Biodiesel and Crude Glycerol from Waste Frying Oil: Production, Characterization and Evaluation of Biodiesel Oxidative Stability with Diesel Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Measurement of WFO, Optimized Biodiesel and Crude Glycerol Properties
2.3. Biodiesel Preparation
2.4. Box–Behnken Design (BBD):
3. Results and Discussion
3.1. Effects of Transesterification Reaction Parameters and Regression Model Validation
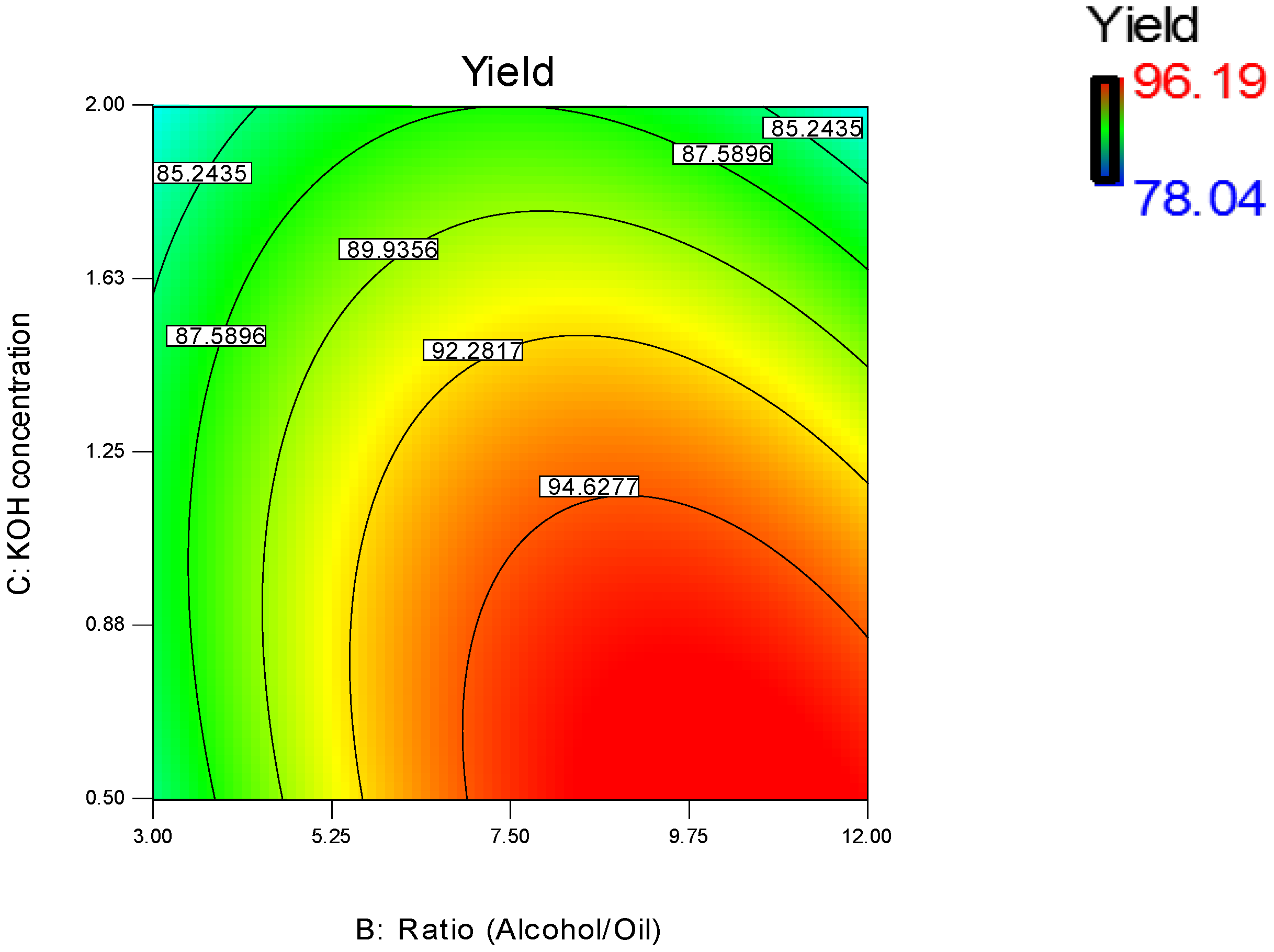
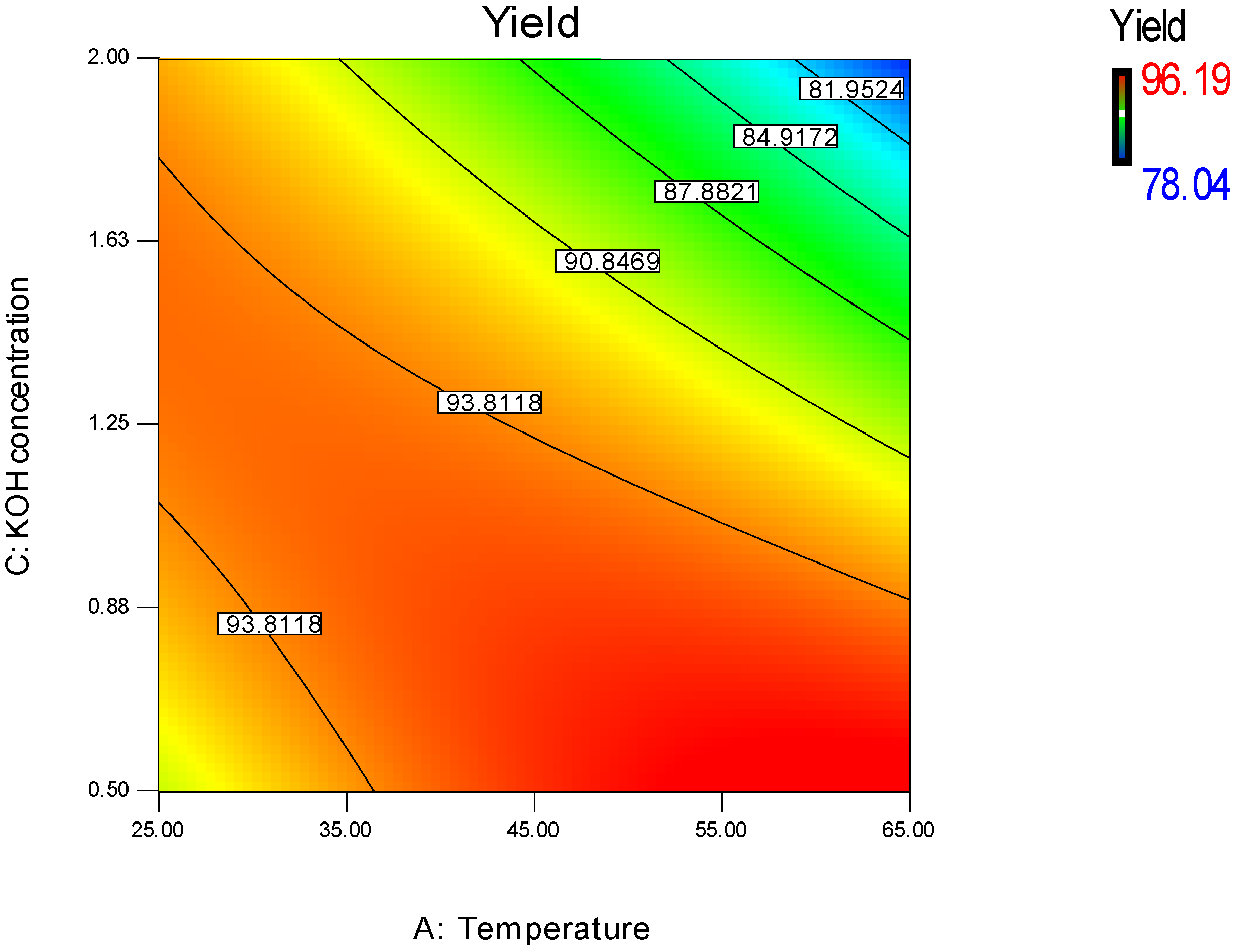
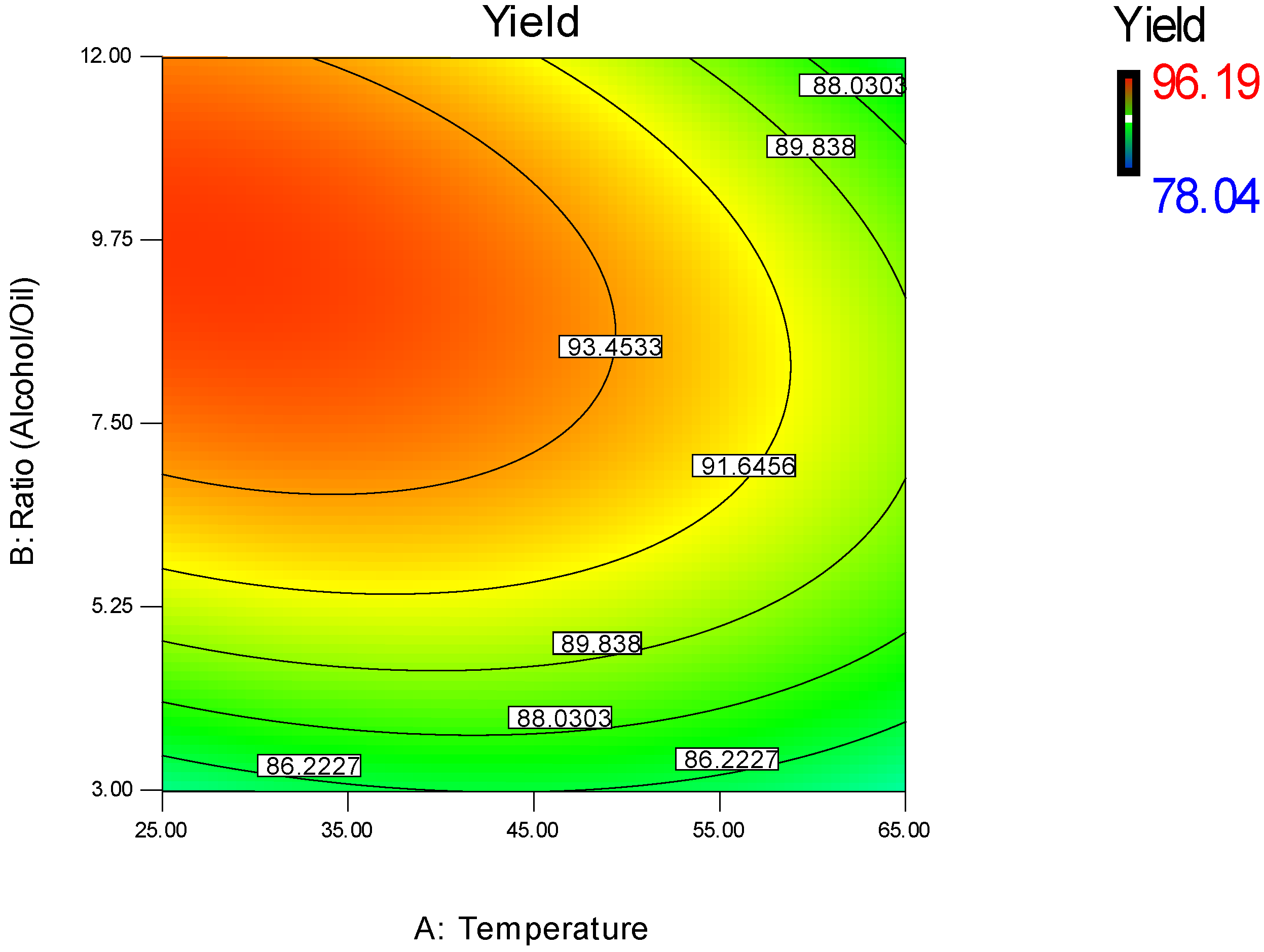
3.1.1. Effect of Methanol/WFO Molar Ratio
3.1.2. Effect of Catalyst Concentration
3.1.3. Effect of Transesterification Temperature
3.1.4. Regression Model Validation
3.2. Parameters Optimization of the Transesterification Process
3.3. Properties of the Optimized Biodiesel
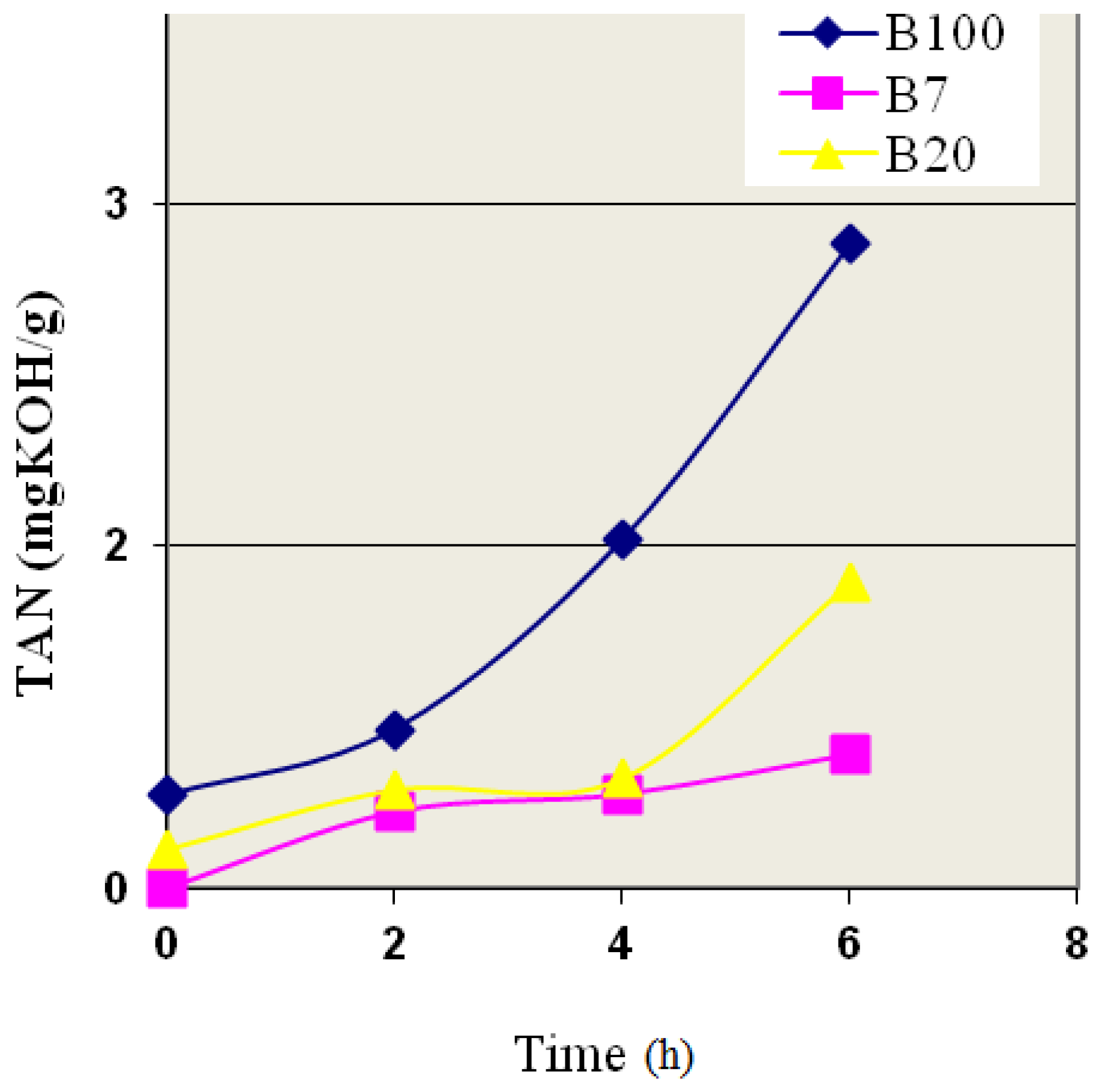
3.4. The Accelerated Oxidation of the Biodiesel and Its Blends
4. Properties of Crude Glycerol as a Byproduct of Optimized Biodiesel
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Recent Trends in Transport Biofuels. Available online: https://www.iea.org/tcep/transport/biofuels/ (accessed on 19 March 2019).
- Da Silva Filho, S.C.; Miranda, A.C.; Silva, T.A.F.; Calarge, F.A.; de Souza, R.R.; Santana, J.C.C.; Tambourgi, E.B. Environmental and techno-economic considerations on biodiesel production from waste frying oil in São Paulo city. J. Clean. Prod. 2018, 183, 1034–1042. [Google Scholar] [CrossRef]
- Ghiaci, M.; Aghabarari, B.; Gil, A. Production of biodiesel by esterification of natural fatty acids over modified organoclay catalysts. Fuel 2011, 90, 3382–3389. [Google Scholar] [CrossRef]
