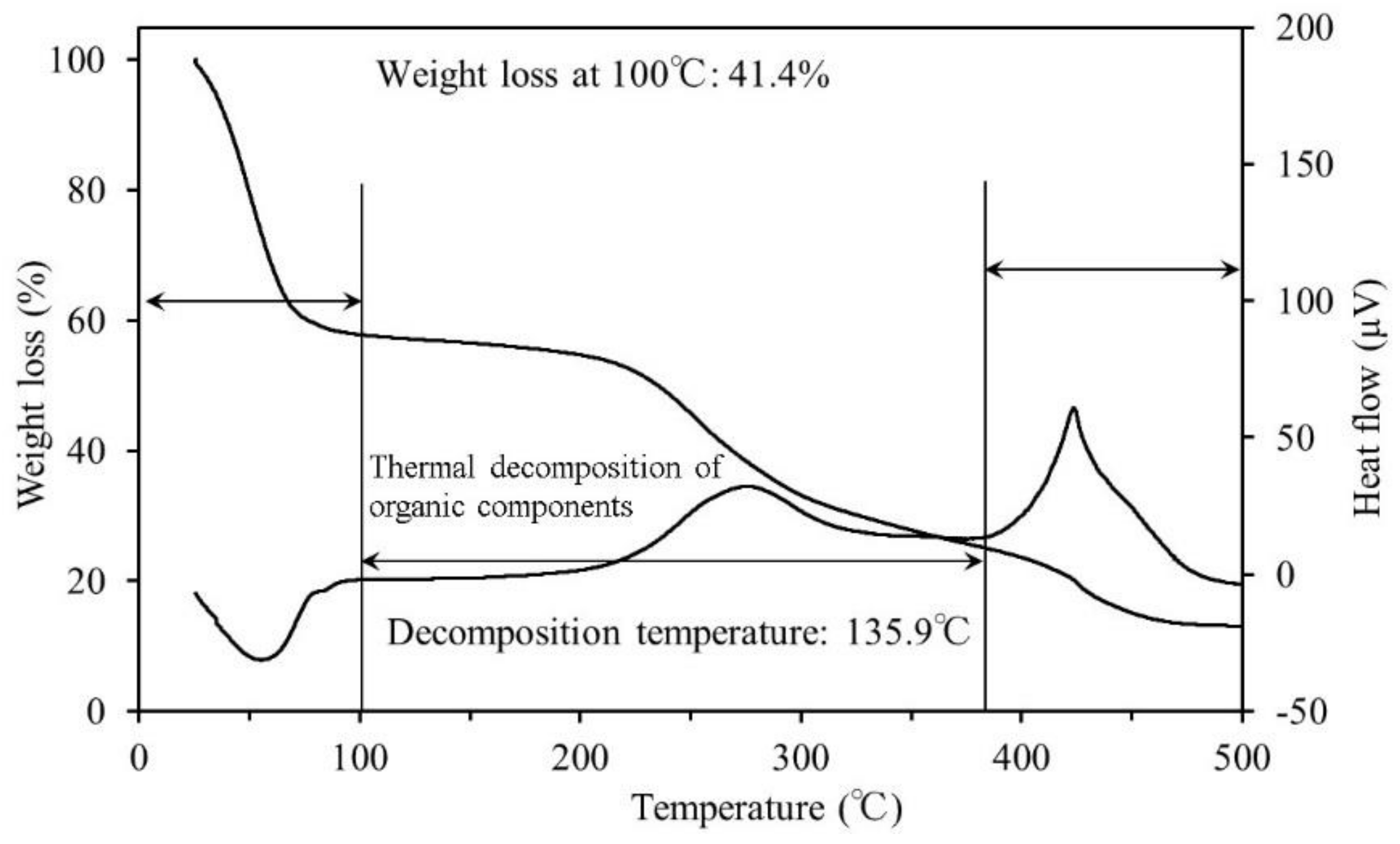
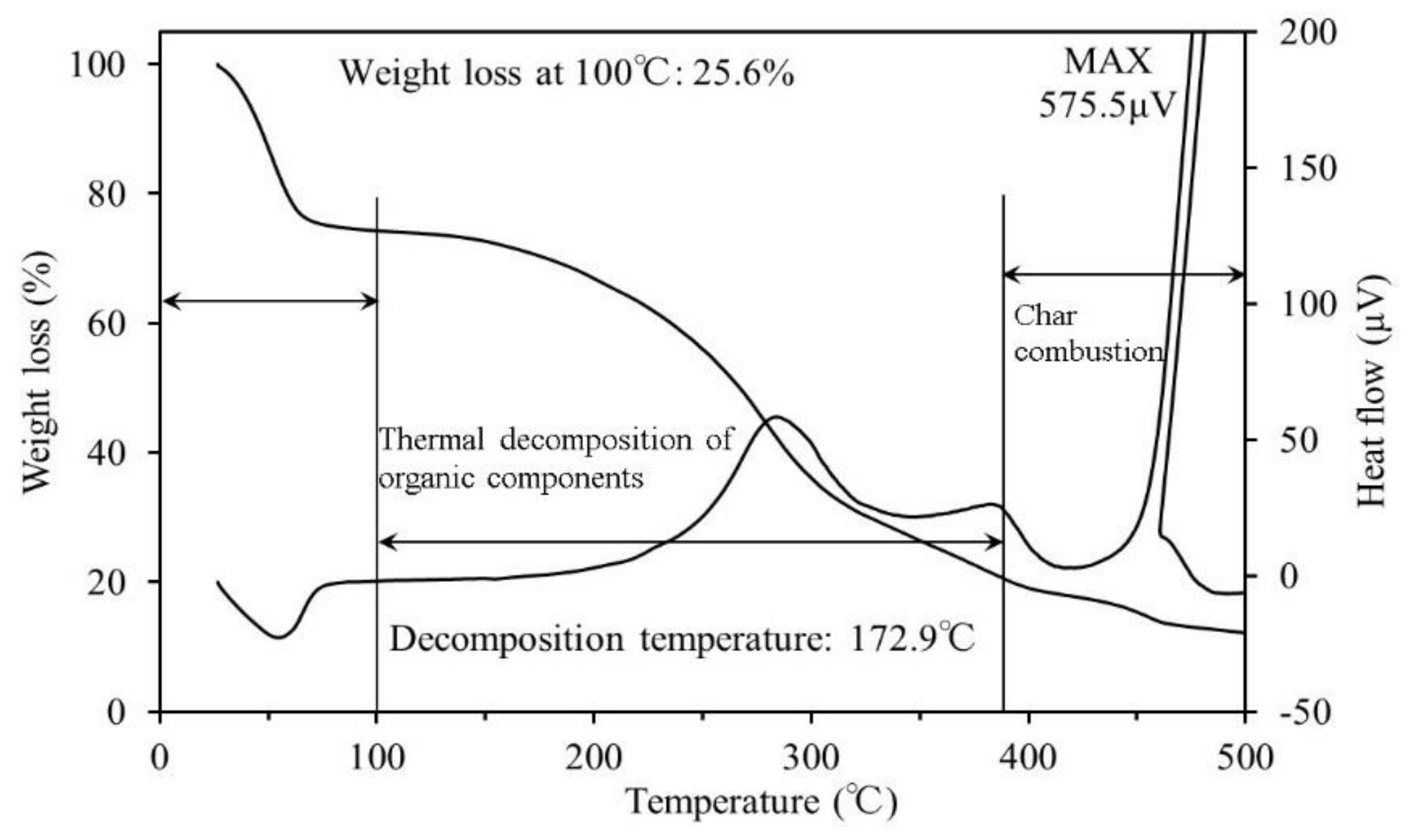
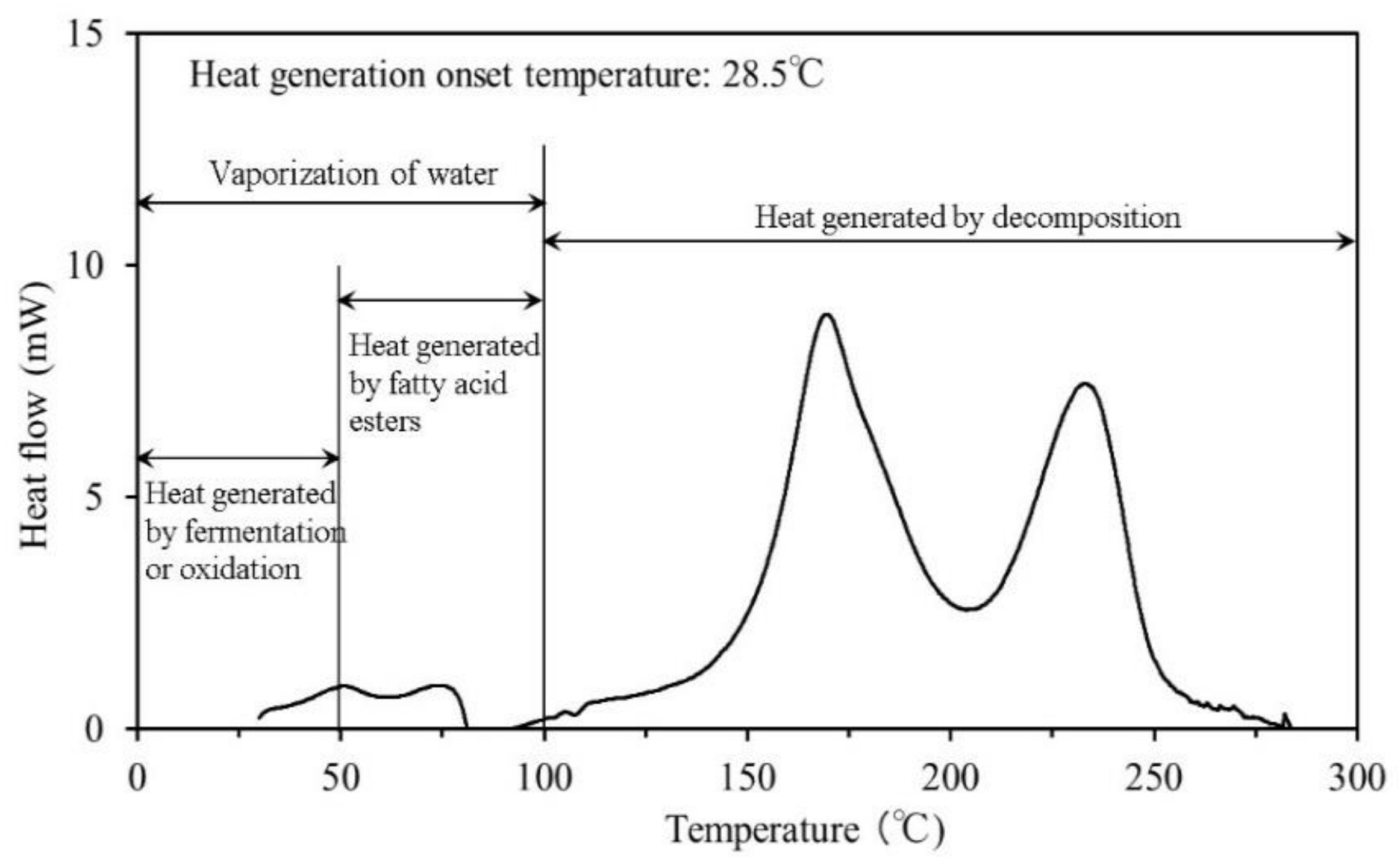
3.1. Thermogravimetric Differential Thermal Analysis
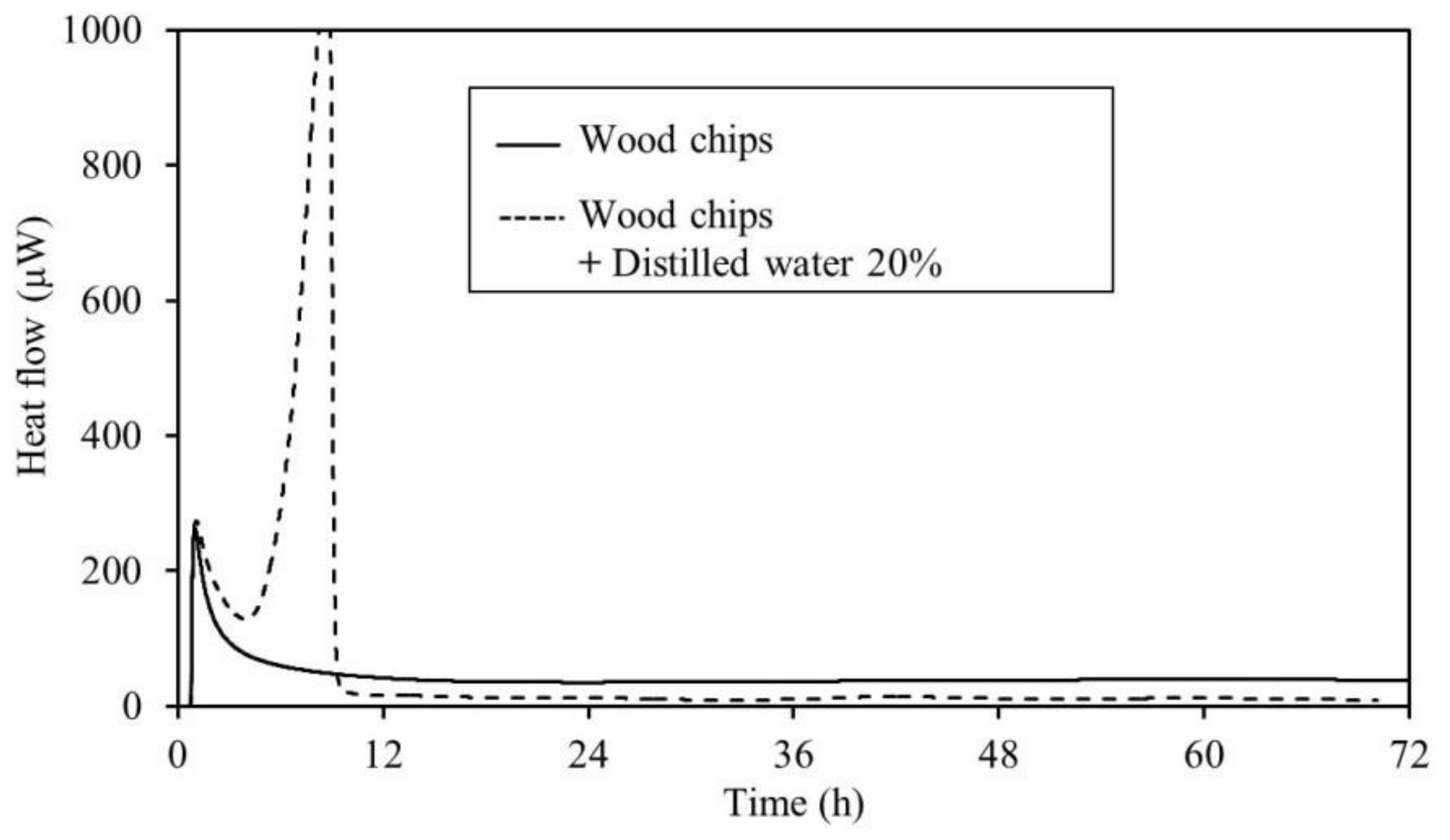
