Short-Term Performance of Sustainable Silica Fume Mortars Exposed to Sulfate Attack
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Curing
2.2. Sulfate Solutions
2.3. Mercury Intrusion Porosimetry
2.4. Determination of Steady-State Ionic Diffusion Coefficient from Resistivity of Sample Obtained Using Impedance Spectroscopy
2.5. Compressive Strength
3. Results and Discussion
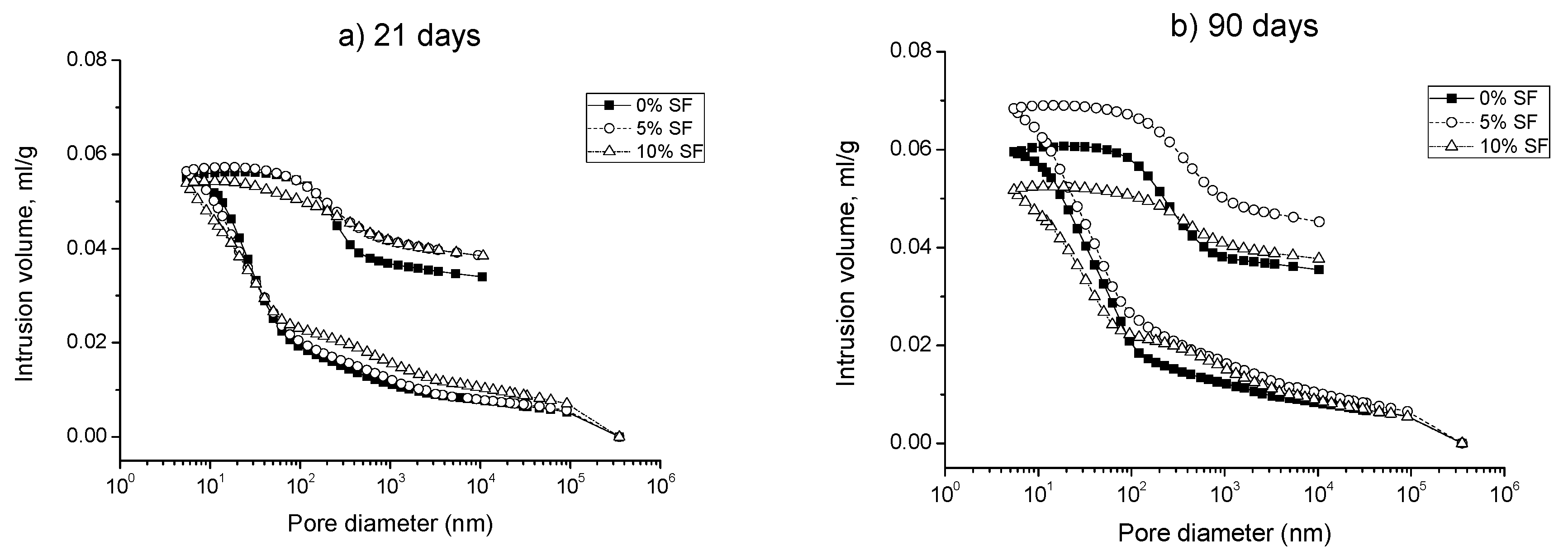
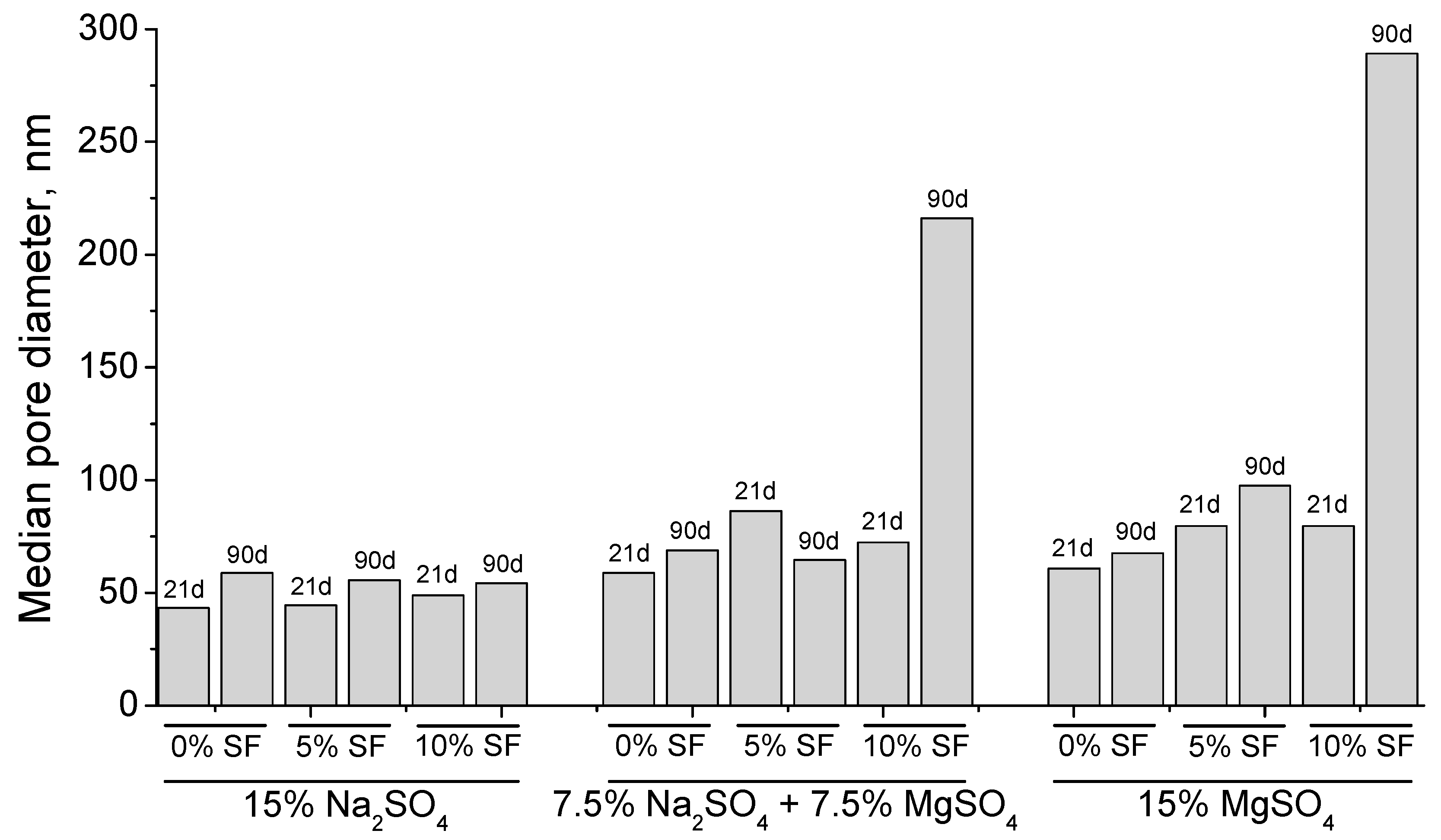
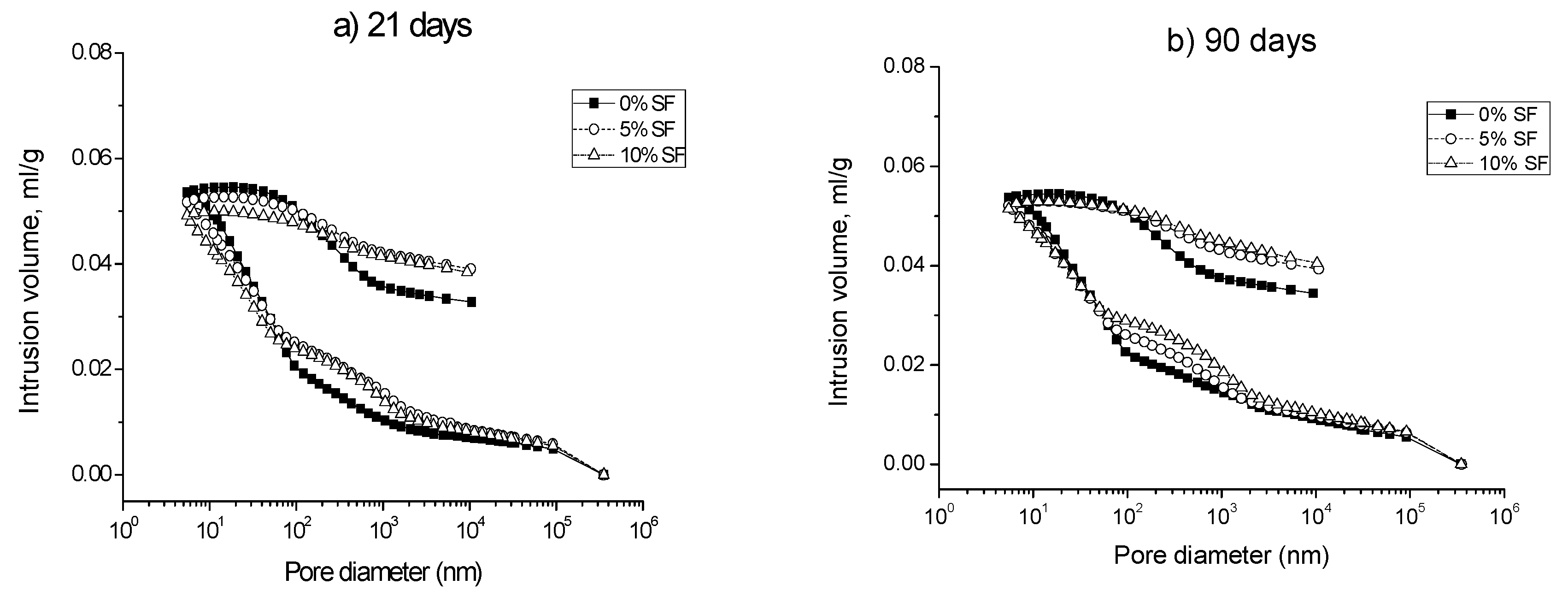
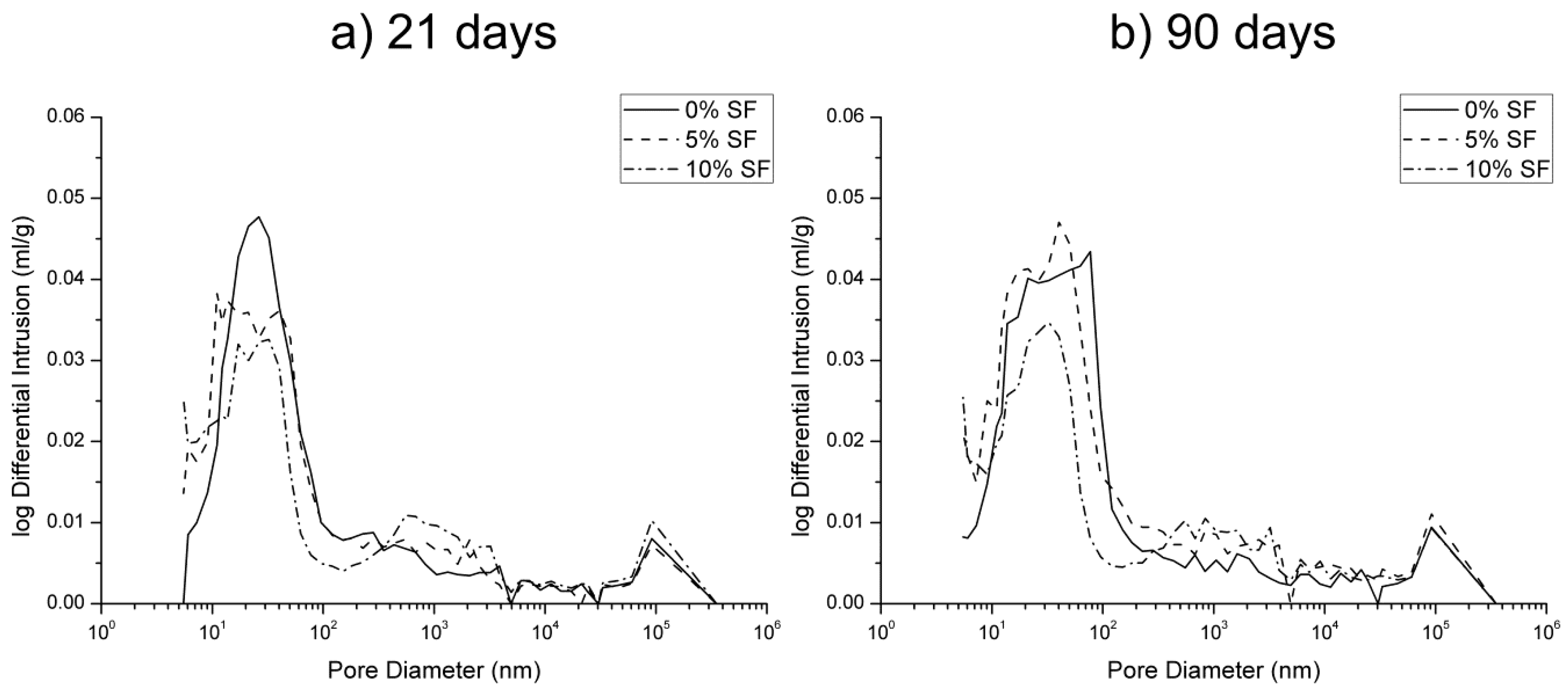
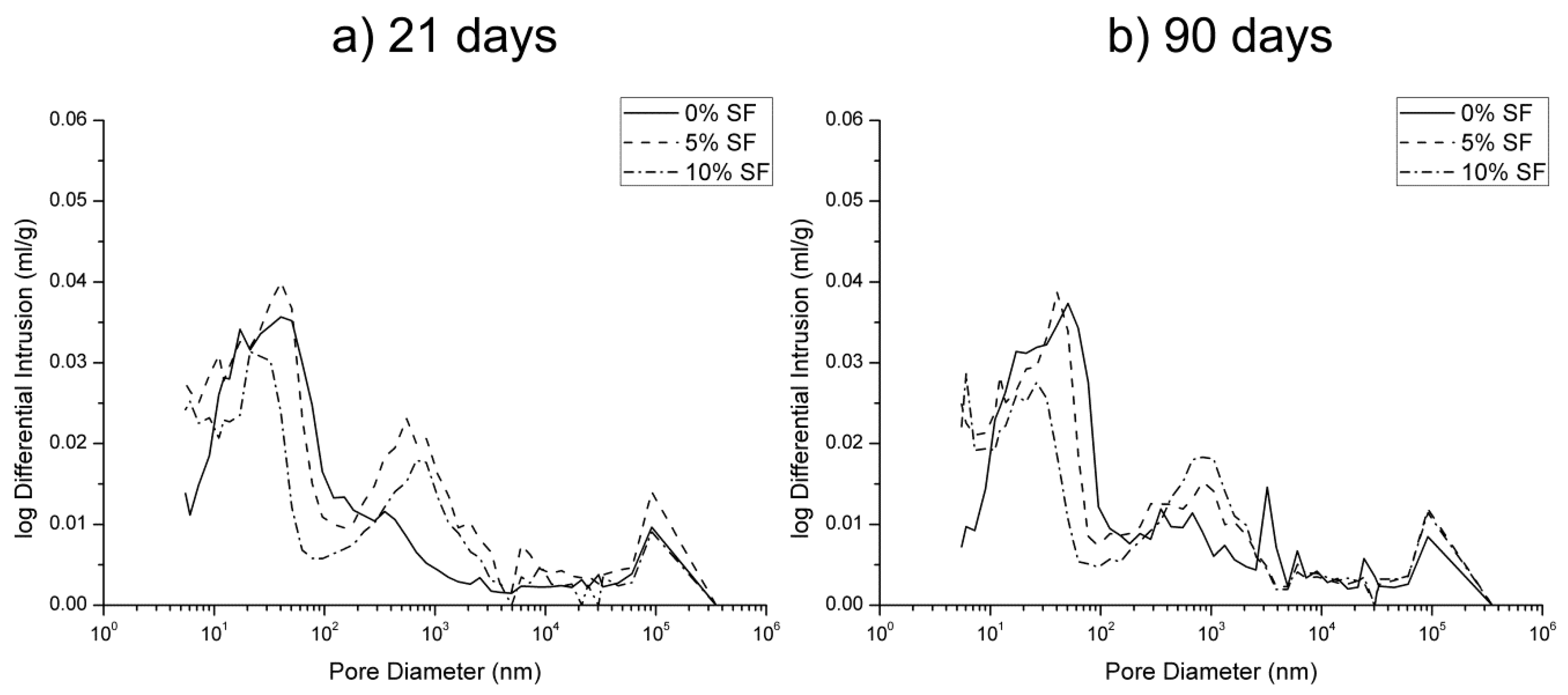
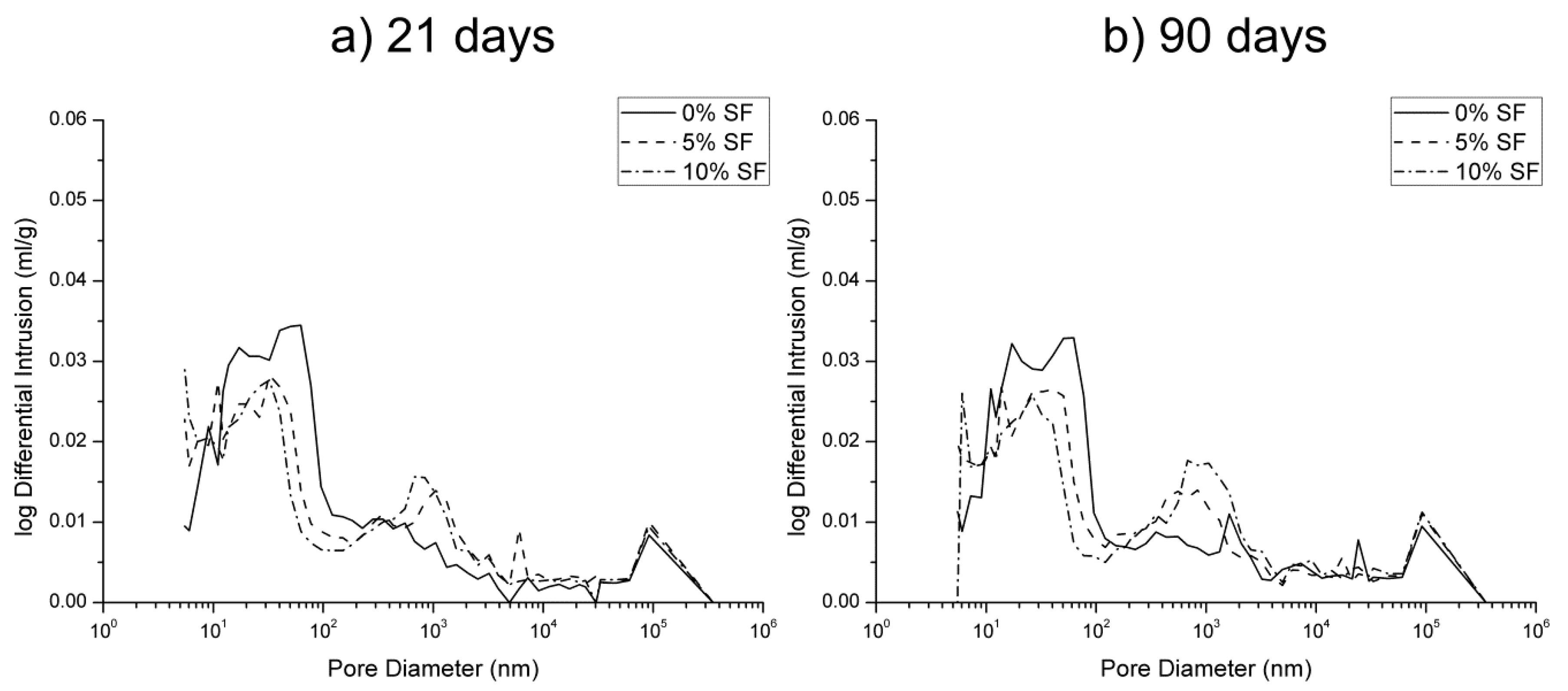
