Assessment of Alkali Activation Potential of a Polish Ferronickel Slag
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Slag Characterisation
3.2. Factors Affecting IP Synthesis
3.2.1. Effect of NaOH Molarity, Selected Molar Ratios in the Reactive Paste and Curing Temperature
3.2.2. Effect of Ageing Period
3.2.3. Effect of Pre-Curing and Curing Period
3.3. Structural Integrity of IPs
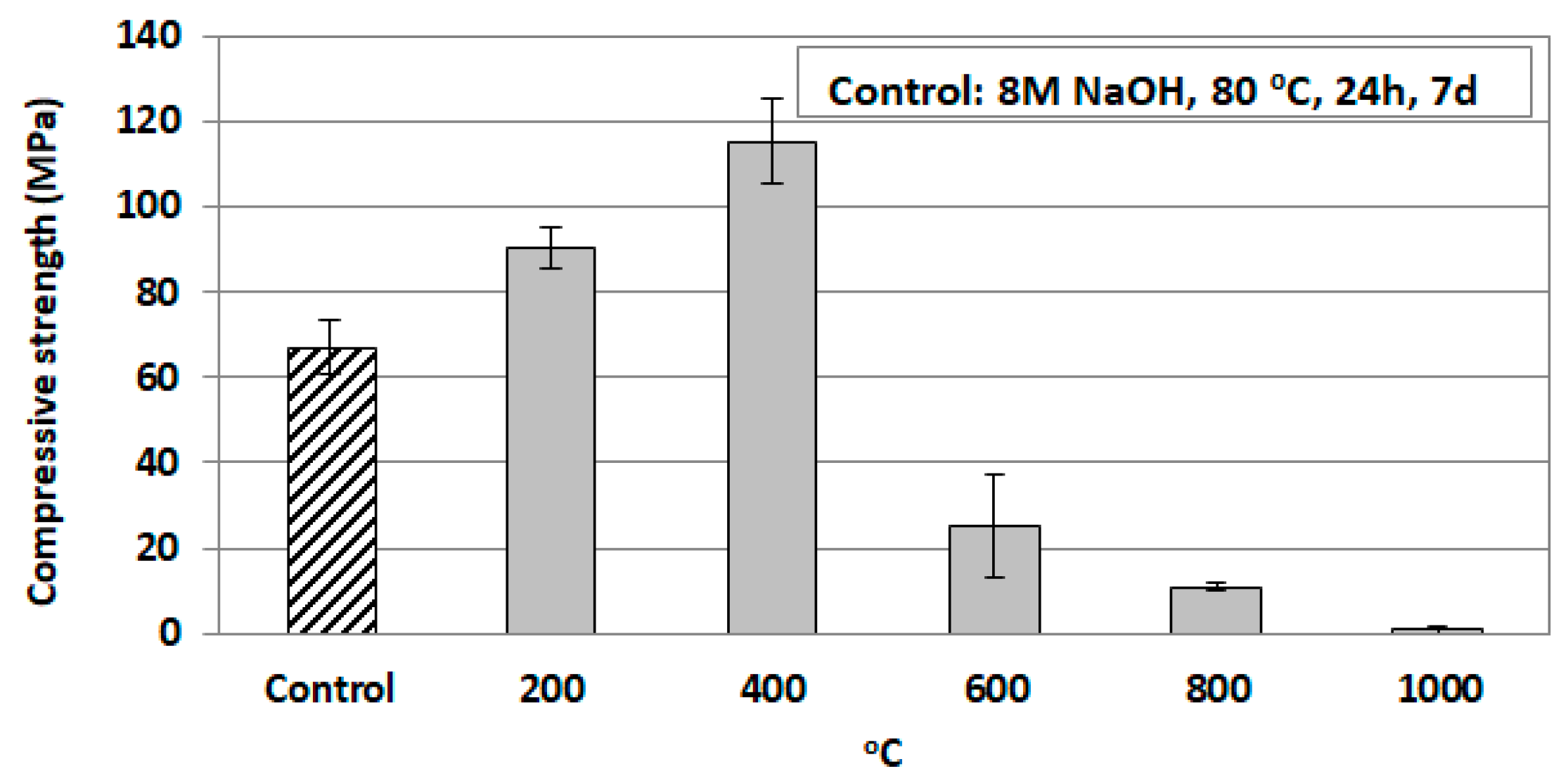
3.3.1. Effect of High-Temperature Firing
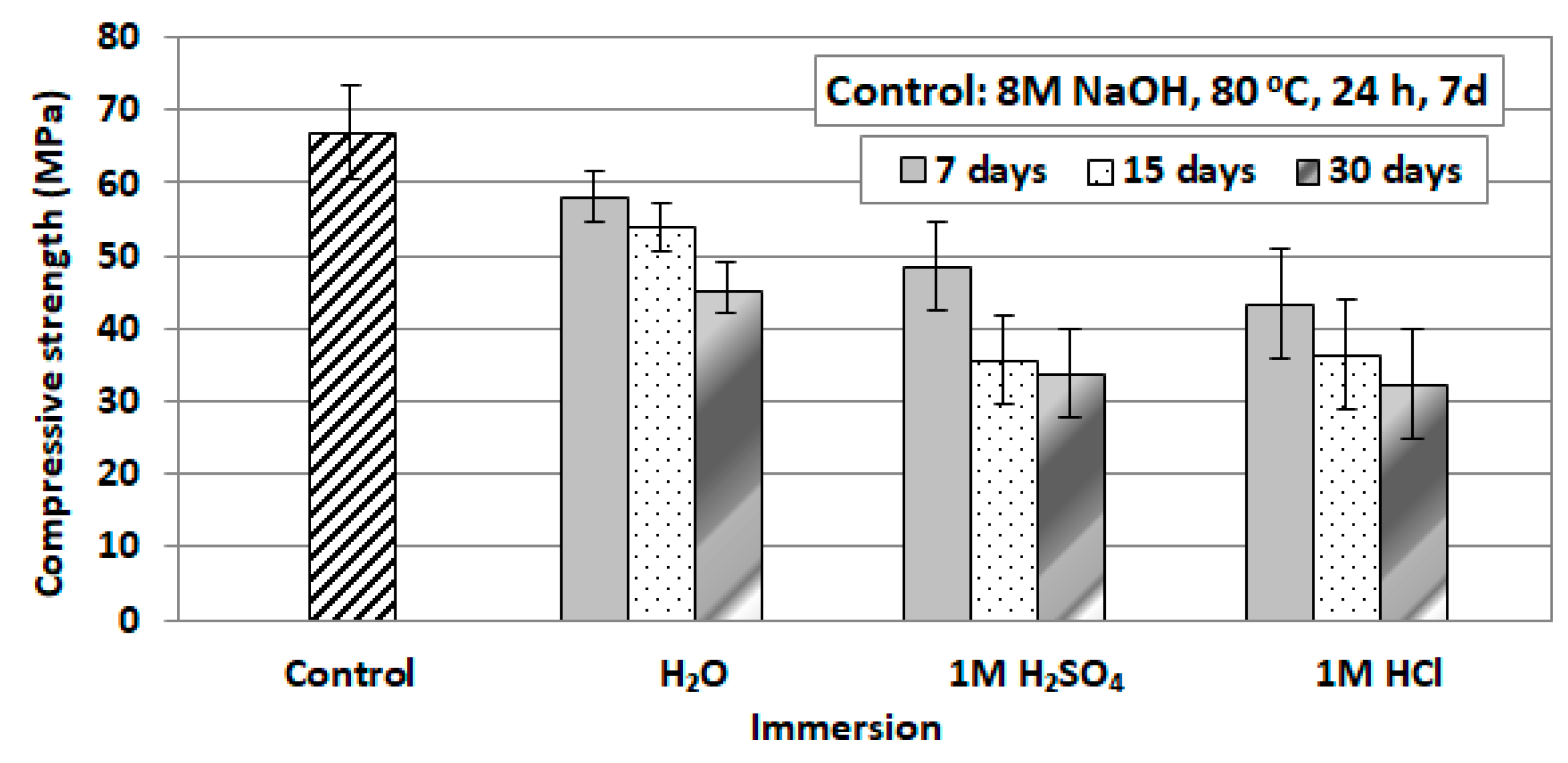
3.3.2. Effect of Immersion of IPs in Distilled Water or Acidic Solutions
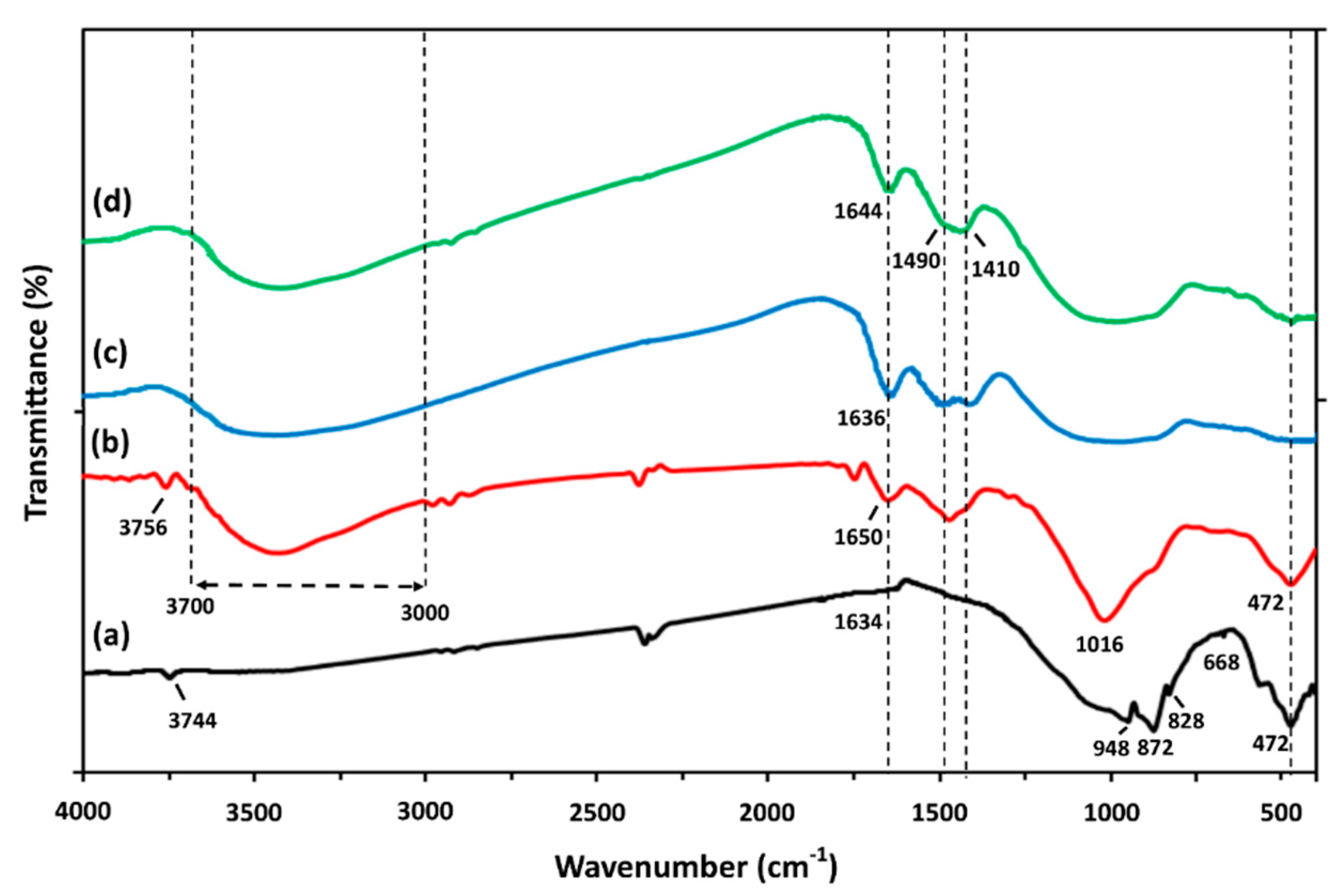
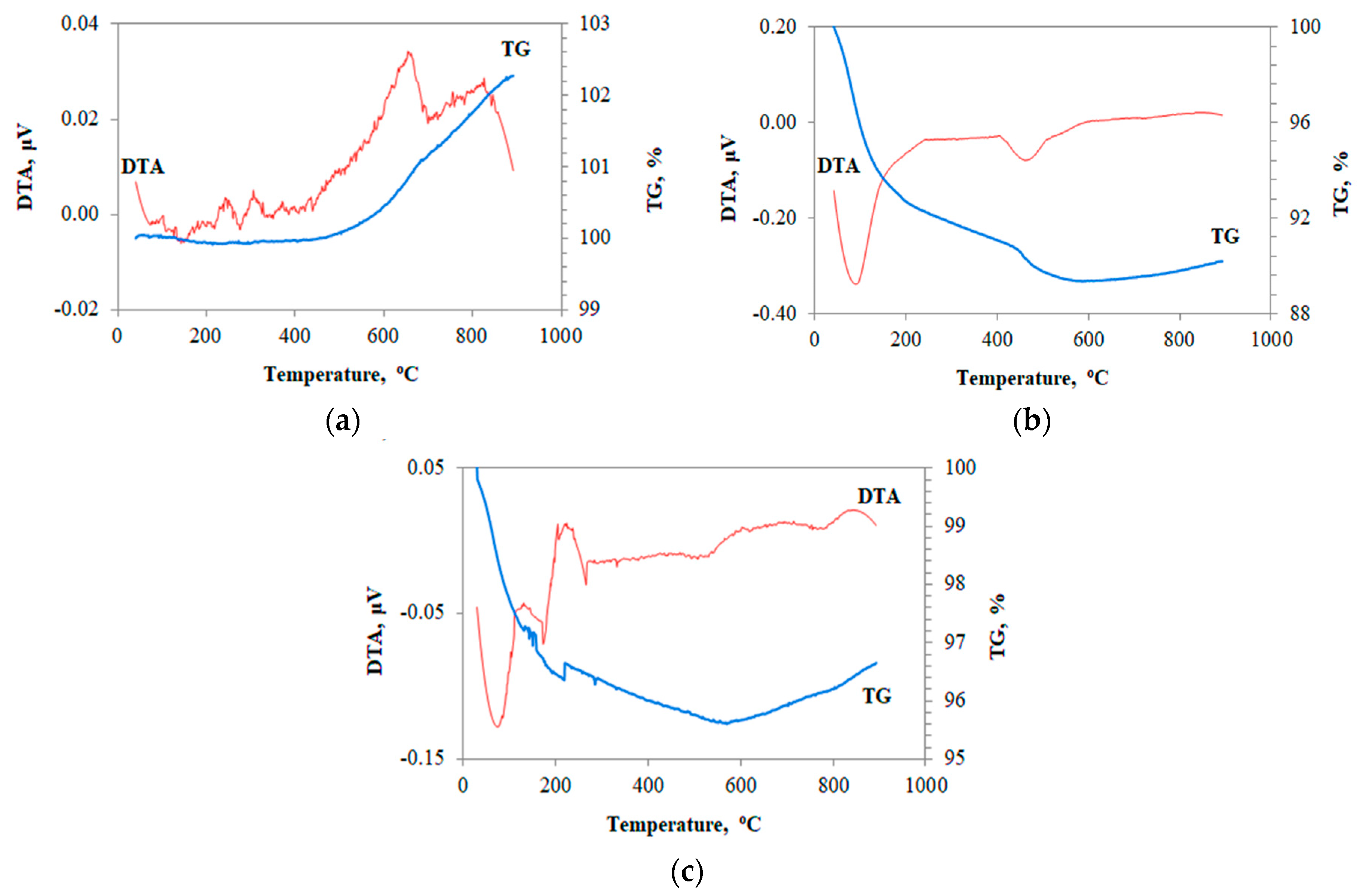
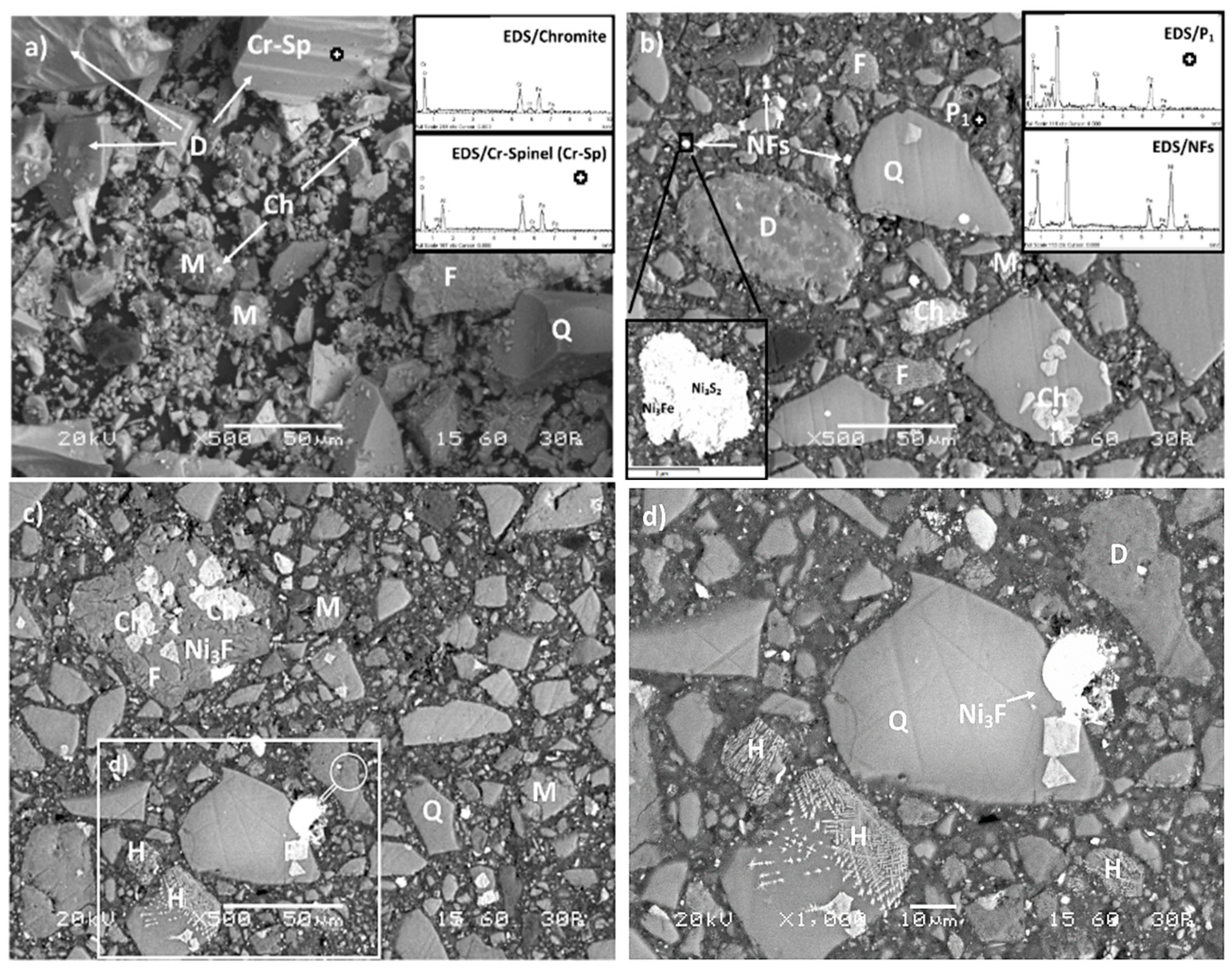
3.4. Morphology-Μicrostructure of Inorganic Polymers
3.5. IP Toxicity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bai, Τ.; Song, Z.G.; Wu, Y.G.; Hu, X.D.; Hua, B. Influence of steel slag on the mechanical properties and curing time of metakaolin Geopolymer. Ceram. Int. 2018, 44, 15706–15713. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Geopolymerisation of low calcium ferronickel slags. J. Mater. Sci. 2007, 42, 3073–3082. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M.; Jin, F.; Al-Tabbaa, A.; Wang, A. Accelerated carbonation and performance of concrete made with steel slag as binding materials and aggregates. Cem. Concr. Compos. 2017, 83, 138–145. [Google Scholar] [CrossRef]
