Crop Diseases and Mycotoxin Accumulation in Temperate Agroforestry Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Experimental Design and Crop Harvest
2.3. Quantification and Identification of Phytopathogenic Fungi
2.4. Determination of Mycotoxins
2.5. Statistical Analysis
3. Results
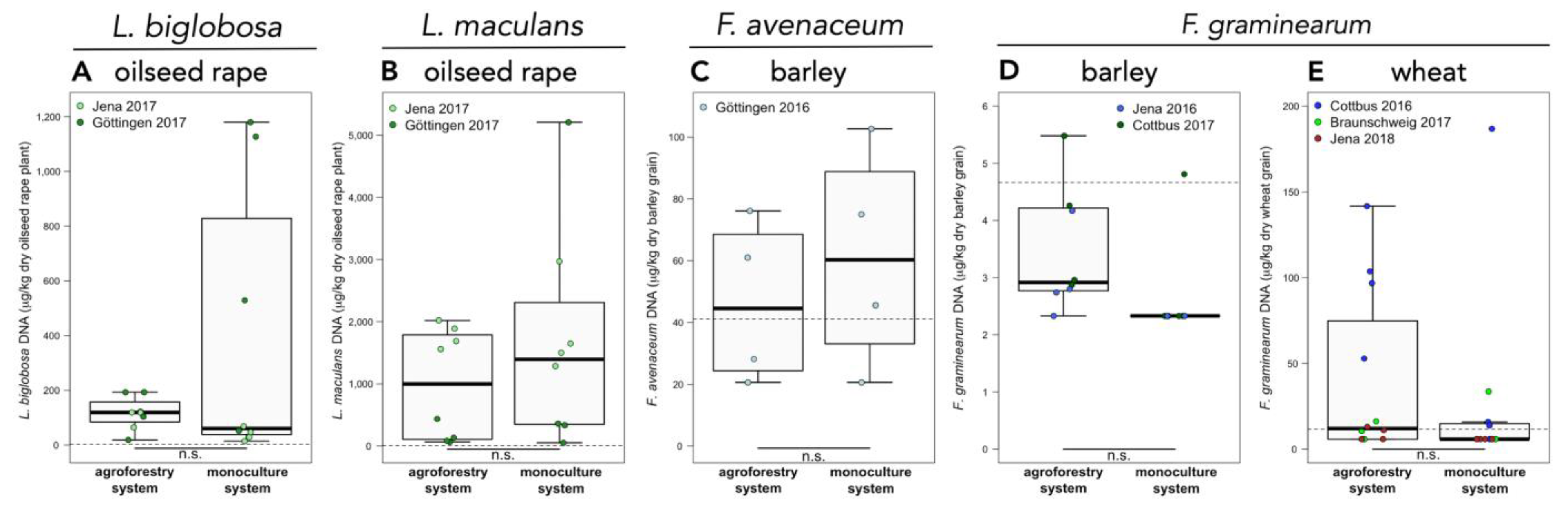
3.1. Occurrence and Abundance of Phytopathogenic Fungi
3.1.1. Oilseed Rape Plants
3.1.2. Barley and Wheat Grain
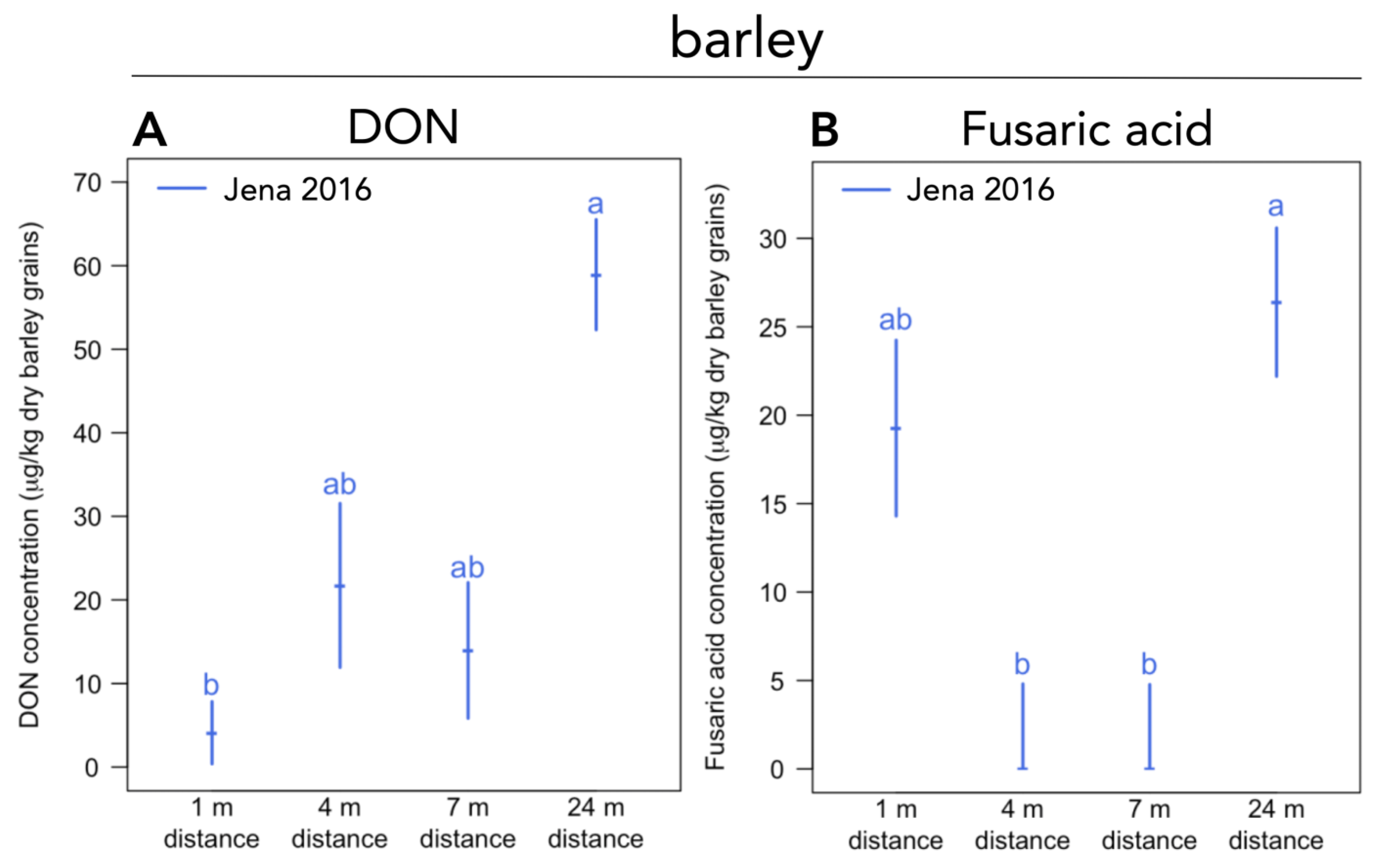
3.2. Mycotoxin Concentrations in Barley and Wheat Grain in Agroforestry versus Monoculture Systems
4. Discussion
4.1. Suppression of V. Longisporum in Oilseed Rape under Agroforestry
4.2. Effect of Agroforestry on L. Maculans and L. Biglobosa in Oilseed Rape
4.3. Effect of Agroforestry on F. Tricinctum in Barley and Wheat Grain
4.4. Mycotoxin Accumulation in Barley and Wheat Grain
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Target Organism | Final MgCl2 Concentration (mM) | Concentration of Each Primer (µM) | Concentration of Each dNTP1 (µM) | Choice of DNA Polymerase2 | Choice of Reaction Buffer2 |
|---|---|---|---|---|---|
| Fusarium avenaceum | 2.5 | 0.4 | 100 | Hot Start Taq | Standard Taq |
| Fusarium culmorum | 4.0 | 0.3 | 200 | Taq | ThermoPol® |
| Fusarium graminearum | 2.5 | 0.3 | 200 | Hot Start Taq | Standard Taq |
| Fusarium poae | 2.0 | 0.3 | 100 | Taq | ThermoPol® |
| Fusarium proliferatum | 2.0 | 0.3 | 125 | Taq | ThermoPol® |
| Fusarium tricinctum | 2.5 | 0.4 | 100 | Hot Start Taq | Standard Taq |
| Leptosphaeria maculans | 2.0 | 0.3 | 100 | Taq | ThermoPol® |
| Leptosphaeria biglobosa | 2.0 | 0.3 | 100 | Taq | ThermoPol® |
| Sclerotinia sclerotiorum | 2.0 | 0.3 | 100 | Taq | ThermoPol® |
| Verticillium longisporum | 3.0 | 0.3 | 200 | Taq | ThermoPol® |
| Target Organism | Primer Pair | Primer Sequence (5’-3’) | Product Size (bp) | Reference |
|---|---|---|---|---|
| Fusarium avenaceum | JIAf | GCTAATTCTTAACTTACTAGGGGCC | 220 | [91] |
| JIAr | CTGTAATAGGTTATTTACATGGGCG | |||
| Fusarium culmorum | OPT18 F | GATGCCAGACCAAGACGAAG | 472 | [92] |
| OPT18 R | GATGCCAGACGCACTAAGAT | |||
| Fusarium graminearum | Fg16N F | ACAGATGACAAGATTCAGGCACA | 280 | [93] |
| Fg16N R | TTCTTTGACATCTGTTCAACCCA | |||
| Fusarium poae | Fp82F | CAAGCAAACAGGCTCTTCACC | 220 | [94] |