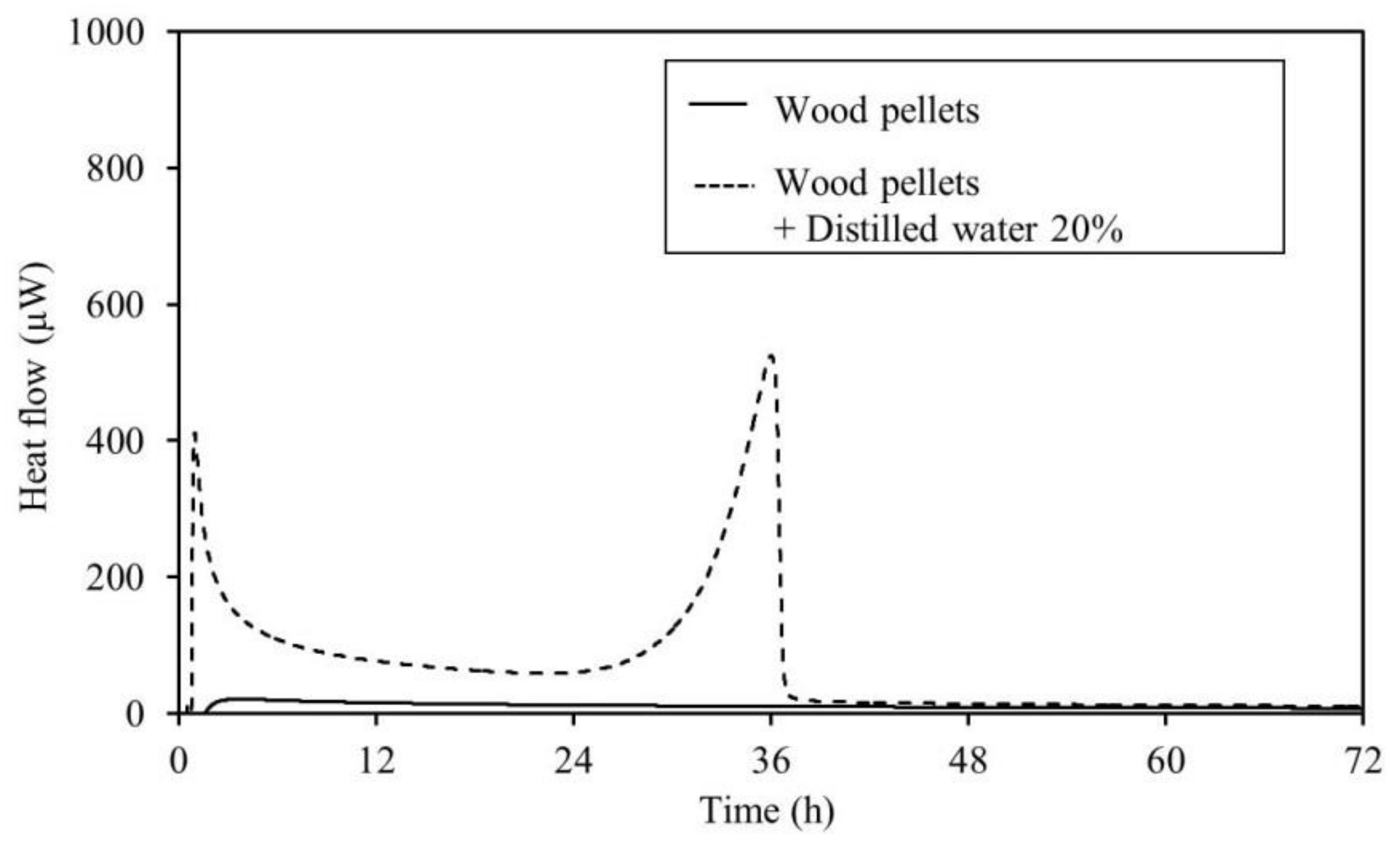
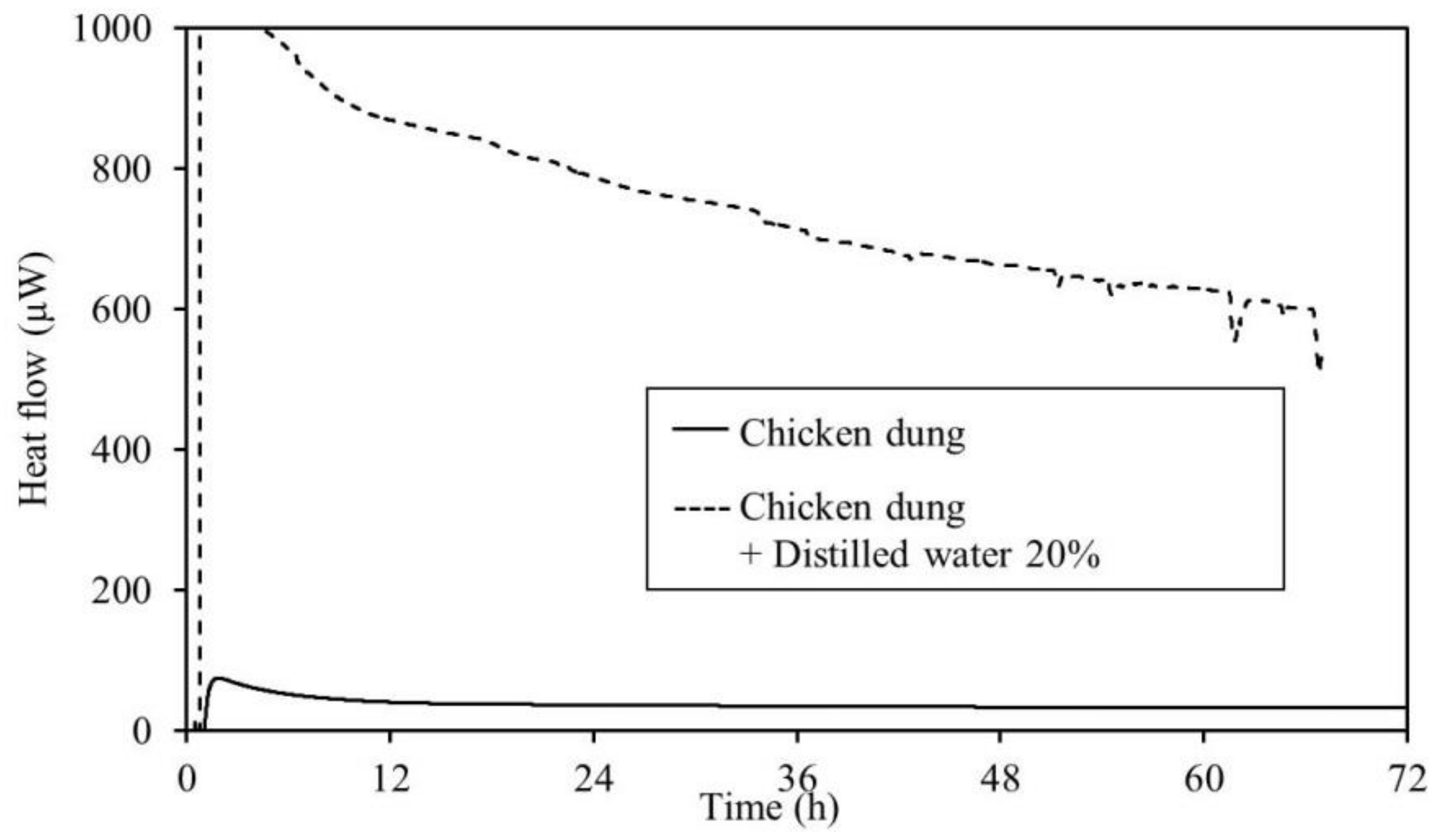
3.2. Calorimetry
3.3. High-Sensitivity Isothermal Calorimeter
3.4. Gas Chromatography
4. Discussion
4.1. Thermogravimetric Differential Thermal Analysis
4.2. Calorimetry
4.3. High-Sensitivity Isothermal Calorimeter
4.4. Gas Chromatography
5. Model Case Leading to Accidents Based on Thermal and Gas Analysis Results in this Study
6. Conclusions
- (1)
- The results of the TG-DTA system measurements showed that the decomposition temperature ranged from approximately 170–180 °C for all samples. To reach ignition conditions, sufficient heat has to be generated to overcome losses and sustain a temperature increase. Fire may occur upon reaching the decomposition temperature, if all other necessary conditions are fulfilled.