- European Commission—IPPC Bureau. Reference Document on Best Available Techniques for Management of Tailings and Waste-Rock in Mining Activities; European Union: Brussels, Belgium, 2009; Available online: http://eippcb.jrc.ec.europa.eu/reference/BREF/mmr_adopted_0109.pdf (accessed on 14 October 2018).
- European Commission. Directive 2006/21/EC of the European Parliament and of the Council of 15 March 2006 on the Management of Waste from Extractive Industries and Amending Directive 2004/35/EC, Official Journal of the European Union, L 102/15-33, 11.4.2006. Available online: https://eur-lex.europa.eu/ resource.html?uri=cellar:c370006a-063e-4dc79b0552c37720740c.0005.02/DOC_1&format=PDF (accessed on 14 October 2018).
- Bartzas, G.; Komnitsas, K. Life cycle assessment of ferronickel production in Greece. Resour. Conserv. Recycl. 2015, 105, 113–122. [Google Scholar] [CrossRef]
- Gee, C.; Ramsey, M.H.; Maskallh, J.; Thornton, I. Mineralogy and weathering processes in historical smelting slags and their effect on the mobilisation of lead. J. Geochem. Explor. 1997, 58, 249–257. [Google Scholar] [CrossRef]
- Pasetto, M.; Baliello, A.; Giacomello, G.; Pasquini, E. Sustainable solutions for road pavements: A multi-scale characterization of warm mix asphalts containing steel slags. J. Clean. Prod. 2017, 166, 835–843. