Improving the Life Cycle Impact Assessment of Metal Ecotoxicity: Importance of Chromium Speciation, Water Chemistry, and Metal Release
Abstract
1. Introduction
2. Materials and Methods
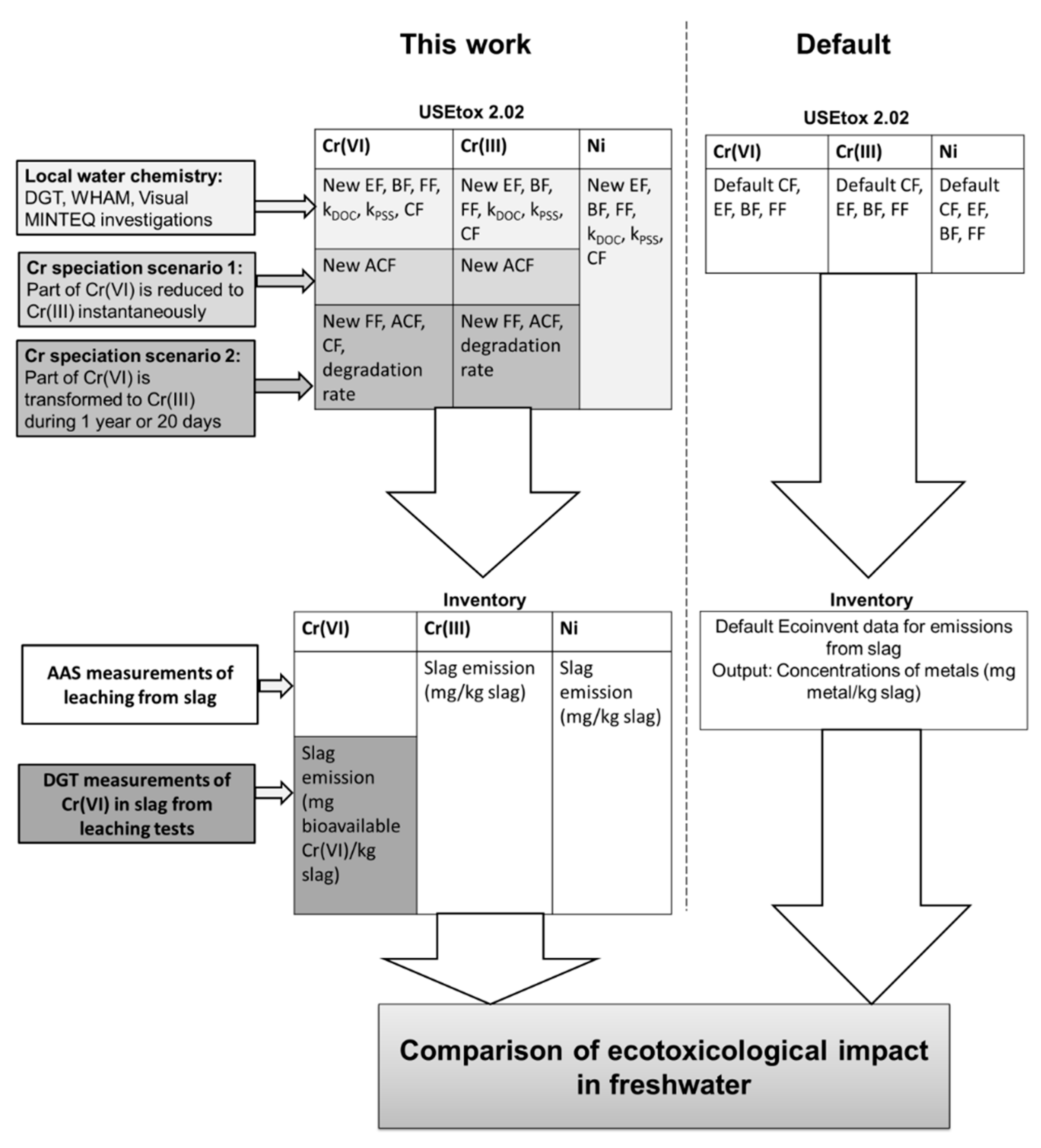
2.1. Construction of Characterization Factors for a Model Freshwater
2.2. Geochemical Modelling of Metal Speciation
2.3. DGT Measurements to Estimate Metal Bioavailability
2.4. Construction of New Effects Factors
2.5. Scenarios for Taking the Reduction of Cr(VI) into Account in LCIA
2.6. LCIA of Stainless Steel Slags Using SimaPro
2.7. Experimental
2.8. Determination of Total Metal Concentrations in Solution
2.9. Diffusive Gradient in Thin-Films
2.10. Total Metal and Labile Metals of Slag Leachates
2.11. Slag Characterization
3. Results and Discussion
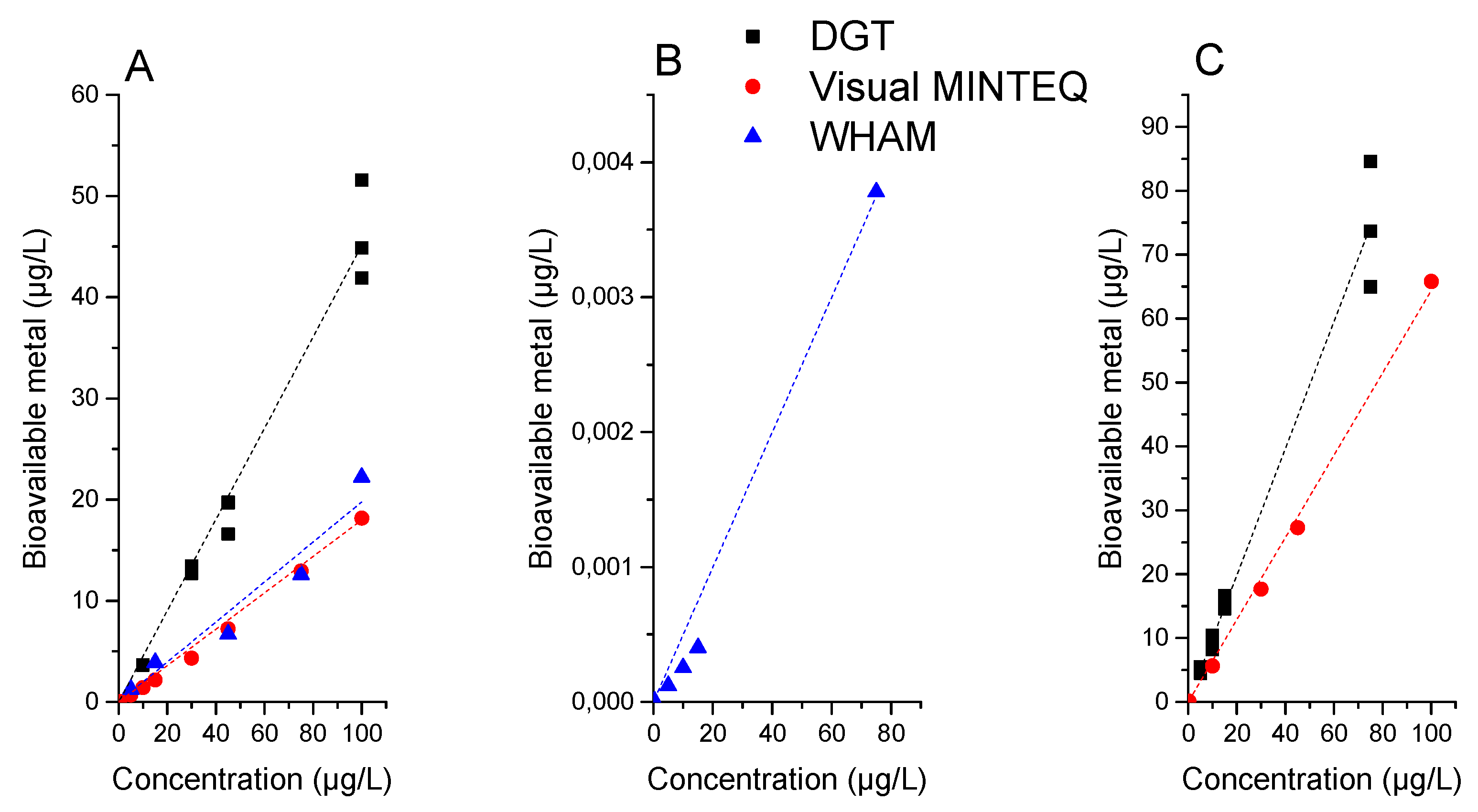
3.1. Comparison between DGT Measurements and Software Simulations for the Construction of Characterization Factors for Cr and Ni in a Model Freshwater
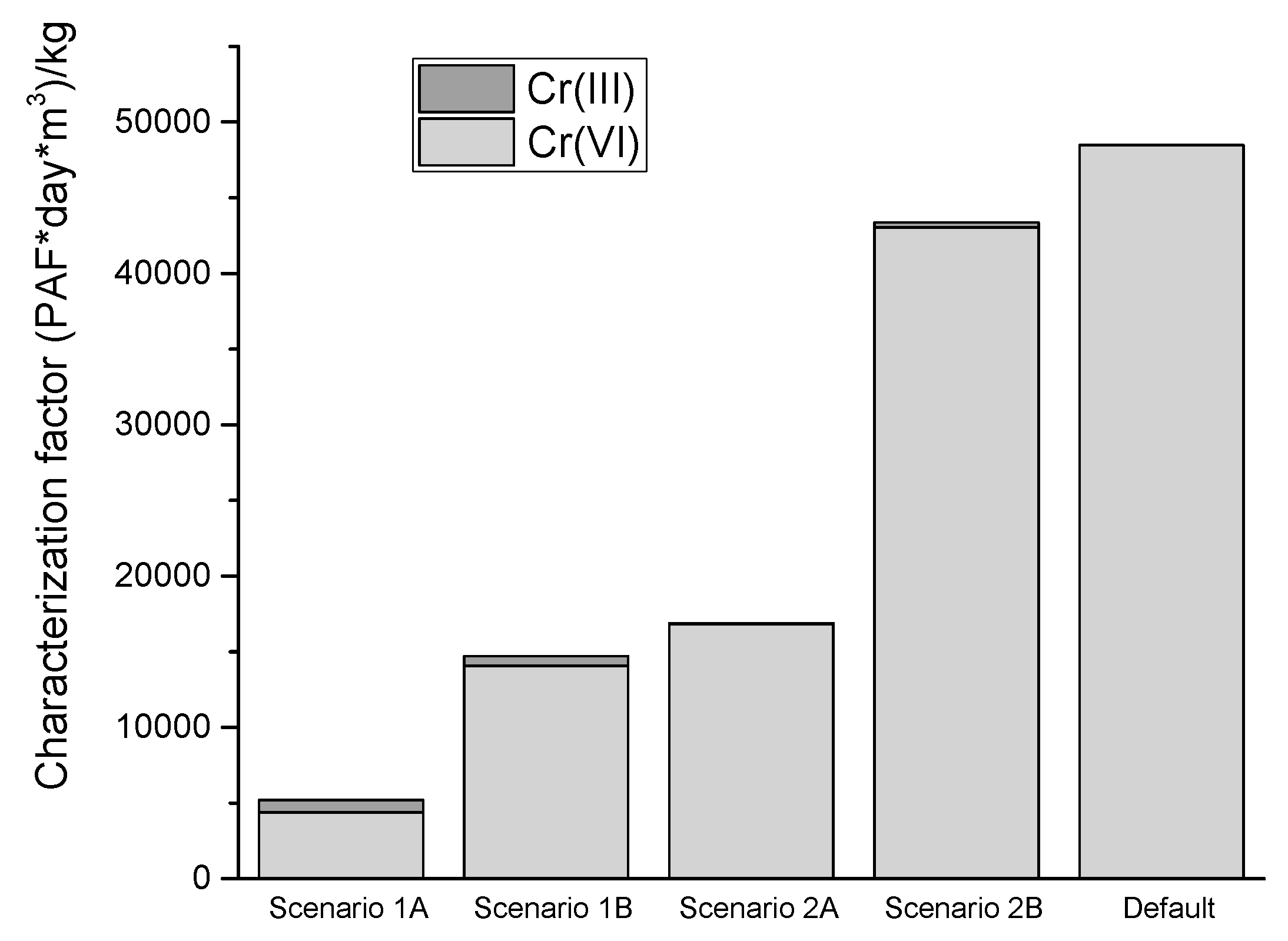
3.2. Comparing Modelling of the Impact of Changes in Cr Oxidation State with the Default Treatment of Cr in USEtox
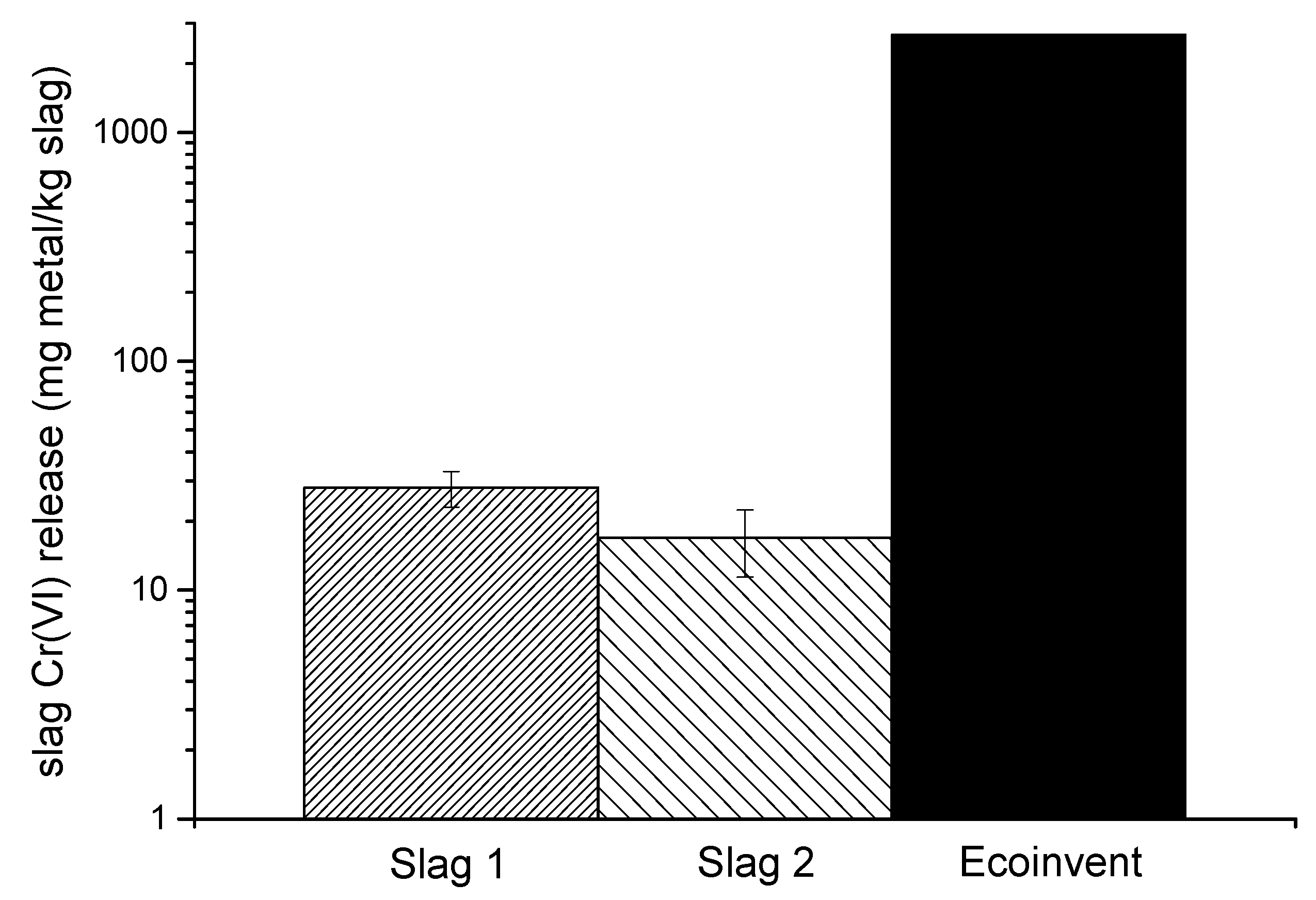
3.3. Implementing Experimental Data in LCIA on Metal Bioavailability of Cr(VI), Cr(III), and Ni Dispersed from Slag and Comparisons between the Use of Data and Default Values in USEtox
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Norgate, T.; Jahanshahi, S.; Rankin, W. Assessing the environmental impact of metal production processes. J. Clean. Prod. 2007, 15, 838–848. [Google Scholar] [CrossRef]