- Hayyan, M.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. A novel technique for separating glycerine from palm oil-based biodiesel using ionic liquids. Fuel Process. Technol. 2010, 91, 116–120. [Google Scholar] [CrossRef]
- Ren, Q.; Zuo, T.; Pan, J.; Chen, C.; Li, W. Preparation of biodiesel from soybean catalyzed by basic ionic liquids [Hnmm] OH. Materials 2014, 7, 8012–8023. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.-W.; Hanna, M.A.; Zhang, Y.-P.; Bhadury, P.S.; Wang, Y.; Song, B.-A.; Yang, S. Biodiesel preparation, optimization, and fuel properties from non-edible feedstock, Datura stramonium L. Fuel 2012, 91, 182–186. [Google Scholar] [CrossRef]
- Yücel, Y. Optimization of biocatalytic biodiesel production from pomace oil using response surface methodology. Fuel Process. Technol. 2012, 99, 97–102. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Reducing volatile organic compound emissions from diesel engines using canola oil biodiesel fuel and blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Asri, N.P.; Soe’eib, S.; Poedjojono, B. Alumina supported zinc oxide catalyst for production of biodiesel from kesambi oil and optimization to achieve highest yields of biodiesel. Euro-Mediterr. J. Environ. Integr. 2018, 3, 3. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Yamin, J. Parametric study of the alkali catalyzed transesterification of waste frying oil for biodiesel production. Energy Convers. Manag. 2014, 79, 246–254. [Google Scholar] [CrossRef]
- Márcio de Almeida, D.; da Silva, M.A.V.; Franca, L.S.; de Oliveira, C.M.; Alexandre, M.O.L.; da Costa Marques, L.G.; Murta, A.L.S.; de Freitas, M.A.V. Comparative study of emissions from stationary engines using biodiesel made from soybean oil, palm oil and waste frying oil. Renew. Sustain. Energy Rev. 2017, 70, 1376–1392. [Google Scholar]