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Agrela, F. Effect of stainless steel slag waste as a replacement for cement in mortars. Mechanical and statistical study. Constr. Build. Mater. 2017, 142, 444–458. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers—Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. Chemistry of Geopolymeric Systems, Terminology. In Proceedings of the Second International Conference on Geopolymers, Saint-Quentin, France, 28–29 June 1999; pp. 9–40. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids. Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Kriven, W.M.; Bell, J.L.; Gordon, M. Microstructure and microchemistry of fully-reacted geopolymers and geopolymer matrix composites. Ceram. Trans. 2003, 153, 227–250. [Google Scholar]
- Krivenko, P.V.; Kovalchuk, G.Y. Directed synthesis of alkaline aluminosilicate minerals in a geocement matrix. J. Mater. Sci. 2007, 42, 2944–2952. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Habert, G.; Lacaillerie, J.B.E.; Russel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- de Toledo Pereira, D.S.; da Silva, F.J.; Porto, A.B.R.; Candido, V.S.; da Silva, A.C.R.; Filho, F.D.C.G.; Monteiro, S.N. Comparative analysis between properties and microstructures of geopolymeric concrete and portland concrete. J. Mater. Res. Technol. 2018, 7, 606–611. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. Geopolymerization of multiple minerals. Miner. Eng. 2002, 15, 1131–1139. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9781845694494. [Google Scholar]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L. Designing precursors for geopolymer cements. J. Am. Ceram. Soc. 2008, 91, 3864–3869. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.J.; Peethamparan, S. Temperature and activator effect on early-age reaction kinetics of alkali-activated slag binders. Constr. Build. Mater. 2016, 133, 783–793. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Wang, K.; Lemougna, P.N.; Tang, Q.; Li, W.; He, Y.; Cui, X. Low temperature depolymerization and polycondensation of a slag-based inorganic polymer. Ceram. Int. 2017, 43, 9067–9076. [Google Scholar] [CrossRef]
- Yan, B.; Yu, Q.L.; Brouwers, H.J.H. Evaluation of slag characteristics on the reaction kinetics and mechanical properties of Na2CO3 activated slag. Constr. Build. Mater. 2017, 131, 334–346. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Shi, M. Characteristics and reactivity of ferronickel slag powder. Constr. Build. Mater. 2017, 156, 773–789. [Google Scholar] [CrossRef]
- Maragkos, I.; Gianopoulou, I.P.; Panias, D. Synthesis of ferronickel slag-based geopolymers. Miner. Eng. 2009, 22, 196–203. [Google Scholar] [CrossRef]