| Fp82R | TGTTCCACCTCAGTGACAGGTT | |||
| Fusarium proliferatum | Fp3-F | CGGCCACCAGAGGATGTG | 230 | [95] |
| Fp4-R | CAACACGAATCGCTTCCTGAC | |||
| Fusarium tricinctum | Tri1 | CGTGTCCCTCTGTACAGCTTTGA | 215 | [96] |
| Tri1 | GTGGTTACCTCCCGATACTCTA | |||
| Leptosphaeria maculans | LmacF | CTTGCCCACCAATTGGATCCCCTA | 331 | [97] |
| LmacR | GCAAAATGTGCTGCGCTCCAGG | |||
| Leptosphaeria biglobosa | LbigF | ATCAGGGGATTGGTGTCAGCAGTTGA | 444 | [97] |
| LmacR | GCAAAATGTGCTGCGCTCCAGG | |||
| Sclerotinia sclerotiorum | SsF | AGTCGAGGGACGGGTACTAA | 225 | [98] |
| SsR | CTTGTCCTCATTGCCGTTT | |||
| Verticillium longisporum | OLG 70 | CAGCGAAACGCGATATGTAG | 261 | [99] |
| OLG 71 | GGCTTGTAGGGGGTTTAGA |
| Target Organism | Initial Denaturation | Denaturation | Annealing | Extension | No. of Cycles | Limit of Quantification (g DNA µL−1 Template) |
|---|---|---|---|---|---|---|
| Fusarium avenaceum | 95 °C, 120 s | 94 °C, 15 s | 60 °C, 15 s | 68 °C, 25 s | 38 | 1.24 × 10−12 |
| Fusarium culmorum | 95 °C, 120 s | 94 °C, 20 s | 62 °C, 40 s | 68 °C, 45 s | 35 | 1.24 × 10−12 |
| Fusarium graminearum | 95 °C, 120 s | 94 °C, 30 s | 61 °C, 30 s | 68 °C, 30 s | 35 | 1.37 × 10−13 |
| Fusarium poae | 95 °C, 120 s | 94 °C, 30 s | 62.5 °C, 30 s | 68 °C, 35 s | 35 | 1.37 × 10−13 |
| Fusarium proliferatum | 95 °C, 120 s | 94 °C, 35 s | 64 °C, 30 s | 68 °C, 35 s | 35 | 1.37 × 10−13 |
| Fusarium tricinctum | 95 °C, 120 s | 94 °C, 20 s | 65 °C, 20 s | 68 °C, 18 s | 38 | 1.52 × 10−14 |
| Leptosphaeria biglobosa | 95 °C, 120 s | 94 °C, 30 s | 68 °C, 35 s1 | 40 | 1.52 × 10−14 | |
| Leptosphaeria maculans | 95 °C, 120 s | 94 °C, 30 s | 68 °C, 35 s1 | 40 | 4.57 × 10−14 | |
| Sclerotinia sclerotiorum | 95 °C, 120 s | 94 °C, 30 s | 56 °C, 30 s | 68 °C, 20 s | 40 | 4.57 × 10−14 |
| Verticillium longisporum | 95 °C, 120 s | 94 °C, 10 s | 60 °C, 15 s | 68 °C, 15 s | 40 | 1.52 × 10−14 |

References
- Kay, S.; Crous-Duran, J.; Ferreiro-Domínguez, N.; García de Jalón, S.; Graves, A.; Moreno, G.; Mosquera-Losada, M.R.; Palma, J.H.N.; Roces-Díaz, J.V.; Santiago-Freijanes, J.J.; et al. Spatial similarities between European agroforestry systems and ecosystem services at the landscape scale. Agrofor. Syst. 2018, 92, 1075–1089. [Google Scholar] [CrossRef]