- (2)
- The results of the C80 measurements showed that the process of heat generation that can lead to the spontaneous ignition of agricultural waste begins with a small amount of heat generated from fermentation or oxidation. Next, the fatty acid esters contained within the materials begin to oxidise as the temperature gradually rises; even if the microorganisms causing fermentation die, the temperature of the material continues to rise which ultimately results in spontaneous ignition.
- (3)
- The results of the TAM measurements showed that for all samples, an increase in heat generation was observed with the addition of distilled water. This micro heat generation is the trigger of fires. Further, it is believed that moderate moisture content of an item encourages fermentation and generates heat more easily. This suggests that caution is required during the warm rainy seasons during periods of repeated rainfall. Moreover, TAM added-moisture results indicate that care should be taken with regard to spontaneous ignition caused by the heat of fermentation during the rainy season in early summer. This is also true in early spring after the snow has melted. Therefore, it is necessary to continuously monitor the area.
- (4)
- The results of the GC measurements showed that an increase in the amount of CO2 generated was observed in all samples when distilled water was added. Furthermore, the addition of distilled water was found to result in the generation of hydrogen. Therefore, anaerobic fermentation involving combustible gases may occur alongside significant heating via aerobic fermentation. Thus, when agricultural waste that is likely to ferment is transported in a tightly sealed container or stored for a long period in a warehouse, caution is required.
- (5)
- Accidents due to oxygen deficiency may occur when a storage facility is well sealed and the amount of oxygen that is circulated is minimal, which allows the oxygen to be consumed by fermentation and would lead to an oxygen deficiency in the storage facility. However, when the amount of oxygen is sufficient, the substance is stored in large deposits, and the facility is well insulated, fermentation likely causes the temperature to increase and leads to the oxidation of fatty acid esters, which may lead to a fire following an increase in temperature. This also applies to storage temperatures near room temperature. Therefore, it is desirable to periodically measure the temperature of stored materials and monitor the generated gases.
Author Contributions
Conflicts of Interest
References
- Li, X.R.; Lim, W.S.; Iwata, Y.; Koseki, H. Thermal characteristics and their relevance to spontaneous ignition of refuse plastics/paper fuel. J. Loss Prev. Process Ind. 2009, 22, 1–6. [Google Scholar] [CrossRef]
- Shimizu, Y.; Wakakura, M.; Arai, M. Heat Accumulations and Fire Accidents of Waste Piles. J. Loss Prev. Process Ind. 2009, 22, 86–90. [Google Scholar] [CrossRef]
- Yasuhara, A.; Amano, Y.; Shibamoto, T. Investigation of the Self-Heating and Spontaneous Ignition of Refuse-Derived Fuel (RDF) During Storage. Waste Manag. 2010, 30, 1161. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, R.; Izato, Y.; Miyake, A. Thermal ignition behavior of waste woods mixed with unsaturated fatty acids. J. Therm. Anal. Calorim. 2015, 121, 361–369. [Google Scholar] [CrossRef]
- Benjamin, M.; Zhang, C.Q.; Utama, N.A.; Farzaneh, H.; Ishihara, K.N. Analysis of Japan’s Post-Fukushima Energy Strategy. Energy Strategy Rev. 2013, 2, 190–198. [Google Scholar]
- Seishu, T.; Hirasawa, T. Research Approaches to Sustainable Biomass Systems; Academic Press: Oxford, UK, 2014. [Google Scholar]
- Hanifzadeh, M.; Nabati, Z.; Longka, P.; Malakul, P.; Apul, D.; Kim, D. Life cycle assessment of superheated steam drying technology as a novel cow manure management method. J. Environ. Manag. 2017, 199, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hanifzadeh, M.; Sarrafzadeh, M.; Nabati, Z.; Tavakoli, O.; Feyzizarnagh, H. Technical, economic and energy assessment of an alternative strategy for mass production of biomass and lipid from microalgae. J. Environ. Chem. Eng. 2018, 6, 866–873. [Google Scholar] [CrossRef]
