Effect of Macro- and Nano-Biosolid Fractions on Sorption Affinity and Transport of Pb in a Loamy Sand Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Preparation
2.2. Biosolid Fractionation and Characterization
2.3. Pb Sorption
2.4. Pb Column Leaching
2.5. Statistical Analysis
3. Results
3.1. Macro- and Nano-Biosolid Properties
3.2. Sorption Affinity of Pb
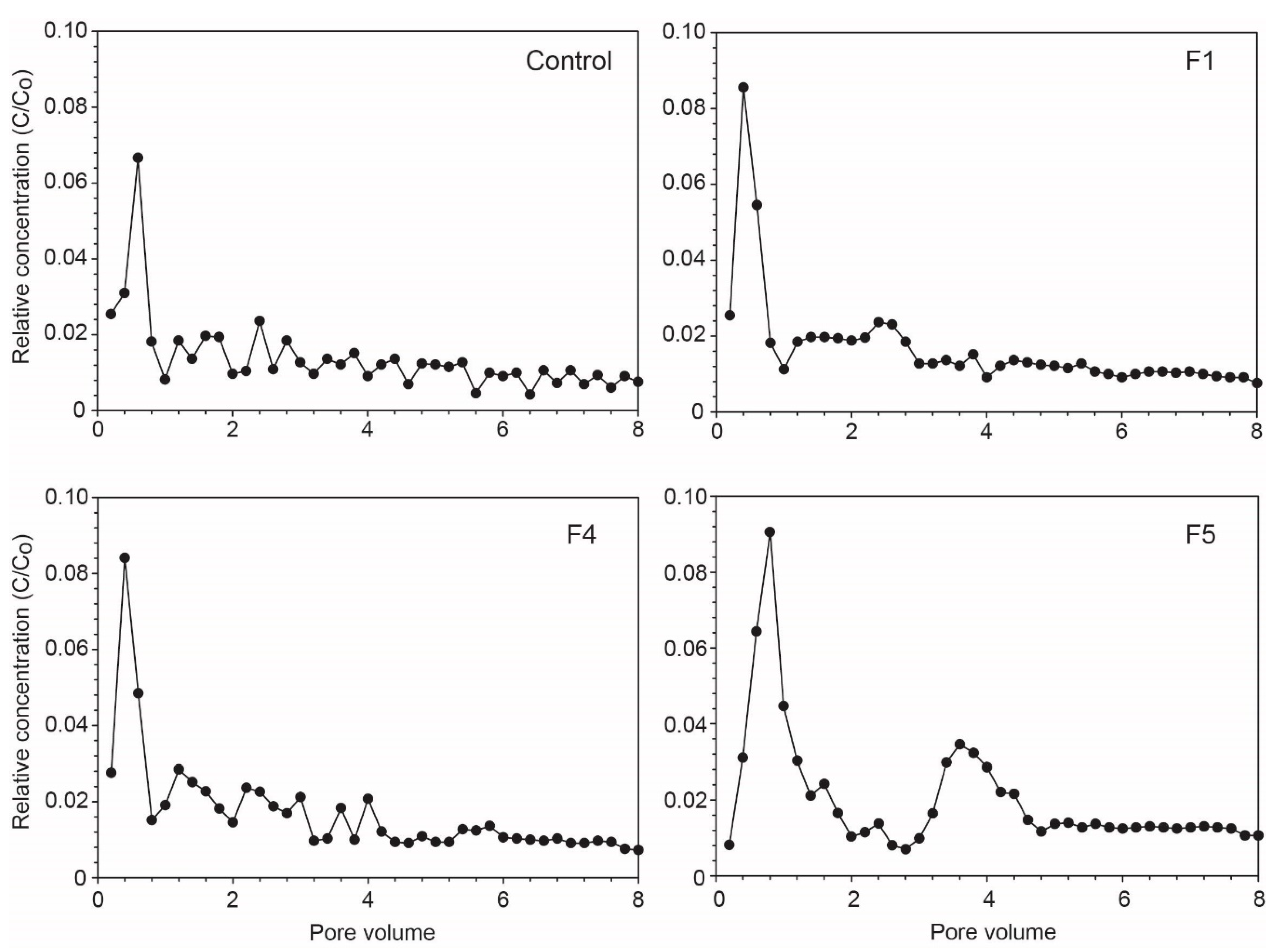
3.3. Breakthrough Curves of Pb
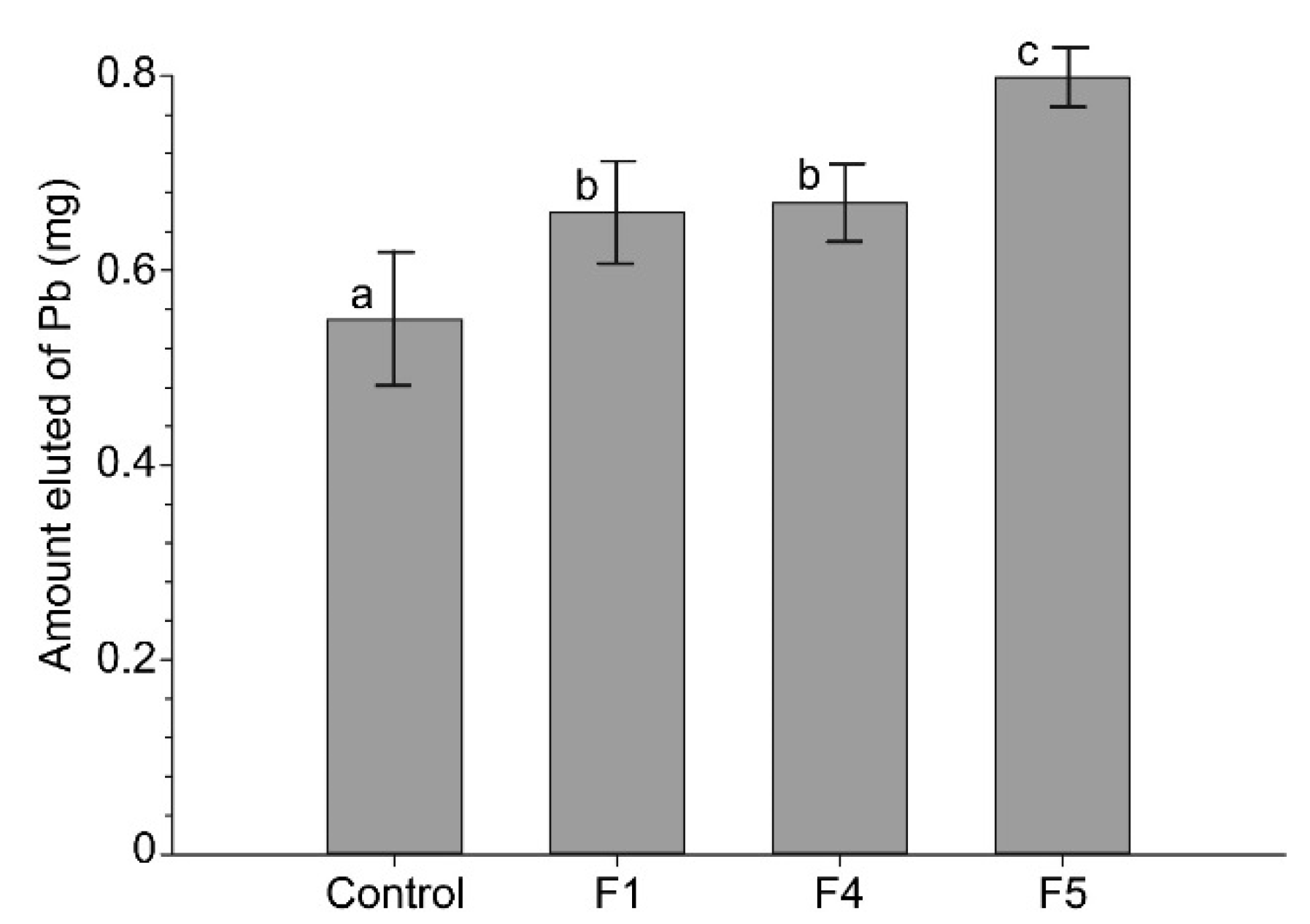
3.4. Amount Eluted of Pb
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Bi-Geochemistry, Bioavailability and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2003. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Alghamdi, A.; Kirkham, M.B.; Presley, D.R.; Hettiarachchi, G.; Murray, L. Rehabilitation of an abandoned mine site with biosolid. In Spoil to Soil: Mine Site Rehabilitation and Revegetation; Bolan, N., Kirkham, M.B., Ok, Y.S., Eds.; Chapter 14; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2018; pp. 241–258. [Google Scholar]
- Karathanasis, A.D.; Johnson, D.M.C.; Matocha, C.J. Biosolid Colloid-Mediated Transport of Copper, Zinc, and Lead in Waste-Amended Soils. J. Environ. Qual. 2005, 34, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Karathanasis, A.D.; Ming, D.A. Colloid-mediated transport of metals associated with lime-stabilized biosolid. Dev. Soil Sci. 2002, 28, 49–62. [Google Scholar]
- Karathanasis, A.D.; Johnson, D.M.C. Stability and Transportability of Biosolid Colloids through Undisturbed Soil Monoliths. Geoderma 2006, 130, 334–345. [Google Scholar] [CrossRef]
- Ghezzi, J.; Karathanasis, A.; Matocha, C.; Unrine, J.; Thompson, Y. Stability of Soil and Biosolid Nanocolloid and Macrocolloid Particles in the Absence and Presence of Arsenic, Selenium, Copper and Lead. Open J. Soil Sci. 2014, 4, 293–304. [Google Scholar] [CrossRef]