- Pardon, P.; Reubens, B.; Reheul, D.; Mertens, J.; De Frenne, P.; Coussement, T.; Janssens, P.; Verheyen, K. Trees increase soil organic carbon and nutrient availability in temperate agroforestry systems. Agric. Ecosyst. Environ. 2017, 247, 98–111. [Google Scholar] [CrossRef]
- Smith, J.; Pearce, B.D.; Wolfe, M.S. Reconciling productivity with protection of the environment: Is temperate agroforestry the answer? Renew. Agric. Food Syst. 2013, 28, 80–92. [Google Scholar] [CrossRef]
- Allen, S.C.; Jose, S.; Nair, P.K.R.; Brecke, B.J.; Nkedi-Kizza, P.; Ramsey, C.L. Safety-net role of tree roots: Evidence from a pecan (Carya illinoensis K. Koch)–cotton (Gossypium hirsutum L.) alley cropping system in the southern United States. For. Ecol. Manag. 2004, 192, 395–407. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Lin, L.; Zepp, H. Agroforestry system reduces subsurface lateral flow and nitrate loss in Jiangxi Province, China. Agric. Ecosyst. Environ. 2011, 140, 441–453. [Google Scholar] [CrossRef]
- Swieter, A.; Langhof, M.; Lamerre, J.; Greef, J.M. Long-term yields of oilseed rape and winter wheat in a short rotation alley cropping agroforestry system. Agrofor. Syst. 2018, 92, 1–12. [Google Scholar] [CrossRef]
- Pardon, P.; Reubens, B.; Mertens, J.; Verheyen, K.; De Frenne, P.; De Smet, G.; Van Waes, C.; Reheul, D. Effects of temperate agroforestry on yield and quality of different arable intercrops. Agric. Syst. 2018, 166, 135–151. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Hiddink, G.A.; Termorshuizen, A.J.; van Bruggen, A.H.C. Mixed Cropping and Suppression of Soilborne Diseases. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 119–146. [Google Scholar]
- Krupinsky, J.M.; Bailey, K.L.; McMullen, M.P.; Gossen, B.D.; Turkington, T.K. Managing Plant Disease Risk in Diversified Cropping Systems. Agron. J. 2002, 94, 198–209. [Google Scholar] [CrossRef]
- Lin, B.B. Resilience in Agriculture through Crop Diversification: Adaptive Management for Environmental Change. BioScience 2011, 61, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: A review. Agron. Sustain. Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Brändle, U.; Koller, B.; Limpert, E.; McDermott, J.M.; Müller, K.; Schaffner, D. Barley mildew in Europe: Population biology and host resistance. Euphytica 1992, 63, 125–139. [Google Scholar] [CrossRef]