3.1. Mercury Intrusion Porosimetry
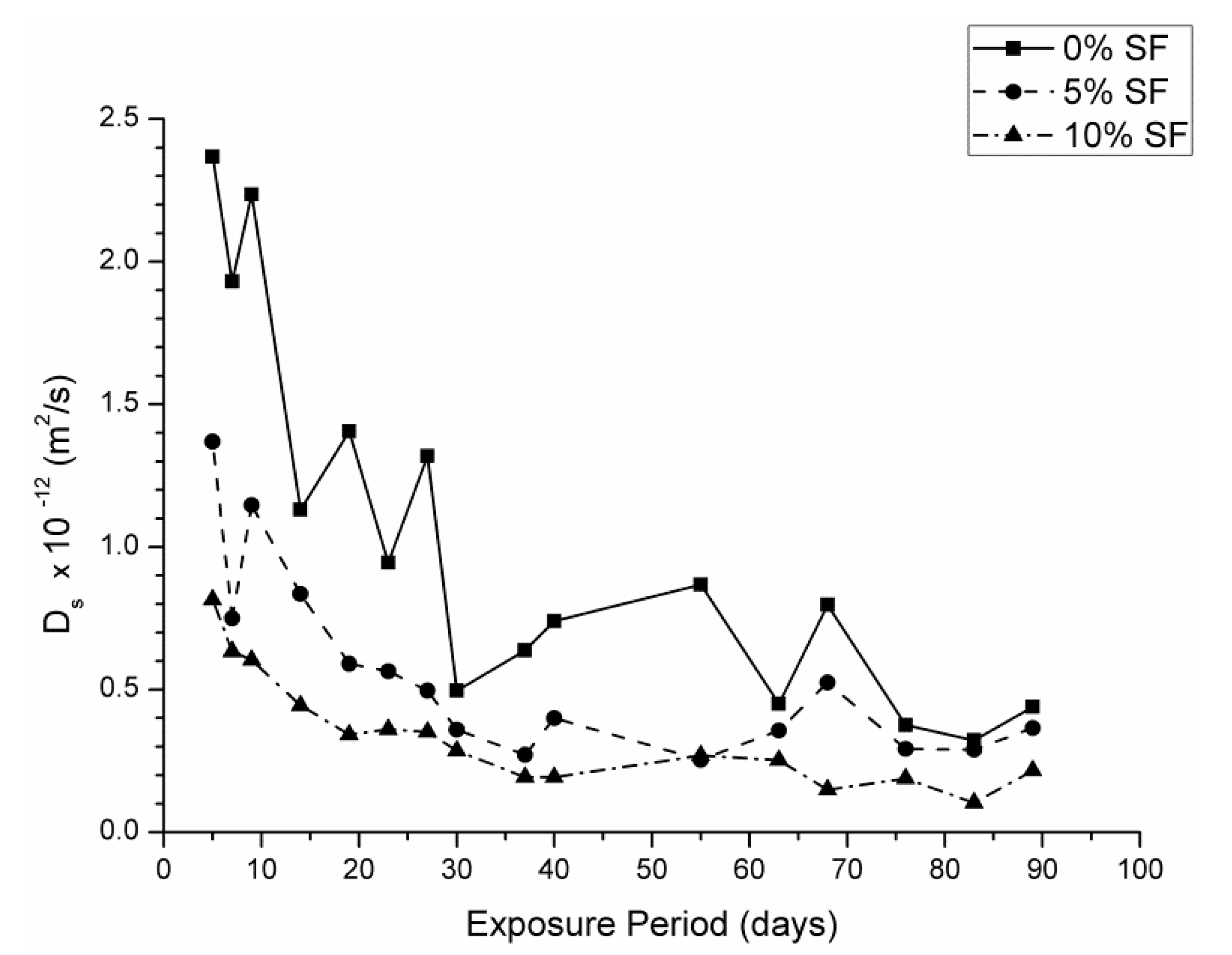
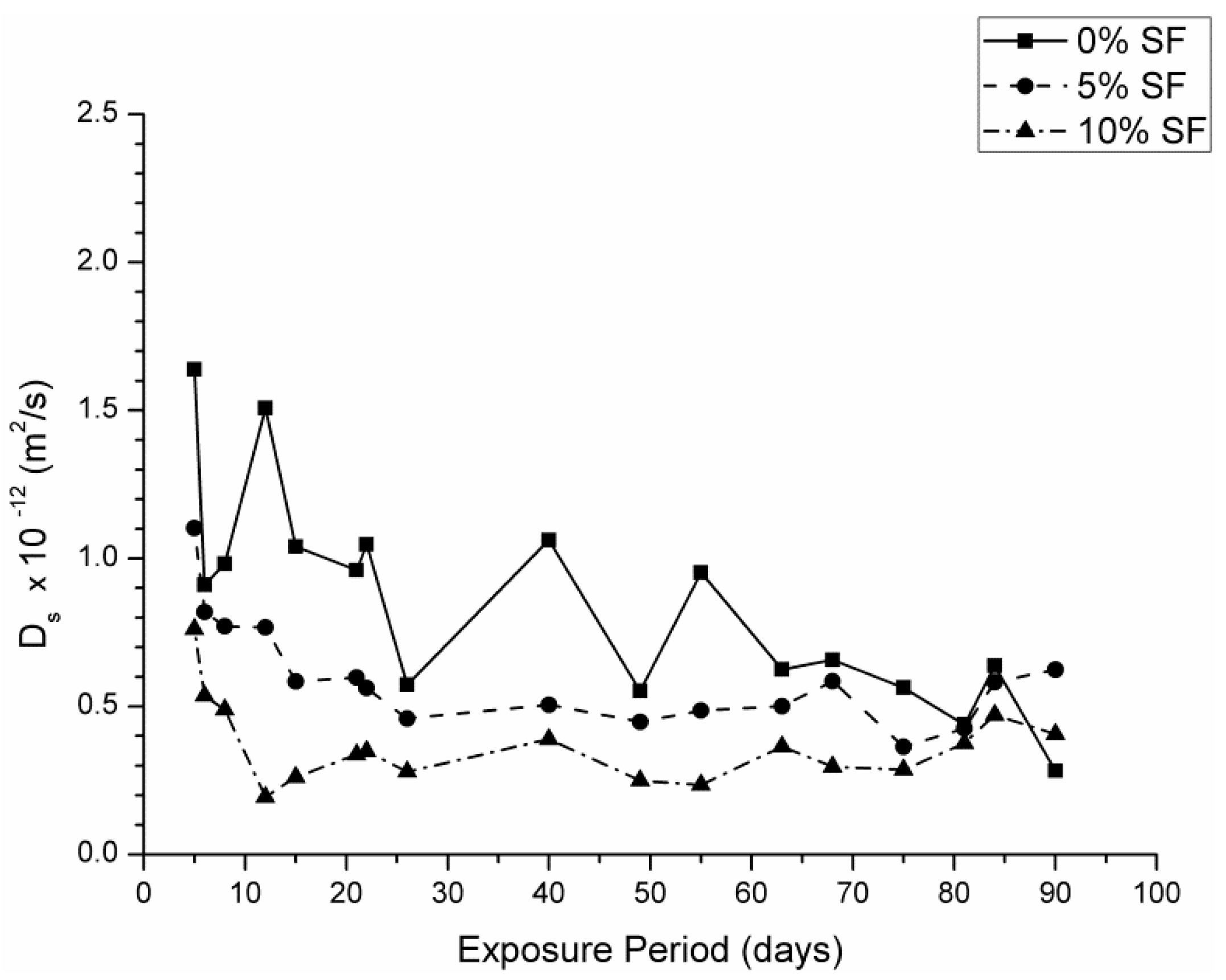
3.2. Steady State Ionic Diffusion Coefficient
3.3. Compressive Strength
4. Conclusions
- (1)
- The pore structure of 0% silica fume samples exposed to sodium sulfate solution was less refined compared to 5% and 10% silica fume ones. This would indicate that the silica fume inclusion improves the resistance to sodium sulfate attack in mortars.
- (2)
- The microstructure of silica fume mortars was more affected by the exposure to the mixed 7.5% MgSO4 + 7.5% Na2SO4 solution than by the sodium sulfate one, as suggested the loss of pore refinement indicated by the appearance of a new pore family with higher diameter. A similar result has been noted for magnesium sulfate solution.
- (3)
- The steady state ionic diffusion coefficient decreases with silica fume content, and appears not to be affected by exposure to sodium sulfate solutions.