- Maurice, P.A.; Hochella, M.F., Jr. Nanoscale Particles and Processes: A New Dimension in Soil Science. Adv. Agron. 2008, 100, 123–153. [Google Scholar]
- Zhao, S.; Liu, X.; Dou, L. Physical and chemical characterization of municipal solid waste compost in different particle size fractions. Pol. J. Environ. Stud. 2012, 21, 509–515. [Google Scholar]
- Sharifi, Z.; Renella, G. Assessment of a particle size fractionation as a technology for reducing heavy metal, salinity and impurities from compost produced by municipal solid waste. Waste Manag. 2015, 38, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Seta, A.K.; Karathanasis, A.D. Stability and transportability of water- dispersible soil colloids. Soil Sci. 1997, 61, 604–611. [Google Scholar] [CrossRef]
- Karathanasis, A.D. Composition and transport behavior of soil nanocolloids in natural porous media. In Nanoparticles in the Water Cycle; Chapter 4; Frimmel, F.H., NieBner, R., Eds.; Springer: Berlin, Germany, 2010. [Google Scholar]
- McCarthy, J.F.; McKay, L.D. Colloid transport in the subsurface: Past, present and future challenges. Vadose Zone J. 2004, 3, 326–337. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Leoppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, G.T.; Sumner, M.E. Methods of Soil Analysis; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Reynolds, W.D.; Elrick, D.E.; Youngs, E.G.; Amoozegar, A.; Booltink, H.W.G.; Bouma, J. Saturated and field-saturated water flow parameters. In Methods of Soil Analysis: Part 4. Physical Methods; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2002. [Google Scholar]
- Vanderborght, M.; Van Griekenm, E. Enrichment of trace metals in water by adsorption on activated carbon. Anal. Chem. 1977, 49, 311–316. [Google Scholar] [CrossRef] [PubMed]
- SPSS, Inc. IBM SPSS Statistics Version 21; International Business Machines Corp: Boston, MA, USA, 2012. [Google Scholar]