- Zaharaki, D.; Galetakis, M.; Komnitsas, K. Valorization of construction and demolition (C&D) and industrial wastes through alkali activation. Constr. Build. Mater. 2016, 121, 686–693. [Google Scholar] [CrossRef]
- Furlani, E.; Maschio, S.; Magnan, M.; Aneggi, E.; Andreatta, F.; Lekka, M.; Lanzutti, A.; Fedrizzi, L. Synthesis and characterization of geopolymers containing blends of unprocessed steel slag and metakaolin: The role of slag particle size. Ceram. Int. 2018, 44, 5226–5232. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, Y.; Zheng, W.; Huang, W. Effect of dosage of sodium carbonate on the strength and drying shrinkage of sodium hydroxide based alkali-activated slag paste. Constr. Build. Mater. 2018, 179, 11–24. [Google Scholar] [CrossRef]
- Pontikes, Y.; Machiels, L.; Onisei, S.; Pandelaers, L.; Geysen, D.; Jones, P.T.; Blanpain, B. Slags with a high Al and Fe content as precursors for inorganic polymers. Appl. Clay Sci. 2013, 73, 93–102. [Google Scholar] [CrossRef]
- Onisei, S.; Pontikes, Y.; Van Gerven, T.; Angelopoulos, G.N.; Velea, T.; Predica, V.; Moldovan, P. Synthesis of inorganic polymers using fly ash and primary lead slag. J. Hazard. Mater. 2012, 205–206, 101–110. [Google Scholar] [CrossRef]
- Kierczak, J.; Neel, C.; Puziewicz, J.; Bril, H. The Mineralogy and weathering of slag produced by the smelting of lateritic Ni ores, Szklary, Southwestern Poland. Can. Miner. 2009, 47, 557–572. [Google Scholar] [CrossRef]