- Finkbeiner, M.; Inaba, A.; Tan, R.; Christiansen, K.; Klüppel, H.-J. The new international standards for life cycle assessment: ISO 14040 and ISO 14044. Int. J. Life Cycle Asses. 2006, 11, 80–85. [Google Scholar] [CrossRef]
- Guinée, J. Handbook on life cycle assessment—Operational guide to the ISO standards. Int. J. Life Cycle Asses. 2001, 6, 255. [Google Scholar] [CrossRef]
- EC—European Commission. International Reference Life Cycle Data System (ILCD) Handbook—General Guide for Life Cycle Assessment—Detailed Guidance; Joint Research Centre Institute for Environment and Sustainability: Ispra (VA), Italy, 2010. [Google Scholar]
- ISO—International Organization for Standardization. Environmental Management—Life Cycle Assessment -Principals and Framework; International Standard ISO 14040; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- ISO—International Organization for Standardization. Environmental Management—Life Cycle Assessment—Requirements and Guidelines; International Standard ISO 14044; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Rosenbaum, R.K.; Bachmann, T.M.; Gold, L.S.; Huijbregts, M.A.; Jolliet, O.; Juraske, R.; Koehler, A.; Larsen, H.F.; MacLeod, M.; Margni, M. USEtox—the UNEP-SETAC toxicity model: Recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int. J. Life Cycle Assess. 2008, 13, 532–546. [Google Scholar] [CrossRef]
- Fantke, P.; Aurisano, N.; Bare, J.; Backhaus, T.; Bulle, C.; Chapman, P.M.; De Zwart, D.; Dwyer, R.; Ernstoff, A.; Golsteijn, L. Toward Harmonizing Ecotoxicity Characterization in Life Cycle Impact Assessment. Environ. Toxicol. Chem. 2018, 37, 2955–2971. [Google Scholar] [CrossRef] [PubMed]