- Kjaergaard, C.; Hansen, H.C.B.; Koch, C.B.; Villholth, K.G. Properties of water-dispersible colloids from macropore deposits and bulk horizons of an Agrudalf. Soil Sci. Soc. Am. J. 2004, 68, 1844–1852. [Google Scholar] [CrossRef]
- Karathanasis, A.D.; Ghezzi, J.L.; Wendroth, O.; Matocha, C.J.; Unrine, J.; Thompson, Y.L. Subsurface transport of As, Se, Cu, and Pb contaminants in association with soil and biosolid nano- and macro-colloid fractions. Austin J. Hydrol. 2014, 1, 1–13. [Google Scholar]
- Jacobsen, O.H.; Moldrup, P.; Larsen, C.; Konnerup, L.; Petersen, L.W. Particle transport in macropores of undisturbed soil columns. J. Hydrol. 1997, 196, 185–203. [Google Scholar] [CrossRef]
- Karathanasis, A.D.; Johnson, D.M.C. Subsurface transport of Cd, Cr, and Mo mediated by biosolid colloids. Sci. Total Environ. 2006, 354, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Logan, E.M.; Pulford, I.D.; Cook, G.T.; Mackenzie, A.B. Complexation of Cu2+ and Pb2+ by peat and humic acid. Eur. J. Soil Sci. 1997, 48, 685–697. [Google Scholar] [CrossRef]

| Properties | Units | Soil | Biosolid |
|---|---|---|---|
| EC | (ds m−1) | 2.96 | 6.1 |
| pH (in water) | - | 7.48 | 7.7 |
| OM | (%) | 0.18 | 71.2 |
| Sand | (%) | 87.5 | - |
| Silt | (%) | 5.4 | - |
| Clay | (%) | 7.1 | - |
| CaCO3 | (%) | 21.05 | - |
| Ks | (cm s−1) | 8.01 × 10−4 | - |
| Cations: | (meq L−1) | ||
| Ca+2 | 5.17 | 13.0 | |
| Mg+2 | 3.33 | 17.0 | |
| Na+1 | 20.61 | 25.1 | |
| K+1 | 1.26 | 5.2 | |
| Anions: | (meq L−1) | ||
| Cl− | 11.33 | 14.0 | |
| HCO3− | 1.17 | 29.3 | |
| SO4−2 | 16.33 | 6.0 | |
| Metals: | (mg g−1) | ||
| Zn | 6.1 | 652.6 | |
| Cu | 12.6 | 280.2 | |
| Ni | 11.1 | 23.7 | |
| As | ND | 1.1 | |
| Cr | 10.9 | 39.2 | |
| Pb | ND | 0.2 |
| Biosolid Fraction | Size | Percent to Whole 1 | Surface Area 2 | Pore Volume 3 | Pore Size 4 | Zeta Potential 5 |
|---|---|---|---|---|---|---|
| (µm) | (%) | (m2 g−1) | (cm3 g−1) | (Å) | (mV) | |