- British Standards Institute. BS EN 1936: Natural Stone Test Methods. Determination of Real Density and Apparent Density and of Total and Open Porosity; NP EN 1936:2006; BSI: London, UK, 2007. [Google Scholar]
- British Standards Institute. BS EN 12457-3: Characterisation of waste. Leaching. In Compliance Test for Leaching of Granular Waste Materials and Sludges. Two Stage Batch Test at a Liquid to Solid Ratio of 2 L/kg and 8 L/kg for Materials with a High Solid Content and with a Particle Size below 4 mm (without or with Size Reduction); British Standards Institue: London, UK, 2002. [Google Scholar]
- van der Sloot, H.A.; Hjelmar, O.; Bjerre Hansen, J.; Woitke, P.; Lepom, P.; Leschber, R.; Bartet, B.; Debrucker, N. Validation of CEN/TC 292 Leaching Tests and Eluate Analysis Methods prEN 12457 1-4, ENV 13370 and ENV 12506 in Co-Operation with CEN/TC 308; ECN: Petten, Netherlands, 2001; Available online: https://publicaties.ecn.nl/PdfFetch.aspx?nr=ECN-C--01-117 (accessed on 9 November 2018).
- European Commission (EC). Council Decision 2003/33/EC of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC; European Commission: Brussels, Belgium, 2003; Available online: https://eur-lex.europa.eu/legalcontent/EN/TXT/PDF/ ?uri=CELEX:32003D0033&from=EN (accessed on 9 November 2018).