- Finckh, M.R.; Mundt, C.C. Stripe rust, yield, and plant competition in wheat cultivar mixtures. Phytopathology 1992, 89, 905–913. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, H.; Fan, J.; Wang, Y.; Li, Y.; Chen, J.; Fan, J.; Yang, S.; Hu, L.; Leung, H.; et al. Genetic diversity and disease control in rice. Nature 2000, 406, 718–722. [Google Scholar] [CrossRef]
- Curl, E.A. Control of plant diseases by crop rotation. Bot. Rev. 1963, 29, 413–479. [Google Scholar] [CrossRef]
- Zogg, H. Crop rotation and biological soil desinfection. Qual. Plant. Mater. Veg. 1969, 18, 256–273. [Google Scholar] [CrossRef]
- Boudreau, M.A. Diseases in intercropping systems. Ann. Rev. Phytopathol. 2013, 51, 499–519. [Google Scholar] [CrossRef]
- Trenbath, B.R. Intercropping for the management of pests and diseases. Field Crop. Res. 1993, 34, 381–405. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Selosse, M.A.; Baudoin, E.; Vandenkoornhuyse, P. Symbiotic microorganisms, a key for ecological success and protection of plants. C. R. Biol. 2004, 327, 639–648. [Google Scholar] [CrossRef]
- Weller, D.M.; Raaijmakers, J.M.; McSpadden Gardener, B.B.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Ann. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Jose, S.; Gillespie, A.R.; Pallardy, S.G. Interspecific interactions in temperate agroforestry. Agrofor. Syst. 2004, 61, 237–255. [Google Scholar]
- Kanzler, M.; Böhm, C.; Mirck, J.; Schmitt, D.; Veste, M. Microclimate effects on evaporation and winter wheat (Triticum aestivum L.) yield within a temperate agroforestry system. Agrofor. Syst. 2018, 92. [Google Scholar] [CrossRef]
- Böhm, C.; Kanzler, M.; Freese, D. Wind speed reductions as influenced by woody hedgerows grown for biomass in short rotation alley cropping systems in Germany. Agrofor. Syst. 2014, 88, 579–591. [Google Scholar] [CrossRef]
- Lovell, D.J.; Parker, S.R.; Van Peteghem, P.; Webb, D.A.; Welham, S.J. Quantification of raindrop kinetic energy for improved prediction of splash-dispersed pathogens. Phytopathology 2002, 92, 497–503. [Google Scholar] [CrossRef]
- Hodges, L.; Brandle, J. Windbreaks: An Important Component in a Plasticulture System. Available online: https://digitalcommons.unl.edu/agronomyfacpub/391/ (accessed on 26 March 2019).