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, M.A.; McLean, D.D.L.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Rao, B.; Mouli, K.C.; Rambabu, N.; Dalai, A.K.; Prasad, R.B.N. Carbon-based solid acid catalyst from de-oiled canola meal for biodiesel production. Catal. Commun. 2011, 14, 20–26. [Google Scholar] [CrossRef]
- Chang, F.; Hanna, M.A.; Zhang, D.-J.; Li, H.; Zhou, Q.; Song, B.-A.; Yang, S. Production of biodiesel from non-edible herbaceous vegetable oil: Xanthium sibiricum Patr. Bioresour. Technol. 2013, 140, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Dalai, A.K. Waste cooking oil an economical source for biodiesel: A review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Nair, P.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Synthesis of biodiesel from low FFA waste frying oil using calcium oxide derived from Mereterix mereterix as a heterogeneous catalyst. J. Clean. Prod. 2012, 29, 82–90. [Google Scholar] [CrossRef]
- Mondala, A.; Liang, K.; Toghiani, H.; Hernandez, R.; French, T. Biodiesel production by in situ transesterification of municipal primary and secondary sludges. Bioresour. Technol. 2009, 100, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Olkiewicz, M.; Fortuny, A.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C. Evaluation of Different Sludges from WWTP as a Potential Source for Biodiesel Production. Procedia Eng. 2012, 42, 634–643. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Bouaziz, M.; Harebi, M.; Mahfoudhi, M.; Jebeur, H.; Bouguerra Neji, S. Biodiesel Production from Raw Tunisian Cas-tor Oil and Its Application as Alternative Fuel. Arch. Pet. Env. Biotechnol. APEB-118 DOI. 2017, 10, 2574–7614. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Atapour, M.; Kariminia, H.-R.; Moslehabadi, P.M. Optimization of biodiesel production by alkali-catalyzed transesterification of used frying oil. Process Saf. Environ. Prot. 2014, 92, 179–185. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Olutoye, M.A.; Hameed, B.H. Synthesis of methyl esters from waste cooking oil using construction waste material as solid base catalyst. Bioresour. Technol. 2013, 128, 788–791. [Google Scholar] [CrossRef]
- Kafuku, G.; Mbarawa, M. Biodiesel production from Croton megalocarpus oil and its process optimization. Fuel 2010, 89, 2556–2560. [Google Scholar] [CrossRef]
- Noshadi, I.; Amin, N.A.S.; Parnas, R.S. Continuous production of biodiesel from waste cooking oil in a reactive distillation column catalyzed by solid heteropolyacid: Optimization using response surface methodology (RSM). Fuel 2012, 94, 156–164. [Google Scholar] [CrossRef]
- Omar, W.N.N.W.; Amin, N.A.S. Optimization of heterogeneous biodiesel production from waste cooking palm oil via response surface methodology. Biomass Bioenergy 2011, 35, 1329–1338. [Google Scholar] [CrossRef]
- Andrade, J.E.; Perez, A.; Sebastian, P.J.; Eapen, D. Retracted: A review of bio-diesel production processes. Biomass Bioenergy 2011, 35, 1008–1020. [Google Scholar] [CrossRef]
- Koh, M.Y.; Ghazi, T.I.M. A review of biodiesel production from Jatropha curcas L. oil. Renew. Sustain. Energy Rev. 2011, 15, 2240–2251. [Google Scholar] [CrossRef]
- Hindarso, H. Production of Fatty Acid Methyl Ester from Microalgae Using Microwave: Kinetic of Transesterification Reaction Using CaO Catalyst. Am. J. Chem. Eng. 2018, 6, 54. [Google Scholar] [CrossRef]