- Saouter, E.; Aschberger, K.; Fantke, P.; Hauschild, M.Z.; Kienzler, A.; Paini, A.; Pant, R.; Radovnikovic, A.; Secchi, M.; Sala, S. Improving substance information in USEtox®, part 2: Data for estimating fate and ecosystem exposure factors. Environ. Toxicol. Chem. 2017, 36, 3463–3470. [Google Scholar] [CrossRef]
- Baumann, H.; Tillman, A. The Hitchhiker’s Guide to LCA, an Orientation in Life Cycle Assessment Methodology and Application; Studentlitteratur: Lund, Sweden, 2004. [Google Scholar]
- Hauschild, M.; Olsen, S.I.; Hansen, E.; Schmidt, A. Gone… but not away—Addressing the problem of long-term impacts from landfills in LCA. Int. J. Life Cycle Assess. 2008, 13, 547. [Google Scholar] [CrossRef]
- Bakas, I.; Hauschild, M.Z.; Astrup, T.F.; Rosenbaum, R.K. Preparing the ground for an operational handling of long-term emissions in LCA. Int. J. Life Cycle Assess. 2015, 20, 1444–1455. [Google Scholar] [CrossRef]
- Hauschild, M.Z.; Huijbregts, M.; Jolliet, O.; Macleod, M.; Margni, M.; van de Meent, D.; Rosenbaum, R.K.; McKone, T.E. Building a model based on scientific consensus for life cycle impact assessment of chemicals: The search for harmony and parsimony. Environ. Sci. Technol. 2008, 42, 7032–7037. [Google Scholar] [CrossRef]
- Dong, Y.; Gandhi, N.; Hauschild, M.Z. Development of Comparative Toxicity Potentials of 14 cationic metals in freshwater. Chemosphere 2014, 112, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Rosenbaum, R.K.; Hauschild, M.Z. Assessment of metal toxicity in marine ecosystems: Comparative toxicity potentials for nine cationic metals in coastal seawater. Environ. Sci. Technol. 