- Schroth, G.; Krauss, U.; Gasparotto, L.; Duarte Aguilar, J.A.; Vohland, K. Pests and diseases in agroforestry systems of the humid tropics. Agrofor. Syst. 2000, 50, 199–241. [Google Scholar] [CrossRef]
- Brandfass, C.; Karlovsky, P. Upscaled CTAB-Based DNA Extraction and Real-Time PCR Assays for Fusarium culmorum and F. graminearum DNA in Plant Material with Reduced Sampling Error. Int. J. Mol. Sci. 2008, 9, 2306–2321. [Google Scholar] [CrossRef] [Green Version]
- Cristina, D.; Turcu, A.G.; Ciuca, M. Molecular Detection of Resistance Genes to Leaf Rust Lr34 And Lr37 in Wheat Germplasm. Agric. Agric. Sci. Procedia 2015, 6, 533–537. [Google Scholar] [CrossRef] [Green Version]
- Reineke, A.; Karlovsky, P.; Zebitz, C.P.W. Preparation and purification of DNA from insects for AFLP analysis. Insect Mol. Biol. 1998, 7, 95–99. [Google Scholar] [CrossRef]
- Nutz, S.; Döll, K.; Karlovsky, P. Determination of the LOQ in real-time PCR by receiver operating characteristic curve analysis: Application to qPCR assays for Fusarium verticillioides and F. proliferatum. Anal. Bioanal. Chem. 2011, 401, 707–726. [Google Scholar] [CrossRef]
- Clarke, J.U. Evaluation of censored data methods to allow statistical comparisons among very small samples with below detection limit observations. Environ. Sci. Technol. 1998, 32, 177–183. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Xiong, S.; Nilsson, C. The effects of plant litter on vegetation: A meta-analysis. J. Ecol. 1999, 87, 984–994. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; Leite, L.F.C.; de Iwata, B.F.; de Lira, M.A.; Xavier, G.R.; Figueiredo, M.D.V.B. Microbiological process in agroforestry systems: A review. Agron. Sustain. Dev. 2012, 32, 215–226. [Google Scholar] [CrossRef]
- Banerjee, S.; Baah-Acheamfour, M.; Carlyle, C.N.; Bissett, A.; Richardson, A.E.; Siddique, T.; Bork, E.W.; Chang, S.X. Determinants of bacterial communities in Canadian agroforestry systems: Co-occurrence patterns of soil bacterial communities. Environ. Microbiol. 2016, 18, 1805–1816. [Google Scholar] [CrossRef]
- Chander, K.; Goyal, S.; Nandal, D.P.; Kapoor, K.K. Soil organic matter, microbial biomass and enzyme activities in a tropical agroforestry system. Biol. Fertil. Soils 1998, 27, 168–172. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, S.R.; Singh, G. Soil carbon, microbial activity and nitrogen availability in agroforestry systems on moderately alkaline soils in northern India. Appl. Soil Ecol. 2000, 15, 283–294. [Google Scholar] [CrossRef]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of Verticillium species. Ann. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Dunker, S.; Keunecke, H.; Steinbach, P.; Tiedemann, A.V. Impact of Verticillium longisporum on yield and morphology of einter oilseed tape (Brassica napus) in relation to systemic spread in the plant. J. Phytopathol. 2008, 156, 698–707. [Google Scholar] [CrossRef]
- Heale, J.B.; Karapapa, V.K. The Verticillium threat to Canada’s major oilseed crop: Canola. Can. J. Plant Pathol. 1999, 21, 1–7. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Q.; Johansson, A.; Dixelius, C. Verticillium longisporum and V. dahliae: Infection and disease in Brassica napus. Plant Pathol. 2006, 55, 137–144. [Google Scholar] [CrossRef]
- Lopisso, D.T.; Knüfer, J.; Koopmann, B.; von Tiedemann, A. The vascular pathogen Verticillium longisporum does not affect water relations and plant responses to drought stress of its host, Brassica napus. Phytopathology 2016, 107, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Debode, J.; Maeyer, K.D.; Perneel, M.; Pannecoucque, J.; Backer, G.D.; Höfte, M. Biosurfactants are involved in the biological control of Verticillium microsclerotia by Pseudomonas spp. J. Appl. Microbiol. 2007, 103, 1184–1196. [Google Scholar] [CrossRef]
- Abuamsha, R.; Salman, M.; Ehlers, R.U. Differential resistance of oilseed rape cultivars (Brassica napus ssp. oleifera) to Verticillium longisporum infection is affected by rhizosphere colonisation with antagonistic bacteria, Serratia plymuthica and Pseudomonas chlororaphis. BioControl 2011, 56, 101–112. [Google Scholar] [CrossRef]
- Berg, G. Rhizobacteria of oilseed rape antagonistic to Verticillium dahliae var. longisporum STARK. J. Plant Dis. Protect. 1996, 103, 20–30. [Google Scholar]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Paoletti, M.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 19–31. [Google Scholar] [Green Version]