- Johnson, D.T.; Taconi, K.A. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007, 26, 338–348. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, N. Scope and opportunities of using glycerol as an energy source. Renew. Sustain. Energy Rev. 2012, 16, 4551–4556. [Google Scholar] [CrossRef]
- Bala-Litwiniak, A.; Radomiak, H. Possibility of the Utilization of Waste Glycerol as an Addition to Wood Pellets. Waste Biomass Valoriz. 2018, 1–7. [Google Scholar] [CrossRef]
- Leoneti, A.B.; Aragão-Leoneti, V.; De Oliveira, S.V.W.B. Glycerol as a by-product of biodiesel production in Brazil: Alternatives for the use of unrefined glycerol. Renew. Energy 2012, 45, 138–145. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Haron, R.; Mat, R.; Abdullah, T.A.T.; Rahman, R.A. Overview on utilization of biodiesel by-product for biohydrogen production. J. Clean. Prod. 2018, 172, 314–324. [Google Scholar] [CrossRef]
- Boey, P.-L.; Ganesan, S.; Maniam, G.P.; Khairuddean, M.; Efendi, J. A new heterogeneous acid catalyst for esterification: Optimization using response surface methodology. Energy Convers. Manag. 2013, 65, 392–396. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, M.P. Optimization of transesterification of chlorella protothecoides oil to biodiesel using box-behnken design method. Waste Biomass Valoriz. 2016, 7, 1105–1114. [Google Scholar] [CrossRef]
- Bouchaala, F.C.; Lazzez, A.; Jabeur, H.; Daoud, L.; Bouaziz, M. Physicochemical characteristics of extra virgin olive oil in function of tree age and harvesting period using chemometric analysis. Sci. Hortic. 2014, 180, 52–58. [Google Scholar] [CrossRef]
- EN ISO 3675:1998 Crude Petroleum and Liquid Petroleum Products—Laboratory Determination of Density—Hydrometer Method 1998; International Organization for Standardization: Geneva, Switzerland, 1998.