2015, 50, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Owsianiak, M.; Holm, P.E.; Fantke, P.; Christiansen, K.S.; Borggaard, O.K.; Hauschild, M.Z. Assessing comparative terrestrial ecotoxicity of Cd, Co, Cu, Ni, Pb, and Zn: The influence of aging and emission source. Environ. Pollut. 2015, 206, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J. Visual MINTEQ ver. 3.0; KTH Department of Land and Water Resources Engineering: Stockholm, Sweden, 2011; (Based on de Allison JD, Brown DS, Novo-Gradac KJ, MINTEQA2 ver. 4, 1991). [Google Scholar]
- Gandhi, N.; Diamond, M.L.; van de Meent, D.; Huijbregts, M.A.; Peijnenburg, W.J.; Guinée, J. New method for calculating comparative toxicity potential of cationic metals in freshwater: Application to copper, nickel, and zinc. Environ. Sci. Technol. 2010, 44, 5195–5201. [Google Scholar] [CrossRef]
- Chattopadhyay, B.; Utpal, S.; Mukhopadhyay, S. Mobility and bioavailability of chromium in the environment: Physico-chemical and microbial oxidation of Cr (III) to Cr (VI). J. Appl. Sci. Environ. Manag. 2010, 14, 97–101. [Google Scholar] [CrossRef]
- Richard, F.C.; Bourg, A.C. Aqueous geochemistry of chromium: A review. Water Res. 1991, 25, 807–816. [Google Scholar] [CrossRef]
- Schwab, O.; Bayer, P.; Juraske, R.; Verones, F.; Hellweg, S. Beyond the material grave: Life Cycle Impact Assessment of leaching from secondary materials in road and earth constructions. Waste Manag. 2014, 34, 1884–1896. [Google Scholar] [CrossRef]