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J. Hazard. Mater. 2009, 161, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.G.D.S.; Strecker, K. Brazilian fly ash based inorganic polymers production using different alkali activator solutions. Ceram. Int. 2017, 43, 9012–9018. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Kaze, C.R.; Djobo, J.N.Y.; Nana, A.; Tchakoute, H.K.; Kamseu, E.; Melo, U.C.; Leonelli, C.; Rahier, H. Effect of silicate modulus on the setting, mechanical strength and microstructure of iron-rich aluminosilicate (laterite) based-geopolymer cured at room temperature. Ceram. Int. 2018, 44, 21442–21450. [Google Scholar] [CrossRef]
- Peys, Α.; White, C.E.; Olds, D.; Rahier, H.; Blanpain, B.; Pontikes, Y. Molecular structure of CaO–FeOx–SiO2 glassy slags and resultant inorganic polymer binders. J. Am. Ceram. Soc. 2018, 101, 5846–5857. [Google Scholar] [CrossRef]
- Onisei, S.; Douvalis, A.P.; Malfliet, A.; Arne Peys, A.; Pontikes, Y. Inorganic polymers made of fayalite slag: On the microstructure and behavior of Fe. J. Am. Ceram. Soc. 2018, 101, 2245–2257. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Chinje Melo, U.F.; Delplancke, M.-P.; Rahier, H. Influence of the activating solution composition on the stability and thermo-mechanical properties of inorganic polymers (geopolymers) from volcanic ash. Constr. Build. Mater. 2013, 48, 278–286. [Google Scholar] [CrossRef]
- Onisei, S.; Malfliet, A.; Gutierrez-Campos, D.; Blanplain, B. Synthesis and high temperature transformations of Fe-rich inorganic polymers. In Proceedings of the 4th International Slag Valorization Symposium, Leuven, Belgium, 15–17 April 2015; Malfliet, A., Pontikes, Y., Eds.; pp. 243–248. Available online: http://www.slag-valorisation-symposium.eu/2015/images/papers/SVS2015-Onisei.pdf (accessed on 21 November 2018).
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of analytical techniques for identification of inorganic polymer gel composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K. Long term behaviour of ferronickel slag inorganic polymers in various environments. Fresenius Environ. Bull. 2012, 21, 2436–2440. [Google Scholar]
- Marangoni, M.; Arnout, L.; Machiels, L.; Pandelaers, L.; Bernardo, E.; Colombo, P.; Pontikes, Y.; Jantzen, C. Porous, sintered glass-ceramics from inorganic polymers based on fayalite slag. J. Am. Ceram. Soc. 2016, 99, 1985–1991. [Google Scholar] [CrossRef]
- Rincón, A.; Desideri, D.; Bernardo, E. Functional glass-ceramic foams from ‘inorganic gel casting’ and sintering of glass/slag mixtures. J. Clean. Prod. 2018, 187, 250–256. [Google Scholar] [CrossRef]
- Nazer, A.; Payá, J.; Borrachero, M.V.; Monzó, J. Use of ancient copper slags in Portland cement and alkali activated cement matrices. J. Environ. Manag. 2016, 167, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, Y.; Zhang, X.; Mukiza, E.; Xu, X.; Li, F. Effect of Fly Ash on the Properties of Ceramics Prepared from Steel Slag. Appl. Sci. 2018, 8, 1187. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, Q.; Lu, S.; He, Y.; Liao, G.; Ke, Y. Effect of CaO/SiO2 ratio on the preparation and crystallization of glass-ceramics from copper slag. Ceram. Int. 2014, 40, 7297–7305. [Google Scholar] [CrossRef]
- Komnitsas, K.; Petrakis, E.; Bartzas, G.; Karmali, V. Column leaching of low-grade saprolitic laterites and valorization of leaching residues. Sci. Total Environ. 2019, 665, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Mužek, M.N.; Zelić, J.; Jozić, D. Microstructural characteristics of geopolymers based on alkali-activated fly ash. Chem. Biochem. Eng. Q. 2012, 26, 89–95. Available online: http://pierre.fkit.hr/hdki/cabeq/ pdf/26_2_2012/Cabeq%202012-02_3.pdf (accessed on 24 November 2018).
- Liu, Y.; Zhang, K.; Feng, E.; Zhao, H.; Liu, F. Synthesis of geopolymer composites from a mixture of ferronickel slag and fly ash. IOP Conf. Ser. Mater. Sci. Eng. 2017, 182, 012038. [Google Scholar] [CrossRef] [Green Version]
- Bernal, S.A.; Rodríguez, E.D.; de Gutiérrez, R.M.; Provis, J.L. Performance at high temperature of alkali-activated slag pastes produced with silica fume and rice husk ash based activators. Mater. Constr. 2015, 65, e049. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Bakri, A.M.M.A.; Kamarudin, H.; Bnhussain, M.; Ismail, K.N.; Yahya, Z.; Razak, R.A. Fly Ash-based Geopolymer Lightweight Concrete Using Foaming Agent. Int. J. Mol. Sci. 2012, 13, 7186–7198. [Google Scholar] [CrossRef] [Green Version]
- Gyurov, S.; Marinkov, N.; Kostova, Y.; Rabadjieva, D.; Kovacheva, D.; Tzvetkova, C.; Gentscheva, G.; Ivan Penkov, I. Technological scheme for copper slag processing. Int. J. Miner. Process. 2017, 158, 1–7. [Google Scholar] [CrossRef]
- Mihailova, I.; Radev, L.; Mehandjie, D. Phase composition, structure and catalytic activity in oxidation reactions of fayalite waste from the flotation of copper slag. J. Chem. Technol. Metall. 2017, 52, 929–939. Available online: https://dl.uctm.edu/journal/node/j2017-5/17_17-69_Irena_p_929-939.pdf (accessed on 14 December 2018).