- ENISO 3104:1996 Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity 1996; SAI GLOBAL: Chicago, IL, USA, 1996.
- EN 14104:2003 Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Acid Value 2003; BSI: London, UK, 2003.
- EN14111:2003 Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Iodine Value 2003; BSI: London, UK, 2003.
- EN ISO 12937:2000 Petroleum Products-Determination of Water—Coulometric Karl Fischer Titration Method 2000; International Organization for Standardization: Geneva, Switzerland, 2000.
- EN ISO 2719:2002 Determination of Flash Point —Pensky-Martens Closed Cup Method 2002; International Organization for Standardization: Geneva, Switzerland, 2002.
- EN ISO 13032:2012 Petroleum Products-Determination of Low Concentration of Sulfur in Automotive Fuels—Energy-Dispersive X-ray Fluorescence Spectrometric Method 2012; International Organization for Standardization: Geneva, Switzerland, 2012.
- Roveda, A.C.; Comin, M.; Caires, A.R.L.; Ferreira, V.S.; Trindade, M.A.G. Thermal stability enhancement of biodiesel induced by a synergistic effect between conventional antioxidants and an alternative additive. Energy 2016, 109, 260–265. [Google Scholar] [CrossRef]
- Fillières, R.; Benjelloun-Mlayah, B.; Delmas, M. Ethanolysis of rapeseed oil: Quantitation of ethyl esters, mono-, di-, and triglycerides and glycerol by high-performance size-exclusion chromatography. J. Am. Oil Chem. Soc. 1995, 72, 427–432. [Google Scholar] [CrossRef]
- Encinar, J.M.; Gonzalez, J.F.; Rodríguez-Reinares, A. Biodiesel from used frying oil. Variables affecting the yields and characteristics of the biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Sianipar, R.N.R.; Ariyani, D.; Nata, I.F. Conversion of palm oil sludge to biodiesel using alum and KOH as catalysts. Sustain. Environ. Res. 2017, 27, 291–295. [Google Scholar]
- Ouanji, F.; Nachid, M.; Kacimi, M.; Liotta, L.F.; Puleo, F.; Ziyad, M. Small scale biodiesel synthesis from waste frying oil and crude methanol in Morocco. Chin. J. Chem. Eng. 2016, 24, 1178–1185. [Google Scholar] [CrossRef]
- Yatish, K.V.; Lalithamba, H.S.; Suresh, R.; Arun, S.B.; Kumar, P.V. Optimization of scum oil biodiesel production by using response surface methodology. Process Saf. Environ. Prot. 2016, 102, 667–672. [Google Scholar] [CrossRef]
- Hamze, H.; Akia, M.; Yazdani, F. Optimization of biodiesel production from the waste cooking oil using response surface methodology. Process Saf. Environ. Prot. 2015, 94, 1–10. [Google Scholar] [CrossRef]
- Rahimi, M.; Aghel, B.; Alitabar, M.; Sepahvand, A.; Ghasempour, H.R. Optimization of biodiesel production from soybean oil in a microreactor. Energy Convers. Manag. 2014, 79, 599–605. [Google Scholar] [CrossRef]
- El-Gendy, N.S.; Deriase, S.F.; Hamdy, A.; Abdallah, R.I. Statistical optimization of biodiesel production from sunflower waste cooking oil using basic heterogeneous biocatalyst prepared from eggshells. Egypt. J. Pet. 2015, 24, 37–48. [Google Scholar] [CrossRef]