- Latz, E.; Eisenhauer, N.; Rall, B.C.; Allan, E.; Roscher, C.; Scheu, S.; Jousset, A. Plant diversity improves protection against soil-borne pathogens by fostering antagonistic bacterial communities. J. Ecol. 2012, 100, 597–604. [Google Scholar] [CrossRef]
- Shoemaker, R.A.; Brun, H. The teleomorph of the weakly aggressive segregate of Leptosphaeria maculans. Can. J. Bot. 2001, 79, 412–419. [Google Scholar]
- West, J.S.; Kharbanda, P.D.; Barbetti, M.J.; Fitt, B.D.L. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathol. 2001, 50, 10–27. [Google Scholar] [CrossRef]
- Danielsson, J.; Reva, O.; Meijer, J. Protection of oilseed rape (Brassica napus) toward fungal pathogens by strains of plant-associated Bacillus amyloliquefaciens. Microb. Ecol. 2007, 54, 134–140. [Google Scholar] [CrossRef]
- Hammoudi, O.; Salman, M.; Abuamsha, R.; Ehlers, R.U. Effectiveness of bacterial and fungal isolates to control Phoma lingam on oilseed rape Brassica napus. Am. J. Plant Sci. 2012, 3, 773–779. [Google Scholar] [CrossRef]
- Huang, Y.J.; Fitt, B.D.L.; Jedryczka, M.; Dakowska, S.; West, J.S.; Gladders, P.; Steed, J.M.; Li, Z.Q. Patterns of ascospore release in relation to phoma stem canker epidemiology in England (Leptosphaeria maculans) and Poland (Leptosphaeria biglobosa). Eur. J. Plant Pathol. 2005, 111, 263–277. [Google Scholar] [CrossRef]
- Travadon, R.; Bousset, L.; Saint-Jean, S.; Brun, H.; Sache, I. Splash dispersal of Leptosphaeria maculans pycnidiospores and the spread of blackleg on oilseed rape. Plant Pathol. 2007, 56, 595–603. [Google Scholar] [CrossRef]
- Czaban, J.; Wróblewska, B.; Sułek, A.; Mikos, M.; Boguszewska, E.; Podolska, G.; Nieróbca, A. Colonisation of winter wheat grain by Fusarium spp. and mycotoxin content as dependent on a wheat variety, crop rotation, a crop management system and weather conditions. Food Addit. Contam. Part A 2015, 32, 874–910. [Google Scholar] [CrossRef]
- Karlsson, I.; Friberg, H.; Kolseth, A.K.; Steinberg, C.; Persson, P. Agricultural factors affecting Fusarium communities in wheat kernels. Int. J. Food Microbiol. 2017, 252, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Leplat, J.; Mangin, P.; Falchetto, L.; Heraud, C.; Gautheron, E.; Steinberg, C. Visual assessment and computer—Assisted image analysis of Fusarium head blight in the field to predict mycotoxin accumulation in wheat grains. Eur. J. Plant Pathol. 2018, 150, 1065–1081. [Google Scholar] [CrossRef]
- Alkadri, D.; Nipoti, P.; Döll, K.; Karlovsky, P.; Prodi, A.; Pisi, A. Study of fungal colonization of wheat kernels in Syria with a focus on Fusarium species. Int. J. Mol. Sci. 2013, 14, 5938–5951. [Google Scholar] [CrossRef]
- Burmeister, H.R.; Plattner, R.D. Enniatin production by Fusarium tricinctum and its effect on germinating wheat seeds. Phytopathology 1987, 77, 1483–1487. [Google Scholar] [CrossRef]
- Chelkowski, J.; Zawadzki, M.; Zajkowski, P.; Logrieco, A.; Bottalico, A. Moniliformin production by fusarium species. Mycotoxin Res. 1990, 6, 41–45. [Google Scholar] [CrossRef]
- Thrane, U. Screening for Fusarin C production by European isolates of Fusarium species. Mycotoxin Res. 1988, 4, 2–10. [Google Scholar] [CrossRef]
- Wachowska, U.; Kucharska, K.; Jedryczka, M.; Lobik, N. Microorganisms as biological control agents against fusarium pathogens in winter wheat. Pol. J. Environ. Stud. 2013, 22, 591–597. [Google Scholar]
- Nourozian, J.; Etebarian, H.R.; Khodakaramian, G. Biological control of Fusarium graminearum on wheat by antagonistic bacteria. Songklanakarin J. Sci. Technol. 2006, 28, 29–38. [Google Scholar]
- Zalila-Kolsi, I.; Ben Mahmoud, A.; Ali, H.; Sellami, S.; Nasfi, Z.; Tounsi, S.; Jamoussi, K. Antagonist effects of Bacillus spp. strains against Fusarium graminearum for protection of durum wheat (Triticum turgidum L. subsp. durum). Microbiol. Res. 2016, 192, 148–158. [Google Scholar] [CrossRef]
- Zhao, Y.; Selvaraj, J.N.; Xing, F.; Zhou, L.; Wang, Y.; Song, H.; Tan, X.; Sun, L.; Sangare, L.; Folly, Y.M.E.; et al. Antagonistic Action of Bacillus subtilis Strain SG6 on Fusarium graminearum. PLoS ONE 2014, 9, e92486. [Google Scholar] [CrossRef]
- Khan, N.; Maymon, M.; Hirsch, A.M. Combating Fusarium Infection Using Bacillus-Based Antimicrobials. Microorganisms 2017, 5, 75. [Google Scholar] [CrossRef]