- (4)
- In silica fume samples exposed to magnesium sulfate solutions, a substantial increase in ionic diffusivity accompanies a loss in compressive strength, suggesting that dissolution type reactions take place in these samples pore network. This attack was more severe in the 7.5% MgSO4 + 7.5% Na2SO4 solution, which implies that magnesium content alone does not determine the severity of attack.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demirboğa, R. Thermal conductivity and compressive strength of concrete incorporation with mineral admixtures. Build. Environ. 2007, 42, 2467–2471. [Google Scholar] [CrossRef]
- Ganjian, E.; Pouya, H.S. The effect of Persian Gulf tidal zone exposure on durability of mixes containing silica fume and blast furnace slag. Constr. Build. Mater. 2009, 23, 644–652. [Google Scholar] [CrossRef]
- Ponikiewski, T.; Gołaszewski, J. The effect of high-calcium fly ash on selected properties of self-compacting concrete. Arch. Civ. Mech. Eng. 2014, 14, 455–465. [Google Scholar] [CrossRef]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Impedance spectroscopy study of the effect of environmental conditions in the microstructure development of OPC and slag cement mortars. Arch. Civ. Mech. Eng. 2015, 15, 569–583. [Google Scholar] [CrossRef]
- Glinicki, M.; Jóźwiak-Niedźwiedzka, D.; Gibas, K.; Dąbrowski, M. Influence of Blended Cements with Calcareous Fly Ash on Chloride Ion Migration and Carbonation Resistance of Concrete for Durable Structures. Materials 2016, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Influence of Environmental Conditions on Durability of Slag Cement Mortars. In Proceedings of the 2nd International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; pp. 277–287. [Google Scholar]
- Nochaiya, T.; Wongkeo, W.; Chaipanich, A. Utilization of fly ash with silica fume and properties of Portland cement–fly ash–silica fume concrete. Fuel 2010, 89, 768–774. [Google Scholar] [CrossRef]
- Estokova, A.; Kovalcikova, M.; Luptakova, A.; Prascakova, M. Testing Silica Fume-Based Concrete Composites under Chemical and Microbiological Sulfate Attacks. Materials 2016, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lo, Y.T.; Wang, W.; Ouyang, D.; Wang, P.; Xing, F. Pozzolanic Reactivity of Silica Fume and Ground Rice Husk Ash as Reactive Silica in a Cementitious System: A Comparative Study. Materials 2016, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Behfarnia, K.; Farshadfar, O. The effects of pozzolanic binders and polypropylene fibers on durability of SCC to magnesium sulfate attack. Constr. Build. Mater. 2013, 38, 64–71. [Google Scholar] [CrossRef]
- Chung, D.D.L. Improving cement-based materials by using silica fume. J. Mater. Sci. 2002, 37, 673–682. [Google Scholar] [CrossRef]
- ACI Committee 234. Guide for the Use of Silica Fume in Concrete (ACI 234R-06); American Concrete Institute: Farmington Hills, MI, USA, 2006; 63p. [Google Scholar]
- Hooton, R.D. Permeability and pore structure of cement pastes containing fly ash, slag and silica fume. In Blended Cements, ASTM Special Technical Publication 897; Frohnsdorff, G., Ed.; American Society for Testing Materials: West Conshohocken, PA, USA, 1986; pp. 128–143. [Google Scholar]
- Wolsiefer, J.T. Silica fume concrete: A solution to steel reinforcement corrosion in concrete. In Utilization of Industrial By-Products for Construction Materials; ASCE: Reston, VA, USA, 1993; pp. 15–29. [Google Scholar]
- Dotto, J.M.R.; Abreu, A.G.D.; Dal Molin, D.C.C.; Müller, I.L. Influence of silica fume addition on concretes physical properties and on corrosion behaviour of reinforcement bars. Cem. Concr. Compos. 2004, 26, 31–39. [Google Scholar] [CrossRef]
- Poon, C.S.; Kou, S.C.; Lam, L. Compressive strength, chloride diffusivity and pore structure of high performance metakaolin and silica fume concrete. Constr. Build. Mater. 2006, 20, 858–865. [Google Scholar] [CrossRef]
- Gjorv, O.E. Effect of condensed silica fume on steel corrosion in concrete. ACI Mater. J. 1995, 92, 591–598. [Google Scholar]
- Ramezanianpour, A.A.; Malhotra, V.M. Effect of curing on the compressive strength, resistance to chloride-ion penetration and porosity of concretes incorporating slag, fly ash or silica fume. Cem. Concr. Compos. 1995, 17, 125–133. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Rehman, A.U.; Qudoos, A.; Kim, H.G.; Ryou, J.-S. Influence of titanium dioxide nanoparticles on the sulfate attack upon ordinary Portland cement and slag-blended mortars. Materials 2018, 11. [Google Scholar] [CrossRef]