- Onukwuli, D.O.; Emembolu, L.N.; Ude, C.N.; Aliozo, S.O.; Menkiti, M.C. Optimization of biodiesel production from refined cotton seed oil and its characterization. Egypt. J. Pet. 2017, 26, 103–110. [Google Scholar] [CrossRef]
- Shu, G.; Dong, L.; Liang, X. A review of experimental studies on deposits in the combustion chambers of internal combustion engines. Int. J. Engine Res. 2012, 13, 357–369. [Google Scholar] [CrossRef]
- Chuah, L.F.; Yusup, S.; Aziz, A.R.A.; Klemeš, J.J.; Bokhari, A.; Abdullah, M.Z. Influence of fatty acids content in non-edible oil for biodiesel properties. Clean Technol. Environ. Policy 2016, 18, 473–482. [Google Scholar] [CrossRef]
- Bajpai, D.; Tyagi, V.K. Biodiesel: Source, production, composition, properties and its benefits. J. Oleo Sci. 2006, 55, 487–502. [Google Scholar] [CrossRef]
- Dantas, M.B.; Albuquerque, A.R.; Barros, A.K.; Rodrigues Filho, M.G.; Antoniosi Filho, N.R.; Sinfrônio, F.S.M.; Rosenhaim, R.; Soledade, L.E.B.; Santos, I.M.G.; Souza, A.G. Evaluation of the oxidative stability of corn biodiesel. Fuel 2011, 90, 773–778. [Google Scholar] [CrossRef]
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J. Chem. Eng. 2010, 27, 944–949. [Google Scholar] [CrossRef]
- Quispe, C.A.; Coronado, C.J.; Carvalho J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]



| Property | Units | Value | |
|---|---|---|---|
| Acid value | mg KOH/g | 0.63 | |
| Peroxide value | meqO2/kg | 6.75 | |
| Iodine value | mg I2/100 g | 109.98 | |
| Density at 15 °C | kg/cm3 | 926 | |
| Kinematic viscosity at 40 °C | mm2/s | 37.31 | |
| Fatty acid composition, wt. % | C12:0 | 0.00 | |
| C14:0 | 0.01 | ||
| C16:0 | 11.54 | ||
| C16:1 | 0.13 | ||
| C17:0 | 0.11 | ||
| C17:1 | 0.06 | ||
| C18:0 | 4.49 | ||
| C18:1 | 23.52 | ||
| C18:2 | 52.81 | ||
| C18:3 | 6.76 | ||
| C20:0 | 0.34 | ||
| C20:1 | 0.23 | ||
| Factors | Units | Code | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Temperature | °C | A | 25 | 45 | 65 |
| Methanol/WFO ratio | mol/mol | B | 3:1 | 7.5:1 | 12:1 |
| Catalyst concentration | % | C | 0.5 | 1.25 | 2 |
| Source | Sum of Squares | df | Mean of Square | F value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 456.80 | 9 | 50.76 | 22.03 | 0.0017 significant |
| A: Temperature | 33.21 | 1 | 33.21 | 14.42 | 0.0127 |
| B: Ratio (Alcohol/Oil) | 60.61 | 1 | 60.61 | 26.31 | 0.0037 |
| C: KOH concentration | 120.13 | 1 | 120.13 | 52.15 | 0.0008 |
| AB | 13.00 | 1 | 13.00 | 5.64 | 0.0635 |
| AC | 100.70 | 1 | 100.70 | 43.72 | 0.0012 |
| BC | 30.42 | 1 | 30.42 | 13.20 | 0.0150 |
| A2 | 9.11 | 1 | 9.11 | 3.95 | 0.1035 |
| B2 | 81.93 | 1 | 81.93 | 35.56 | 0.0019 |
| C2 | 17.80 | 1 | 17.80 | 7.73 | 0.0389 |
| Residual | 11.52 | 5 | 2.30 | ||
| Lack of Fit | 9.15 | 3 | 3.05 | 2.57 | 0.2922 not significant |
| Pure Error | 2.37 | 2 | 1.19 | ||
| Cor Total | 468.31 | 14 | |||
| R2 = 0.972 | |||||
| Run No. | Temperature (A, °C) | Methanol/WFO Ratio Molar (B, mol/mol) | Catalyst Concentration (C, wt. %) | Observed Yield (Y1, %) | Predicted Yield (Y1, %) |
|---|---|---|---|---|---|
| 1 | 25 | 7.5 | 2 | 94.27 | 93.10 |