- Ghorbani, R.; Wilcockson, S.; Koocheki, A.; Leifert, C. Soil management for sustainable crop disease control: A review. Environ. Chem. Lett. 2008, 6, 149–162. [Google Scholar] [CrossRef]
- Hope, R.; Aldred, D.; Magan, N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett. Appl. Microbiol. 2005, 40, 295–300. [Google Scholar] [CrossRef]
- Ramirez, M.L.; Chulze, S.; Magan, N. Temperature and water activity effects on growth and temporal deoxynivalenol production by two Argentinean strains of Fusarium graminearum on irradiated wheat grain. Int. J. Food Microbiol. 2006, 106, 291–296. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. Modelling the relationship between environmental factors, transcriptional genes and deoxynivalenol mycotoxin production by strains of two Fusarium species. J. R. Soc. Interface 2011, 8, 117–126. [Google Scholar] [CrossRef]
- Llorens, A.; Mateo, R.; Hinojo, M.J.; Valle-Algarra, F.M.; Jimenez, M. Influence of environmental factors on the biosynthesis of type B trichothecenes by isolates of Fusarium spp. from Spanish crops. Int. J. Food Microbiol. 2004, 94, 43–54. [Google Scholar] [CrossRef]
- Ryu, D.; Bullerman, L.B. Effect of cycling temperatures on the production of deoxynivalenol and zearalenone by Fusarium graminearum NRRL 5883. J. Food Prot. 1999, 62, 1451–1455. [Google Scholar] [CrossRef]
- Bacon, C.W.; Porter, J.K.; Norred, W.P.; Leslie, J.F. Production of fusaric acid by Fusarium species. Appl. Environ. Microbiol. 1996, 62, 4039–4043. [Google Scholar] [Green Version]
- Chawla, H.S.; Wenzel, G. In vitro Selection for fusaric acid resistant barley plants. Plant Breed. 1987, 99, 159–163. [Google Scholar] [CrossRef]
- Smith, T.K.; Graça Sousadias, M. Fusaric acid content of swine feedstuffs. J. Agric. Food Chem. 1993, 41, 2296–2298. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Pharmacological activities of fusaric acid (5-butylpicolinic acid). Life Sci. 1999, 65, 849–856. [Google Scholar] [CrossRef]
- Bacon, C.W.; Porter, J.K.; Norred, W.P. Toxic interaction of fumonisin B1 and fusaric acid measured by injection into fertile chicken egg. Mycopathologia 1995, 129, 29–35. [Google Scholar] [CrossRef]
- Dowd, P.F. Toxicological and biochemical interactions of the fungal metabolites fusaric acid and kojic acid with xenobiotics in Heliothis zea (F.) and Spodoptera frugiperda (J.E. Smith). Pestic. Biochem. Physiol. 1988, 32, 123–134. [Google Scholar] [CrossRef]
- Smith, T.K.; McMillan, E.G.; Castillo, J.B. Effect of feeding blends of Fusarium mycotoxin-contaminated grains containing deoxynivalenol and fusaric acid on growth and feed consumption of immature swine. J. Anim. Sci. 1997, 75, 2184–2191. [Google Scholar] [CrossRef]
- Porter, J.K.; Bacon, C.W.; Wray, E.M.; Hagler, W.M. Fusaric acid in Fusarium moniliforme cultures, corn, and feeds toxic to livestock and the neurochemical effects in the brain and pineal gland of rats. Nat. Toxins 1995, 3, 91–100. [Google Scholar] [CrossRef]
- Smith, T.K.; MacDonald, E.J. Effect of fusaric acid on brain regional neurochemistry and vomiting behavior in swine. J. Anim. Sci. 1991, 69, 2044–2049. [Google Scholar] [CrossRef]
- Hidaka, H.; Nagatsu, T.; Takeya, K.; Takeuchi, T.; Suda, H. Fusaric acid, a hypotensive agent produced by fungi. J. Antibiot. (Tokyo) 1969, 22, 228–230. [Google Scholar] [CrossRef]
- Yabuta, T.; Kobe, K.; Hayashi, T. Biochemical studies of the “bakanae” fungus of rice. I. Fusarinic acid, a new product of the “Bakanae” fungus. Z. Pflanzenkrankh. Pflanzenpathol. Pflanzenschutz 1934, 10, 1059–1068. [Google Scholar]
- López-Díaz, C.; Rahjoo, V.; Sulyok, M.; Ghionna, V.; Martín-Vicente, A.; Capilla, J.; Pietro, A.D.; López-Berges, M.S. Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Plant Pathol. 2018, 19, 440–453. [Google Scholar] [CrossRef]
- Venter, S.L.; Steyn, P.J. Correlation between fusaric acid production and virulence of isolates of Fusarium oxysporum that causes potato dry rot in South Africa. Potato Res. Neth. 1998, 41, 289–294. [Google Scholar] [CrossRef]
- Amato, B.; Pfohl, K.; Tonti, S.; Nipoti, P.; Dastjerdi, R.; Pisi, A.; Karlovsky, P.; Prodi, A. Fusarium proliferatum and fumonisin B1 co-occur with Fusarium species causing Fusarium Head Blight in durum wheat in Italy. J. Appl. Bot. Food Qual. 2015, 88, 228–233. [Google Scholar]