- Kim, D.; Hanifzadeh, M.; Kumar, A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustain. Energy 2018, 37, 7–19. [Google Scholar] [CrossRef]
- Li, X.R.; Koseki, H.; Iwata, Y. Risk Assessment on Processing Facility of Raw Organic Garbage. J. Hazard. Mater. 2008, 154, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Murasawa, N.; Koseki, H.; Iwata, Y. Lessons Learned from Accidents of Soy Sauce Squeezing Residue—Risks of Spontaneous Ignition and Oxygen Deficiency. Loss Prev. Bull. 2012, 224, 14–17. [Google Scholar]
- Tabata, T.; Okuda, T. Life cycle assessment of woody biomass energy utilization: Case study in Gifu Prefecture, Japan. Energy 2012, 45, 944–951. [Google Scholar] [CrossRef]
- Li, X.R.; Lim, W.S.; Iwata, Y.; Koseki, H. Thermal Behavior of Sewage Sludge Derived Fuels. Therm. Sci. 2008, 12, 137–148. [Google Scholar] [CrossRef]
- Murasawa, N.; Koseki, H.; Iwata, Y. Causes of accidents by Soy Sauce Squeezing Residue and Fish meal. J. Mater. Cycle Waste Manag. 2013, 15, 42–48. [Google Scholar] [CrossRef]
- Gillespie, E.H.; Jackson, J.M.; Owen, G.R. Ethylene oxide sterilisation-is it safe? J. Clin. Pathol. 1979, 32, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Murasawa, N.; Koseki, H.; Iwata, Y.; Shibata, Y. Determination of Spontaneous Ignition of SSSR and Fish meal during Transport and Storage. J. Food Res. 2012, 1, 320–329. [Google Scholar] [CrossRef]
- Murasawa, N.; Koseki, H.; Iwata, Y.; Gao, L. Study on Spontaneous Ignition of Stored Food Waste to be Used for Recycling. Fire Mater. 2013, 37, 520–529. [Google Scholar] [CrossRef]
- Gao, M.; Ling, B.; Yang, S.; Zhao, M. Flame retardance of wood treated with guanidine compounds characterized by thermal degradation behavior. J. Anal. Appl. Pyrolysis 2005, 73, 151–156. [Google Scholar] [CrossRef]
- TranVan, L.; Legrand, V.; Jacquemin, F. Thermal decomposition kinetics of balsa wood: Kinetics and degradation mechanisms comparison between dry and moisturized materials. Polym. Degrad. Stab. 2014, 110, 208–215. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, C.; Hu, Y.; Zhu, X.; Qing, Y.; Wu, Q. Comparative Performance of Three Magnesium Compounds on Thermal Degradation Behavior of Red Gum Wood. Materials 2014, 7, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Howard, B.H. Impact of Thermal Pretreatment Temperatures on Woody Biomass Chemical Composition, Physical Properties and Microstructure. Energies 2018, 11, 25. [Google Scholar] [CrossRef]
- Lohrer, C.; Krause, U.; Steinbach, J. Self-Ignition of Combustible Bulk Materials under Various Ambient Conditions. Process Saf. Environ. Prot. 2005, 83, 145–150. [Google Scholar] [CrossRef]
- Li, X.R.; Koseki, H.; Momota, M. Evaluation of danger from fermentation-induced spontaneous ignition of wood chips. J. Hazard. Mater. 2006, 135, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Bilitewski, B. The temporary storage of municipal solid waste—Recommendations for a safe operation of interim storage facilities. Waste Manag. 2009, 29, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Göransson, G.; Kaczala, F.; Hogland, W.; Marques, M. Characterization of municipal solid waste temporary storage sites: Risks posed to surrounding areas as a consequence of fire incidents. Waste Manag. 2013, 33, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Matunaga, A.; Yasuhara, A.; Shimizu, Y.; Wakakura, M. Investigation on the spontaneous combustion of refuse-derived fuels during storage using a chemiluminescence technique. Waste Manag. Res. 2008, 26, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Plank, M.; Wachtmeister, G.; Thuneke, K.; Remmele, E.; Emberger, P. Effect of fatty acid composition on ignition behavior of straight vegetable oils measured in a constant volume combustion chamber apparatus. Fuel 2017, 207, 293–301. [Google Scholar] [CrossRef]
- Fu, Z.M.; Li, X.R.; Koseki, H. Heat Generation of Refuse Derived Fuel with Water. J. Loss Prev. Process Ind. 2005, 18, 27–33. [Google Scholar] [CrossRef]
- Fu, Z.M.; Koseki, H.; Iwata, Y. Investigation on Spontaneous Ignition of Two Kinds of Organic Material with Water. Thermochim. Acta 2006, 400, 68–74. [Google Scholar] [CrossRef]