- Zuo, Z.; Yu, Q.; Wei, M.; Xie, H.; Duan, W.; Wang, K.; Qin, Q. Thermogravimetric study of the reduction of copper slag by biomass. Therm. Anal. Calorim. 2016, 126, 481. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]




| Fe2O3 | SiO2 | Al2O3 | Cr2O3 | MgO | NiO | K2O | Na2O | TiO2 | CoO | MnO | CaO | P2O5 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40.62 | 30.18 | 7.60 | 1.98 | 1.80 | 0.95 | 0.89 | 0.44 | 0.69 | 0.03 | 0.28 | 13.0 | 0.02 | 98.48 |
| NaOH Molarity (M) | Compressive Strength (MPa) * | (SiO2 + Al2O3)/Na2O | H2O/Na2O | Na/Al | Fe2O3/Na2O |
|---|---|---|---|---|---|
| 6 | 63.3 | 11.9 | 16.3 | 0.7 | 5.1 |
| 8 | 67.0 | 8.6 | 12.9 | 0.9 | 3.7 |
| 10 | 41.7 | 6.8 | 10.3 | 1.2 | 2.9 |
| 12 | 30.2 | 6.4 | 8.5 | 1.3 | 2.7 |
| Temperature (°C) | Compressive Strength (MPa) | Shrinkage (%) | Mass Loss (%) | Apparent Density (g cm−3) |
|---|---|---|---|---|
| 200 | 90 | 4.0 | 5.1 | 2.2 |
| 400 | 115 | 4.8 | 7.3 | 2.7 |
| 600 | 25 | 3.6 | 9.6 | 2.0 |
| 800 | 11 | 2.2 | 10.2 | 1.8 |
| 1000 | 2 | −7.1 | NM 1 | NM |
| Element | Polish Slag (mg kg−1) | Inorganic Polymer (mg kg−1) | Limit Values (mg kg−1) * | ||||
|---|---|---|---|---|---|---|---|
| Control IP | IP After Firing at 400 °C | For Wastes Accepted at Landfills for Inert Wastes | For Non- Hazardous Wastes | For Hazardous Wastes Accepted at Landfills for Non-Hazardous Wastes | For Wastes Accepted at Landfills for Hazardous Wastes | ||
| Fe | 32.1 | 29.1 | 164.3 | ||||
| Mn | 0.4 | 0.2 | 1.0 | ||||
| Al | 9.0 | 88.4 | 97.6 | ||||
| Ni | 6.3 | 0.3 | 2.2 | 0.4 | 10 | 10 | 40 |
| Cu | 0.1 | 0.9 | 0.7 | 2 | 50 | 50 | 100 |
| Zn | 3.6 | 0.8 | 2.9 | 4 | 50 | 50 | 200 |
| As | 0.1 | 0.4 | 1.2 | 0.5 | 2 | 2 | 25 |
| Mo | <DL | 0.2 | 0.5 | 0.5 | 10 | 10 | 30 |
| Cd | <DL | <DL | <DL | 0.04 | 1 | 1 | 5 |
| Crtotal | 0.6 | 0.3 | 2.4 | 0.5 | 10 | 10 | 50 |
| Pb | <DL | <DL | <DL | 0.5 | 10 | 10 | 50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komnitsas, K.; Bartzas, G.; Karmali, V.; Petrakis, E.; Kurylak, W.; Pietek, G.; Kanasiewicz, J. Assessment of Alkali Activation Potential of a Polish Ferronickel Slag. Sustainability 2019, 11, 1863. https://doi.org/10.3390/su11071863
Komnitsas K, Bartzas G, Karmali V, Petrakis E, Kurylak W, Pietek G, Kanasiewicz J. Assessment of Alkali Activation Potential of a Polish Ferronickel Slag. Sustainability. 2019; 11(7):1863. https://doi.org/10.3390/su11071863
Chicago/Turabian StyleKomnitsas, Konstantinos, Georgios Bartzas, Vasiliki Karmali, Evangelos Petrakis, Witold Kurylak, Grzegorz Pietek, and Jarosław Kanasiewicz. 2019. "Assessment of Alkali Activation Potential of a Polish Ferronickel Slag" Sustainability 11, no. 7: 1863. https://doi.org/10.3390/su11071863