- Allegrini, E.; Butera, S.; Kosson, D.; Van Zomeren, A.; Van der Sloot, H.; Astrup, T.F. Life cycle assessment and residue leaching: The importance of parameter, scenario and leaching data selection. Waste Manag. 2015, 38, 474–485. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Asses. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- SLU—Institutionen för Vatten och Miljö. Ö Storsjön, 1,2 km V Stensängsholmen. Available online: http://info1.ma.slu.se/max/www_max.acgi$Station?ID=Intro&pID=38&sID=2219 (accessed on 3 March 2017).
- SLU—Institutionen för Vatten och Miljö. V Storsjön. 1,2 km O Gravholmen. Available online: http://info1.ma.slu.se/max/www_max.acgi$Station?ID=Intro&pID=38&sID=2218 (accessed on 3 March 2017).
- Zhang, H.; Davison, W. Use of diffusive gradients in thin-films for studies of chemical speciation and bioavailability. Environ. Chem. 2015, 12, 85–101. [Google Scholar] [CrossRef]
- Davison, W.; Zhang, H. Progress in understanding the use of diffusive gradients in thin films (DGT)–back to basics. Environ. Chem. 2012, 9, 1–13. [Google Scholar] [CrossRef]
- Sjöstedt, C.S.; Gustafsson, J.P.; Köhler, S.J. Chemical equilibrium modeling of organic acids, pH, aluminum, and iron in Swedish surface waters. Environ. Sci. Technol. 2010, 44, 8587–8593. [Google Scholar] [CrossRef] [PubMed]
- Tipping, E. WHAMC—A chemical equilibrium model and computer code for waters, sediments, and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances. Comput. Geosci. 1994, 20, 973–1023. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Anal. Chem. 1995, 67, 3391–3400. [Google Scholar] [CrossRef]