| F0 | >2000 | 18.2 | - | - | - | −13.6 a |
| F1 | 2000–1000 | 17.6 | 0.22 a | 0.00194 a | 354.2 a | −14.4 b |
| F2 | 1000–500 | 22.6 | 0.23 a | 0.00202 a | 352.6 a | −14.7 c |
| F3 | 500–100 | 31.5 | 1.75 b | 0.01125 b | 257.3 b | −14.8 cd |
| F4 | <100 | 8.9 | 1.89 c | 0.01122 b | 237.6 c | −14.9 d |
| F5 | 0.35 | 1.2 | 2.24 d | 0.01148 c | 205.1 d | −15.6 e |
| Properties | Units | Biosolid Fraction | |||||
|---|---|---|---|---|---|---|---|
| F0 | F1 | F2 | F3 | F4 | F5 | ||
| EC | (dS m−1) | 5.9 a | 6.5 b | 6.1 a | 5.9 a | 6.2 a | 6.1 a |
| pH | - | 7.7 a | 7.9 a | 7.5 b | 7.6 b | 7.6 b | 7.9 a |
| Cations: | (meq L−1) | ||||||
| Ca+2 | 14.0 a | 13.6 a | 12.0 c | 17.6 d | 17.2 d | 15.6 e | |
| Mg+2 | 17.0 a | 17.6 b | 20.0 c | 18.4 d | 17.2 ab | 21.6 e | |
| Na+1 | 25.1 a | 26.8 b | 26.1 c | 26.9 b | 26.9 b | 25.7 d | |
| K+1 | 5.2 a | 5.8 b | 6.1 c | 6.5 d | 6.2 c | 6.0 bc | |
| Anions: | (meq L−1) | ||||||
| Cl− | 12.0 a | 11.6 b | 12.0 a | 14.0 c | 13.0 d | 12.8 d | |
| HCO3− | 29.4 a | 29.0 a | 27.0 b | 28.0 c | 30.0 d | 29.0 a | |
| SO4−2 | 6.0 a | 5.9 a | 6.0 a | 6.1 a | 6.1 a | 6.1 a | |
| Metals: | (mg kg−1) | ||||||
| Zn | 700.8 a | 719.7 a | 663.8 b | 667.5 b | 623.5 c | 607.4 c | |
| Cu | 271.2 a | 214.3 b | 309.2 c | 264.6 a | 338.6 d | 336.9 d | |
| Ni | 20.7 a | 21.8 a | 19.7 a | 21.4 a | 32.8 b | 30.8 b | |
| As | ND | ND | ND | ND | ND | 1.1 a | |
| Cr | 34.5 a | 37.6 a | 34.8 a | 36.3 a | 58.6 b | 58.2 b | |
| Biosolid Fraction | Concentration (mg L−1) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | |
| (mg L−1) | ||||||
| F1 | 0.140 a | 0.229 a | 0.471 a | 0.804 a | 1.213 a | 2.245 a |
| F2 | 0.139 a | 0.220 b | 0.417 b | 0.667 b | 1.005 b | 2.068 b |
| F3 | 0.148 b | 0.195 b | 0.289 c | 0.380 c | 0.911 c | 1.380 c |
| F4 | 0.137 c | 0.151 c | 0.220 d | 0.253 d | 1.393 d | 0.974 d |
| F5 | 0.136 c | 0.142 d | 0.244 d | 0.355 e | 1.009 b | 1.075 e |