- Back, E.L. Auto-ignition in Hygroscopic, Organic Materials—Especially Forest Products -as Initiated by Moisture Absorption from the Ambient Atmosphere. Fire Saf. J. 1981, 4, 185–196. [Google Scholar] [CrossRef]
- Thauer, R.K.; Jungermann, K.; Decker, K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 1977, 41, 100–180. [Google Scholar] [PubMed]
- Wang, Q.; Narita, J.-Y.; Xie, W.; Ohsumi, Y.; Kusano, K.; Shirai, Y.; Ogawa, H. Effects of anaerobic/aerobic incubation and storage temperature on preservation and deodorization of kitchen garbage. Bioresour. Technol. 2002, 84, 213–220. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, G.; Chen, Y.; Li, X.M. Effect of polyhydroxyalkanoates on dark fermentative hydrogen production from waste activated sludge. Water Res. 2015, 73, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Hogland, W.; Marques, M. Physical, biological and chemical processes during storage and spontaneous combustion of waste fuel. Resour. Conserv. Recycl. 2003, 40, 53–69. [Google Scholar] [CrossRef]














| Sample | Heat Generation (J/g) 0–24 h | Heat generation (J/g) 24–72 h | Heat generation (J/g) 0–72 h |
|---|---|---|---|
| Wood chip | 4.8 | 6.3 | 11.1 |
| Wood chip + Distilled water 20% | 12.3 | 1.9 | 14.2 |
| Wood pellet | 1.3 | 1.6 | 2.9 |
| Wood pellet + Distilled water 20% | 8.0 | 10.4 | 18.3 |
| Sludge fuel | 17.7 | 11.1 * | 28.9 ** |
| Sludge fuel + Distilled water 20% | 35.1 | 8.52 | 43.6 |
| Chicken dung | 3.7 | 5.7 | 9.4 |
| Chicken dung + Distilled water 20% | 80.4 | 96.8 * | 177.2 ** |
| Soy sauce squeezing residue | 11.1 | 4.2 | 15.3 |
| Soy sauce squeezing residue + Distilled water 20% | 12.3 | 3.8 | 16.1 |
| Sample | GC Results % | |||||
|---|---|---|---|---|---|---|
| O2 | N2 | H2 | CO | CH4 | CO2 | |
| Wood chip | 5.3 | 77.8 | - | - | - | 12.1 |
| Wood chip + Distilled water 20% | 1.5 | 78.5 | 0.1 | - | - | 14.4 |
| Wood pellet | 19.6 | 77.0 | <0.1 | - | - | 0.7 |
| Wood pellet + Distilled water 20% | 1.4 | 68.9 | <0.1 | - | - | 21.7 |
| Sludge fuel | 19.5 | 77.3 | - | - | - | 0.5 |
| Sludge fuel + Distilled water 20% | 17.2 | 76.2 | - | - | - | 2.3 |
| Chicken dung | 9.5 | 70.1 | - | - | - | 14.2 |
| Chicken dung + Distilled water 20% | 1.5 | 73.8 | 0.3 | - | - | 22.0 |
| Soy sauce squeezing residue | 1.7 | 79.4 | - | - | - | 16.1 |
| Soy sauce squeezing residue + Distilled water 20% | 1.7 | 79.1 | - | - | - | 18.2 |
| Sample | GC Results % | |||||
|---|---|---|---|---|---|---|
| O2 | N2 | H2 | CO | CH4 | CO2 | |
| EOG Wood chip | 20.3 | 76.7 | - | - | - | 0.1 |
| EOG Wood chip + Distilled water 20% | 20.3 | 77.9 | - | - | - | 0.9 |
| EOG Wood pellet | 20.5 | 77.7 | - | - | - | <0.1 |
| EOG Wood pellet + Distilled water 20% | 17.6 | 76.3 | - | - | - | 2.5 |
| EOG Sludge fuel | 19.2 | 77.2 | - | - | - | 0.1 |
| EOG Sludge fuel + Distilled water 20% | 19.8 | 77.2 | - | - | - | 0.5 |
| EOG Chicken dung | 19.0 | 77.9 | - | - | - | <0.1 |
| EOG Chicken dung + Distilled water 20% | 18.3 | 78.3 | - | - | - | 0.3 |
| EOG Soy sauce squeezing residue | 18.9 | 76.7 | - | - | - | 0.1 |
| EOG Soy sauce squeezing residue + Distilled water 20% | 18.9 | 77.8 | - | - | - | 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murasawa, N.; Koseki, H.; Iwata, Y.; Sakamoto, T. Evaluating the Fires and Oxygen Deficiency Risks Caused by Stored Agricultural Waste. Sustainability 2018, 10, 1116. https://doi.org/10.3390/su10041116
Murasawa N, Koseki H, Iwata Y, Sakamoto T. Evaluating the Fires and Oxygen Deficiency Risks Caused by Stored Agricultural Waste. Sustainability. 2018; 10(4):1116. https://doi.org/10.3390/su10041116
Chicago/Turabian StyleMurasawa, Naoharu, Hiroshi Koseki, Yusaku Iwata, and Takabumi Sakamoto. 2018. "Evaluating the Fires and Oxygen Deficiency Risks Caused by Stored Agricultural Waste" Sustainability 10, no. 4: 1116. https://doi.org/10.3390/su10041116
APA StyleMurasawa, N., Koseki, H., Iwata, Y., & Sakamoto, T. (2018). Evaluating the Fires and Oxygen Deficiency Risks Caused by Stored Agricultural Waste. Sustainability, 10(4), 1116. https://doi.org/10.3390/su10041116