- Unsworth, E.R.; Warnken, K.W.; Zhang, H.; Davison, W.; Black, F.; Buffle, J.; Cao, J.; Cleven, R.; Galceran, J.; Gunkel, P.; et al. Model Predictions of Metal Speciation in Freshwaters Compared to Measurements by In Situ Techniques. Environ. Sci. Technol. 2006, 40, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Munn, S.; Allanou, R.; Aschberger, K.; Berthault, F.; Cosgrove, O.; Luotamo, M.; Pakalin, S.; Paya-Perez, A.; Pellegrini, G.; Schwarz-Schulz, B. Chromium trioxide, sodium chromate, sodium dichromate, ammonium dichromate, potassium dichromate, EUR 21508 EN. Eur. Union Risk Assess. Rep. 2005, 53. [Google Scholar]
- Woldegiorgis, A. Results from the Swedish Screening Programme 2006. Subreport 5: Hexavalent Chromium, Cr (IV). IVL Swedish Environmental Research Institute; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2007. [Google Scholar]
- Crommentuijn, T.; Sijm, D.; De Bruijn, J.; Van den Hoop, M.; Van Leeuwen, K.; Van de Plassche, E. Maximum permissible and negligible concentrations for metals and metalloids in the Netherlands, taking into account background concentrations. J. Environ. Manag. 2000, 60, 121–143. [Google Scholar] [CrossRef]
- Ableitung und Erprobung von Zielvorgaben zum Schutz Oberirdischer Binnengewässer für die Schwermetalle Blei, Cadmium, Chrom, Kupfer, Nickel, Quecksilber und Zink. (Derivation and Proof Testing of Target Settings for the Protection of the Aboveground Inland Waters for the Heavy Metals Lead, Cadmium, Chrome, Copper, Mercury and Zinc.); Kulturbuchverlag: Berlin, Germany, 1994; ISBN 3-88961-216-4.