- Guo, Z.; Pfohl, K.; Karlovsky, P.; Dehne, H.W.; Altincicek, B. Fumonisin B1 and beauvericin accumulation in wheat kernels after seed-borne infection with Fusarium proliferatum. Agric. Food Sci. 2016, 25, 138–145. [Google Scholar] [CrossRef]
- Turner, A.S.; Lees, A.K.; Rezanoor, H.N.; Nicholson, P. Refinement of PCR-detection of Fusarium avenaceum and evidence from DNA marker studies for phenetic relatedness to Fusarium tricinctum. Plant Pathol. 1998, 47, 278–288. [Google Scholar] [CrossRef]
- Schilling, A.G.; Moller, E.M.; Geiger, H.H. Polymerase chain reaction-based assays for species-specific detection of Fusarium culmorum, F. graminearum, and F. avenaceum. Phytopathology 1996, 86, 515–522. [Google Scholar] [CrossRef]
- Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Lees, A.K.; Parry, D.W.; Joyce, D. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant Pathol. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- Parry, D.W.; Nicholson, P. Development of a PCR assay to detect Fusarium poae in wheat. Plant Pathol. 1996, 45, 383–391. [Google Scholar] [CrossRef]
- Jurado, M.; Vázquez, C.; Marín, S.; Sanchis, V.; Teresa González-Jaén, M. PCR-based strategy to detect contamination with mycotoxigenic Fusarium species in maize. Syst. Appl. Microbiol. 2006, 29, 681–689. [Google Scholar] [CrossRef]
- Kulik, T. Detection of Fusarium tricinctum from cereal grain using PCR assay. J. Appl. Genet. 2008, 49, 305–311. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, Z.; Fitt, B.D.L.; Evans, N.; Foster, S.J.; Huang, Y.J.; Latunde-Dada, A.O.; Lucas, J.A. Resistance to Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape) induced by L. biglobosa and chemical defence activators in field and controlled environments. Plant Pathol. 2006, 55, 401–412. [Google Scholar] [CrossRef]
- Yin, Y.; Ding, L.; Liu, X.; Yang, J.; Ma, Z. Detection of Sclerotinia sclerotiorum in Planta by a Real-time PCR Assay. J. Phytopathol. 2009, 157, 465–469. [Google Scholar] [CrossRef]
- Eynck, C.; Koopmann, B.; Grunewaldt-Stoecker, G.; Karlovsky, P.; von Tiedemann, A. Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. Eur. J. Plant Pathol. 2007, 118, 259–274. [Google Scholar] [CrossRef]


| Study Site | ||||
|---|---|---|---|---|
| Jena | Cottbus | Göttingen | Braunschweig | |
| Mean annual air temperature 1981–2010 | 9.9 ± 0.1 °C a | 9.6 ± 0.2 °C b | 9.2 ± 0.1 °C c | 9.6 ± 0.2 °C d |
| Mean annual air temperature 2016 | 10.8 °C | 10.3 °C | 9.8 °C | 10.3 °C |
| Mean annual air temperature 2017 | 10.9 °C | 10.3 °C | 9.9 °C | 10.3 °C |
| Mean annual air temperature 2018 | 11.5 °C | 11.2 °C | 10.6 °C | 11.2 °C |
| Mean annual precipitation 1981–2010 | 608 ± 21 mm a | 568 ± 21 mm b | 651 ± 24 mm c | 637 ± 23 mm d |
| Annual precipitation 2016 | 528 mm | 593 mm | 544 mm | 504 mm |
| Annual precipitation 2017 | 648 mm | 621 mm | 777 mm | 819 mm |
| Annual precipitation 2018 | 415 mm | 429 mm | 430 mm | 380 mm |
| Meters above sea level | 289 m | 67 m | 329 m | 82 m |
| Year of agroforestry system establishment | 2007 | 2010 | 2011 | 2008 |
| Harvest dates of the aboveground tree biomass of the agroforestry system | winter 2014/15 | winter 2014/15, winter 2017/18 | winter 2014/15 | winter 2013/14 |
| Soil type | Calcaric Phaeozem | Gleyic Cambisol | Eutric Cambisol | Vertic Cambisol |
| Study Site | Crop Rotation | |||
|---|---|---|---|---|
| Crop 2015 a | Crop 2016 | Crop 2017 | Crop 2018 | |
| Jena | summer barley | summer barley | winter oilseed rape | winter wheat |
| Cottbus | maize | winter wheat | winter barley | maize b |
| Göttingen | summer wheat | winter barley | winter oilseed rape | winter wheat a |
| Braunschweig | winter barley | winter oilseed rape c | winter wheat | winter wheat |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beule, L.; Lehtsaar, E.; Rathgeb, A.; Karlovsky, P. Crop Diseases and Mycotoxin Accumulation in Temperate Agroforestry Systems. Sustainability 2019, 11, 2925. https://doi.org/10.3390/su11102925
Beule L, Lehtsaar E, Rathgeb A, Karlovsky P. Crop Diseases and Mycotoxin Accumulation in Temperate Agroforestry Systems. Sustainability. 2019; 11(10):2925. https://doi.org/10.3390/su11102925
Chicago/Turabian StyleBeule, Lukas, Ena Lehtsaar, Anna Rathgeb, and Petr Karlovsky. 2019. "Crop Diseases and Mycotoxin Accumulation in Temperate Agroforestry Systems" Sustainability 11, no. 10: 2925. https://doi.org/10.3390/su11102925