- Yu, C.; Sun, W.; Scrivener, K. Mechanism of expansion of mortars immersed in sodium sulfate solutions. Cem. Concr. Res. 2013, 43, 105–111. [Google Scholar] [CrossRef]
- Al-Amoudi, O.S.B. Performance of 15 reinforced concrete mixtures in magnesium-sodium sulphate environments. Constr. Build. Mater. 1995, 9, 149–158. [Google Scholar] [CrossRef]
- Baghabra Al-Amoudi, O.S. Attack on plain and blended cements exposed to aggressive sulfate environments. Cem. Concr. Compos. 2002, 24, 305–316. [Google Scholar] [CrossRef]
- Ganjian, E.; Pouya, H.S. Effect of magnesium and sulfate ions on durability of silica fume blended mixes exposed to the seawater tidal zone. Cem. Concr. Res. 2005, 35, 1332–1343. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U.; Maslehuddin, M.; Al-Zahrani, M.M.; Sharif, A.M.; Shameem, M.; Ibrahim, M. Sulfate resistance of plain and blended cements exposed to varying concentrations of sodium sulfate. Cem. Concr. Compos. 2003, 25, 429–437. [Google Scholar] [CrossRef]
- Lee, S.T.; Moon, H.Y.; Swamy, R.N. Sulfate attack and role of silica fume in resisting strength loss. Cem. Concr. Compos. 2005, 27, 65–76. [Google Scholar] [CrossRef]
- Hooton, R.D. Influence of silica fume replacement of cement on physical properties and resistance to sulfate attack, freezing and thawing, and alkali-silica reactivity. ACI Mater. J. 1993, 90, 143–151. [Google Scholar]
- Cohen, M.D.; Bentur, A. Durability of Portland cement-Silica fume pastes in magnesium sulfate and sodium sulfate solutions. ACI Mater. J. 1988, 85, 148–157. [Google Scholar]
- Türker, F.; Aköz, F.; Koral, S.; Yüzer, N. Effects of magnesium sulfate concentration on the sulfate resistance of mortars with and without silica fume. Cem. Concr. Res. 1997, 27, 205–214. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look. Part 1: Summary of experimental results. Cem. Concr. Res. 2002, 32, 915–921. [Google Scholar] [CrossRef]
- Asociación Española de Normalización y Certificación (AENOR). UNE-EN 197-1:2011. Composición, Especificaciones y Criterios de Conformidad de Los Cementos Comunes; AENOR: Madrid, Spain, 2011; p. 30. (In Spanish) [Google Scholar]
- Asociación Española de Normalización y Certificación (AENOR). UNE-EN 196-1:2005. Métodos de Ensayo de Cementos. Parte 1: Determinación de Resistencias Mecánicas; AENOR: Madrid, Spain, 2005; p. 36. (In Spanish) [Google Scholar]
- Ortega, J.M.; Esteban, M.D.; Rodríguez, R.R.; Pastor, J.L.; Ibanco, F.J.; Sánchez, I.; Climent, M.A. Long-Term Behaviour of Fly Ash and Slag Cement Grouts for Micropiles Exposed to a Sulphate Aggressive Medium. Materials 2017, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.M.; Esteban, M.D.; Rodríguez, R.R.; Pastor, J.L.; Sánchez, I. Microstructural Effects of Sulphate Attack in Sustainable Grouts for Micropiles. Materials 2016, 9, 11. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution ASTM C 1012-04; ASTM International: Wesk Conshohocken, PA, USA, 2004; p. 6. [Google Scholar]
- Diamond, S. Mercury porosimetry. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Diamond, S. Aspects of concrete porosity revisited. Cem. Concr. Res. 1999, 29, 1181–1188. [Google Scholar] [CrossRef]
- Ortega, J.M.; Pastor, J.L.; Albaladejo, A.; Sánchez, I.; Climent, M.A. Durability and compressive strength of blast furnace slag-based cement grout for special geotechnical applications. Mater. Constr. 2014, 64. [Google Scholar] [CrossRef] [Green Version]
- Ortega, J.M.; Ferrandiz, V.; Antón, C.; Climent, M.A.; Sánchez, I. Influence of curing conditions on the mechanical properties and durability of cement mortars. In Materials Characterisation IV: Computational Methods and Experiments; Mammoli, A.A., Brebbia, C.A., Eds.; WIT Press: Southampton, UK, 2009; pp. 381–392. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C.; Arteaga, A.; Tanner, P. Methodology based on the electrical resistivity for the calculation of reinforcement service life. In Proceedings of the 5th CANMET/ACO International Conference on Durability of Concrete, Supplementary Papers; Malhotra, V.M., Ed.; American Concrete Institute: Barcelona, Spain, 2000; pp. 899–915. [Google Scholar]
- Sánchez, I.; Nóvoa, X.R.; de Vera, G.; Climent, M.A. Microstructural modifications in Portland cement concrete due to forced ionic migration tests. Study by impedance spectroscopy. Cem. Concr. Res. 2008, 38, 1015–1025. [Google Scholar] [CrossRef]
- Cabeza, M.; Merino, P.; Miranda, A.; Nóvoa, X.R.; Sanchez, I. Impedance spectroscopy study of hardened Portland cement paste. Cem. Concr. Res. 2002, 32, 881–891. [Google Scholar] [CrossRef]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Impedance spectroscopy study of the effect of environmental conditions on the microstructure development of sustainable fly ash cement mortars. Materials 2017, 10. [Google Scholar] [CrossRef]