- Garnier, J.-M.; Ciffroy, P.; Benyahya, L. Implications of short and long term (30 days) sorption on the desorption kinetic of trace metals (Cd, Zn, Co, Mn, Fe, Ag, Cs) associated with river suspended matter. Sci. Total Environ. 2006, 366, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Guan, D.-X.; Zhao, D.; Luo, J.; Zhang, H.; Davison, W.; Ma, L.Q. Novel speciation method based on diffusive gradients in thin-films for in situ measurement of CrVI in aquatic systems. Environ. Sci. Technol. 2015, 49, 14267–14273. [Google Scholar] [CrossRef]
- Gandhi, N.; Diamond, M.; Huijbregts, M.J.; Guinée, J.; Peijnenburg, W.G.M.; Meent, D. Implications of considering metal bioavailability in estimates of freshwater ecotoxicity: Examination of two case studies. Int. J. Life Cycle Assess. 2011, 16, 774–787. [Google Scholar] [CrossRef]
- Owsianiak, M.; Rosenbaum, R.K.; Huijbregts, M.A.; Hauschild, M.Z. Addressing geographic variability in the comparative toxicity potential of copper and nickel in soils. Environ. Sci. Technol. 2013, 47, 3241–3250. [Google Scholar] [CrossRef]
- Cornelis, G.; Johnson, C.A.; Van Gerven, T.; Vandecasteele, C. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: A review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
- Loncnar, M.; Zupan, M.; Bukovec, P.; Jakli, A. The effect of water cooling on the leaching behaviour of EAF slag from stainless steel production. Mater. Technol. Mater. Technol. 2009, 43, 315–321. [Google Scholar]
- Shen, H.; Forssberg, E.; Nordström, U. Physicochemical and mineralogical properties of stainless steel slags oriented to metal recovery. Resour. Conserv. Recy. 2004, 40, 245–271. [Google Scholar] [CrossRef]
- Lopez, F.; Lopez-Delgado, A.; Balcazar, N. Physico-Chemical and Mineralogical Properties of EAF and AOD Slags; Associazione Italiana di Metallurgia: Milano, Italy, 1997; pp. 417–426. [Google Scholar]
- Schmukat, A.; Duester, L.; Ecker, D.; Heininger, P.; Ternes, T. Determination of the long-term release of metal (loid) s from construction materials using DGTs. J. Hazard. Mater. 2013, 260, 725–732. [Google Scholar] [CrossRef]
- Astrup, T.; Mosbæk, H.; Christensen, T.H. Assessment of long-term leaching from waste incineration air-pollution-control residues. Waste Manag. 2006, 26, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A.; Beaven, R.P.; Woodman, N.D. Evaluating landfill aftercare strategies: A life cycle assessment approach. Waste Manag. 2017, 63, 417–431. [Google Scholar] [CrossRef] [PubMed]


| pH | Dissolved Organic Carbon, DOC (mg/L) | Alkalinity (mg CaCO3/L) | Temperature (°C) | Suspended Particulate Matter (mg/L) |
|---|---|---|---|---|
| 7.3 | 11 | 16 | 11 | 7.2 |
| DGT Device | Metals Investigated | Functional Resin | Thickness Resin (cm) | Thickness Diffusive Gel (cm) | Elution Factor | Diffusion Coefficient at 25 °C (m2/s) | Membrane Area (cm2) |
|---|---|---|---|---|---|---|---|