| (mg g−1) | ||||||
| F1 | −0.028 a | 0.354 a | 0.706 a | 1.039 a | 1.358 a | 1.551 a |
| F2 | −0.028 a | 0.356 a | 0.717 a | 1.067 b | 1.399 b | 1.586 b |
| F3 | −0.030 b | 0.361 b | 0.742 b | 1.124 c | 1.418 b | 1.724 c |
| F4 | −0.027 a | 0.370 c | 0.756 b | 1.149 d | 1.321 c | 1.805 d |
| F5 | −0.027 a | 0.372 c | 0.751 b | 1.129 d | 1.398 b | 1.785 e |
| Removal efficiency (%) | ||||||
| F1 | - | 88.6 a | 88.2 a | 86.6 a | 84.8 a | 77.6 a |
| F2 | - | 89.0 a | 89.6 b | 88.9 b | 87.4 b | 79.3 b |
| F3 | - | 90.2 b | 92.8 c | 93.7 c | 88.6 c | 86.2 c |
| F4 | - | 92.5 c | 94.5 d | 95.8 d | 82.6 d | 90.3 d |
| F5 | - | 92.9 c | 93.9 e | 94.1 e | 84.4 e | 89.3 e |
| Biosolid Fraction | Freundlich Parameters | Langmuir Parameters | |||||
|---|---|---|---|---|---|---|---|
| 1/n | R2 | R2 | |||||
| (L g−1) | (-) | - | (mg g−1) | (L mg−1) | (-) | - | |
| F1 | 1.07 a | 0.661 a | 0.95 | 4.35 a | 0.392 a | 0.338 a | 0.99 |
| F2 | 1.19 b | 0.676 a | 0.91 | 5.16 b | 0.348 b | 0.363 b | 0.98 |
| F3 | 1.55 c | 0.696 a | 0.83 | 21.1 c | 0.102 c | 0.641 c | 0.87 |
| F4 | 1.49 c | 0.505 b | 0.66 | 4.58 a | 0.721 d | 0.225 d | 0.76 |
| F5 | 1.67 d | 0.651 a | 0.88 | 5.33 d | 0.567 e | 0.266 e | 0.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, A.G.; Ibrahim, H.M. Effect of Macro- and Nano-Biosolid Fractions on Sorption Affinity and Transport of Pb in a Loamy Sand Soil. Sustainability 2019, 11, 3460. https://doi.org/10.3390/su11123460
Alghamdi AG, Ibrahim HM. Effect of Macro- and Nano-Biosolid Fractions on Sorption Affinity and Transport of Pb in a Loamy Sand Soil. Sustainability. 2019; 11(12):3460. https://doi.org/10.3390/su11123460
Chicago/Turabian StyleAlghamdi, Abdulaziz G., and Hesham M. Ibrahim. 2019. "Effect of Macro- and Nano-Biosolid Fractions on Sorption Affinity and Transport of Pb in a Loamy Sand Soil" Sustainability 11, no. 12: 3460. https://doi.org/10.3390/su11123460