- Ortega, J.M.; Esteban, M.D.; Rodríguez, R.R.; Pastor, J.L.; Ibanco, F.J.; Sánchez, I.; Climent, M.A. Influence of Silica Fume Addition in the Long-Term Performance of Sustainable Cement Grouts for Micropiles Exposed to a Sulphate Aggressive Medium. Materials 2017, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.L.; Ortega, J.M.; Flor, M.; López, M.P.; Sánchez, I.; Climent, M.A. Microstructure and durability of fly ash cement grouts for micropiles. Constr. Build. Mater. 2016, 117, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Keddam, M.; Takenouti, H.; Nóvoa, X.R.; Andrade, C.; Alonso, C. Impedance measurements on cement paste. Cem. Concr. Res. 1997, 27, 1191–1201. [Google Scholar] [CrossRef]
- Ortega, J.M.; Sánchez, I.; Antón, C.; De Vera, G.; Climent, M.A. Influence of environment on durability of fly ash cement mortars. ACI Mater. J. 2012, 109, 647–656. [Google Scholar]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Durability related transport properties of OPC and slag cement mortars hardened under different environmental conditions. Constr. Build. Mater. 2012, 27, 176–183. [Google Scholar] [CrossRef]
- Bonakdar, A.; Mobasher, B. Multi-parameter study of external sulfate attack in blended cement materials. Constr. Build. Mater. 2010, 24, 61–70. [Google Scholar] [CrossRef]
- Duan, P.; Shui, Z.; Chen, W.; Shen, C. Effects of metakaolin, silica fume and slag on pore structure, interfacial transition zone and compressive strength of concrete. Constr. Build. Mater. 2013, 44, 1–6. [Google Scholar] [CrossRef]
- Aköz, F.; Türker, F.; Koral, S.; Yüzer, N. Effects of sodium sulfate concentration on the sulfate resistance of mortars with and without silica fume. Cem. Concr. Res. 1995, 25, 1360–1368. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U. Sulfate resistance of plain and blended cements exposed to magnesium sulfate solutions. Constr. Build. Mater. 2007, 21, 1792–1802. [Google Scholar] [CrossRef]



| Compound | CEM I 42.5R (%) | Silica Fume (%) |
|---|---|---|
| CaO | 64.5 | 0.4 |
| SiO2 | 20.0 | 94.1 |
| Al2O3 | 5.7 | 0.3 |
| Fe2O3 | 2.5 | 0.1 |
| SO3 | 3.2 | 0.1 |
| MgO | 0.9 | 0.3 |
| Na2O | 0.1 | 0.6 |
| K2O | 1.0 | 0.6 |
| Solution Name | Mg2+ (g/L) | Na+ (g/L) | SO42− (g/L) | Mass Ratio Mg2+/SO42− | Mass Ratio Na+/SO42− | Mg2+ (mol/L) | Na+ (mol/L) | SO42− (mol/L) | Molar Ratio Mg2+/SO42− | Molar Ratio Na+/SO42− |
|---|---|---|---|---|---|---|---|---|---|---|
| 15% Na2SO4 | 0 | 48.558 | 101.442 | 0 | 0.479 | 0 | 1.056 | 1.056 | 0 | 1.000 |
| 7.5% MgSO4 + 7.5% Na2SO4 | 15.145 | 24.279 | 110.576 | 0.136 | 0.220 | 0.622 | 0.529 | 1.151 | 0.540 | 0.460 |
| 15% MgSO4 | 30.290 | 0 | 119.710 | 0.253 | 0 | 1.246 | 0 | 1.246 | 1.000 | 0 |
| Mortar Type | 15% Na2SO4 Solution | Mixed Solution | 15% MgSO4 Solution | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 21 Days | 90 Days | Variation | 21 Days | 90 Days | Variation | 21 Days | 90 Days | Variation | |
| 0% SF | 43.3 | 58.8 | 35.8% | 58.8 | 68.8 | 17.0% | 60.8 | 67.5 | 11.0% |
| 5% SF | 44.4 | 55.6 | 25.2% | 86.3 | 64.6 | −25.1% | 79.7 | 97.5 | 22.3% |
| 10% SF | 48.8 | 54.3 | 11.3% | 72.3 | 216.1 | 198.9% | 79.6 | 289.2 | 263.3% |
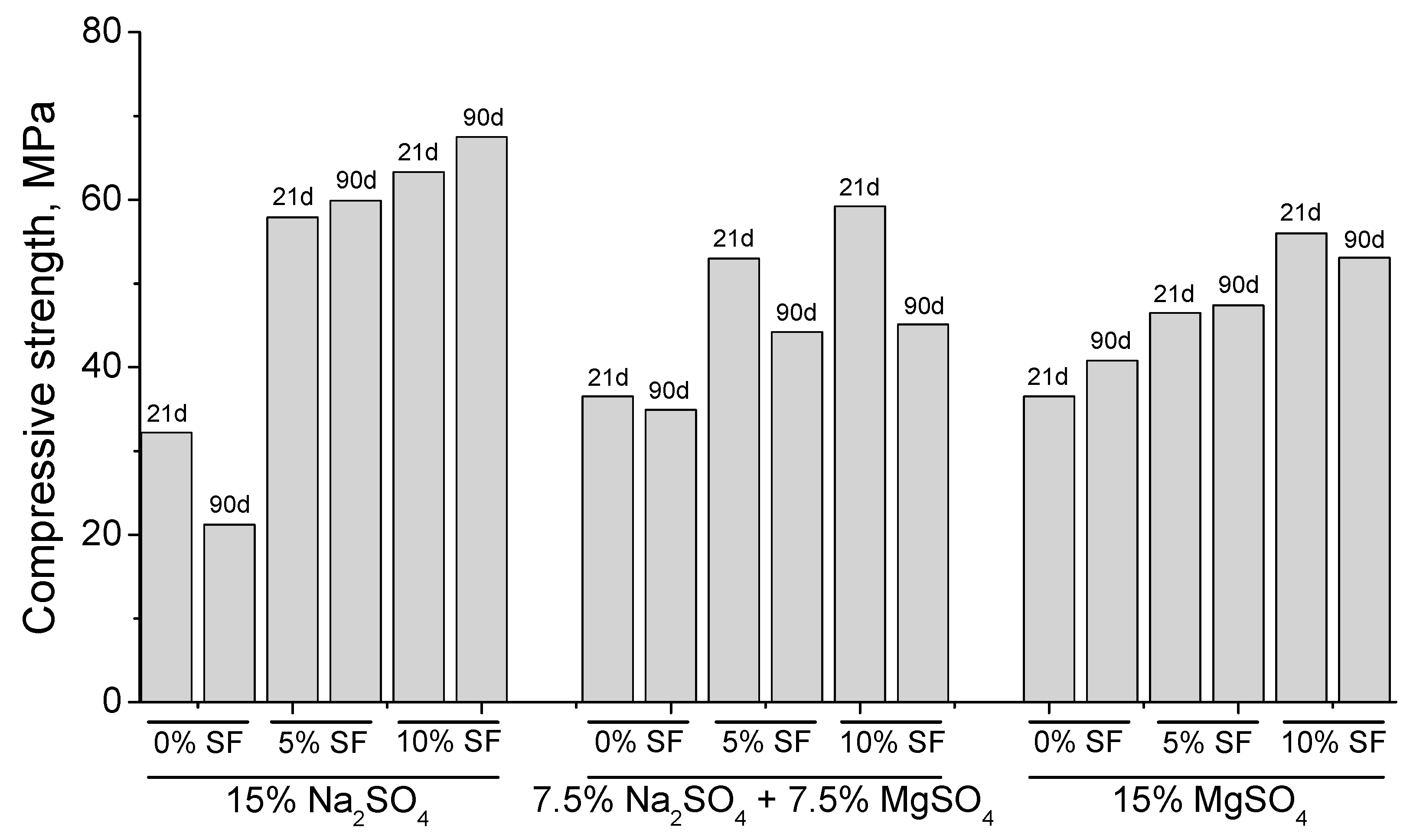
| Mortar Type | 15% Na2SO4 Solution | Mixed Solution | 15% MgSO4 Solution | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 21 Days | 90 Days | Variation | 21 Days | 90 Days | Variation | 21 Days | 90 Days | Variation | |
| 0% SF | 32.3 | 21.2 | −34.4% | 36.5 | 34.9 | −4.4% | 36.5 | 40.8 | 11.8% |
| 5% SF | 57.9 | 59.9 | 3.5% | 53.0 | 44.2 | −16.6% | 46.2 | 47.4 | 2.6% |
| 10% SF | 63.3 | 67.5 | 6.6% | 59.2 | 45.1 | −23.8% | 56.0 | 53.1 | −5.2% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, J.M.; Esteban, M.D.; Williams, M.; Sánchez, I.; Climent, M.Á. Short-Term Performance of Sustainable Silica Fume Mortars Exposed to Sulfate Attack. Sustainability 2018, 10, 2517. https://doi.org/10.3390/su10072517
Ortega JM, Esteban MD, Williams M, Sánchez I, Climent MÁ. Short-Term Performance of Sustainable Silica Fume Mortars Exposed to Sulfate Attack. Sustainability. 2018; 10(7):2517. https://doi.org/10.3390/su10072517
Chicago/Turabian StyleOrtega, José Marcos, María Dolores Esteban, Mark Williams, Isidro Sánchez, and Miguel Ángel Climent. 2018. "Short-Term Performance of Sustainable Silica Fume Mortars Exposed to Sulfate Attack" Sustainability 10, no. 7: 2517. https://doi.org/10.3390/su10072517