| LSNM | Cr(III), Ni | Chelex 100 (cross-linked polystyrene matrix with aminodiacetic acid as active group) | 0.04 | 0.074 | 0.8 | 5.77·10−6 | 3.14 |
| LSNE | Cr(VI) | N-methyl-D-glucamine (DMDG) | 0.05 | 0.078 | 0.712 | 8.18·10−6 | 3.14 |
| kdoc [L/kg] | kpss [L/kg] | HC50 [kg/m3] | ||||
|---|---|---|---|---|---|---|
| Model Freshwater | Default | Model Freshwater | Default | Model Freshwater | Default | |
| Ni | 1.45·105 | 7.6·104 | 3.43·104 | 2.4·103 | 1.23·10−4 | 3.61·10−4 |
| Cr(III) | 8.77·109 | 3.8·109 | 2.76·108 | 8.9·107 | 1.64·10−6 | 4.96·10−7 |
| Cr(VI) | 1.00·102 | 1.00·102 | 9.58·104 | 1.6·104 | N/A | 3.61·10−4 |
| BF-Bioavailability Factor * | FF-Fate Factor [d] | EF-Effect Factor [PAF·m3·kg−1] | CF-Characterization Factor [PAF·m3·d/kg emitted] | |||||
|---|---|---|---|---|---|---|---|---|
| Model Freshwater | Default | Model Freshwater | Default | Model Freshwater | Default | Model Freshwater | Default | |
| Ni | 2.0·10−1 | 7.1·10−1 | 6.6·101 | 1.1·102 | 4.1·103 | 3.9·103 | 9.9·104 | 3.0·105 |
| Cr(III) | 5.0·10−5 | 7.4·10−4 | 1.1·101 | 1.1·101 | 3.1·105 | 1.0·106 | 1.7·102 | 8.1·104 |
| Cr(VI) | 6.4·10−1 | 8.1·10−1 | 5.5·101 | 9.3·101 | N/A | 1.4·103 | 4.9·104 | 1.1·105 |
| Degradation Rate [s−1] | Fate Factor [d] | Accessibility Factor | Characterization Factor, Cr(VI) [PAF·m3·d/kg emitted] | Characterization Factor, Cr(III) [PAF·m3·d/kg emitted] | Characterization Factor, Total [PAF·m3·d/kg emitted] | |
|---|---|---|---|---|---|---|
| Scenario 1A | 1·10−20 | 5.5·101 | 0.09 | 3.4·103 | 8.4·102 | 5.2·103 |
| Scenario 1B | 1·10−20 | 5.5·101 | 0.29 | 1.4·104 | 6.5·102 | 1.5·104 |
| Scenario 2A | 2.2·10−8 | 5.2·101 | 0.93 | 4.3·103 | 6.5·101 | 4.3·104 |
| Scenario 2B | 4.0·10−7 | 3.0·101 | 0.63 | 1.7·107 | 3.4·102 | 1.7·104 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hedberg, J.; Fransson, K.; Prideaux, S.; Roos, S.; Jönsson, C.; Odnevall Wallinder, I. Improving the Life Cycle Impact Assessment of Metal Ecotoxicity: Importance of Chromium Speciation, Water Chemistry, and Metal Release. Sustainability 2019, 11, 1655. https://doi.org/10.3390/su11061655
Hedberg J, Fransson K, Prideaux S, Roos S, Jönsson C, Odnevall Wallinder I. Improving the Life Cycle Impact Assessment of Metal Ecotoxicity: Importance of Chromium Speciation, Water Chemistry, and Metal Release. Sustainability. 2019; 11(6):1655. https://doi.org/10.3390/su11061655
Chicago/Turabian StyleHedberg, Jonas, Kristin Fransson, Sonja Prideaux, Sandra Roos, Christina Jönsson, and Inger Odnevall Wallinder. 2019. "Improving the Life Cycle Impact Assessment of Metal Ecotoxicity: Importance of Chromium Speciation, Water Chemistry, and Metal Release" Sustainability 11, no. 6: 1655. https://doi.org/10.3390/su11061655
APA StyleHedberg, J., Fransson, K., Prideaux, S., Roos, S., Jönsson, C., & Odnevall Wallinder, I. (2019). Improving the Life Cycle Impact Assessment of Metal Ecotoxicity: Importance of Chromium Speciation, Water Chemistry, and Metal Release. Sustainability, 11(6), 1655. https://doi.org/10.3390/su11061655