| 2 | 45 | 3 | 2 | 82.88 | 82.91 |
| 3 | 45 | 7.50 | 1.25 | 94.94 | 93.68 |
| 4 | 65 | 7.5 | 2 | 78.04 | 78.99 |
| 5 | 25 | 3 | 1.25 | 83.74 | 84.88 |
| 6 | 65 | 12 | 1.25 | 87.46 | 86.31 |
| 7 | 25 | 12 | 1.25 | 93.02 | 93.99 |
| 8 | 45 | 7.5 | 1.25 | 93.08 | 93.68 |
| 9 | 65 | 3 | 1.25 | 85.39 | 84.41 |
| 10 | 45 | 12 | 0.5 | 96.19 | 96.16 |
| 11 | 45 | 12 | 2 | 82.70 | 82.90 |
| 12 | 45 | 3 | 0.5 | 85.34 | 85.14 |
| 13 | 65 | 7.5 | 0.5 | 95.6 | 96.77 |
| 14 | 25 | 7.5 | 0.5 | 91.76 | 90.81 |
| 15 | 45 | 7.5 | 1.25 | 93.03 | 93.68 |
| Optimum Conditions | Yield % | References |
|---|---|---|
| KOH 0.5 wt. %, 7.3:1, 58.30 °C, 600 rpm, 60 min | 96.33 | This work |
| KOH 1.5 wt. %, 20:1, 60 °C, 300 rpm, 60 min | 93.00 | [51] |
| KOH 1.2 wt. %, 6:1, 60 °C | 80.80 | [52] |
| KOH 1.2 wt. %, 4.5:1, 62 °C, 600 rpm, 75 min | 93.00 | [53] |
| KOH 1.4 wt. %, 7.5:1, 65 °C, 500 rpm, 60 min | 99.39 | [54] |
| KOH 1.2 wt. %, 9:1, 60°C, 0.43 min | 89.00 | [55] |
| NaOH 0.72 wt. %, 9:1, 65 °C, 45 min | 92.05 | [24] |
| Property | Units | Biodiesel | Diesel | [56] | [57] | EN14214 |
|---|---|---|---|---|---|---|
| Methyl ester | % (w/w) | 100 | - | - | - | Min 96 |
| Density at 15 °C | kg/m3 | 889.3 | 834.5 | 884.0 | 881.7 | 860–900 |
| Kinematic viscosity at 40 °C | mm2/s | 4.57 | 2.70 | 4.63 | 6.81 | 3.5–5.00 |
| Flash point | °C | 156 | 58 | 161 | 173 | Min 120 |
| Sulfur content | % (w/w) | 0.0058 | 0.1571 | - | - | 0.05 |
| Water content | ppm | 100 | - | 468 | 530 | Max 500 |
| Iodine value | mg I2/100 g | 105.96 | - | 108 | 125.28 | Min 120 |
| TAN | mg KOH/g | 0.41 | - | 0.6 | 0.22 | Max 0.5 |
| Peroxide value | meqO2/kg | 78.16 | - | - | 26.01 | - |
| Crude Glycerol | Results | Kongjao et al. [62] | Quality Specifications [63] |
|---|---|---|---|
| Color | Dark Brown | Dark Brown | |
| Glycerol yield (wt. %) | 30.40 | 28.56 | 40%–88% |
| pH | 9.5 | 10–11 | 4–9 |
| Density at 15 °C (g/cm3) | 1.01 | 1.01 | NA |
| Viscosity at 40 °C (mm2/s) | 26.35 | 42.41 | NA |
| Water content (wt. %) | 7.2 | 6.7 | 12% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harabi, M.; Neji Bouguerra, S.; Marrakchi, F.; P. Chrysikou, L.; Bezergianni, S.; Bouaziz, M. Biodiesel and Crude Glycerol from Waste Frying Oil: Production, Characterization and Evaluation of Biodiesel Oxidative Stability with Diesel Blends. Sustainability 2019, 11, 1937. https://doi.org/10.3390/su11071937
Harabi M, Neji Bouguerra S, Marrakchi F, P. Chrysikou L, Bezergianni S, Bouaziz M. Biodiesel and Crude Glycerol from Waste Frying Oil: Production, Characterization and Evaluation of Biodiesel Oxidative Stability with Diesel Blends. Sustainability. 2019; 11(7):1937. https://doi.org/10.3390/su11071937
Chicago/Turabian StyleHarabi, Mariem, Soumaya Neji Bouguerra, Fatma Marrakchi, Loukia P. Chrysikou, Stella Bezergianni, and Mohamed Bouaziz. 2019. "Biodiesel and Crude Glycerol from Waste Frying Oil: Production, Characterization and Evaluation of Biodiesel Oxidative Stability with Diesel Blends" Sustainability 11, no. 7: 1937. https://doi.org/10.3390/su11071937





