Edge Effects Reflect the Impact of the Agricultural Matrix on the Corticolous Lichens Found in Fragments of Cerrado Savanna in Central Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collection of the Phytosociological Data
2.3. Collection of the Data on the Lichen Community
2.4. Statistical Analyses
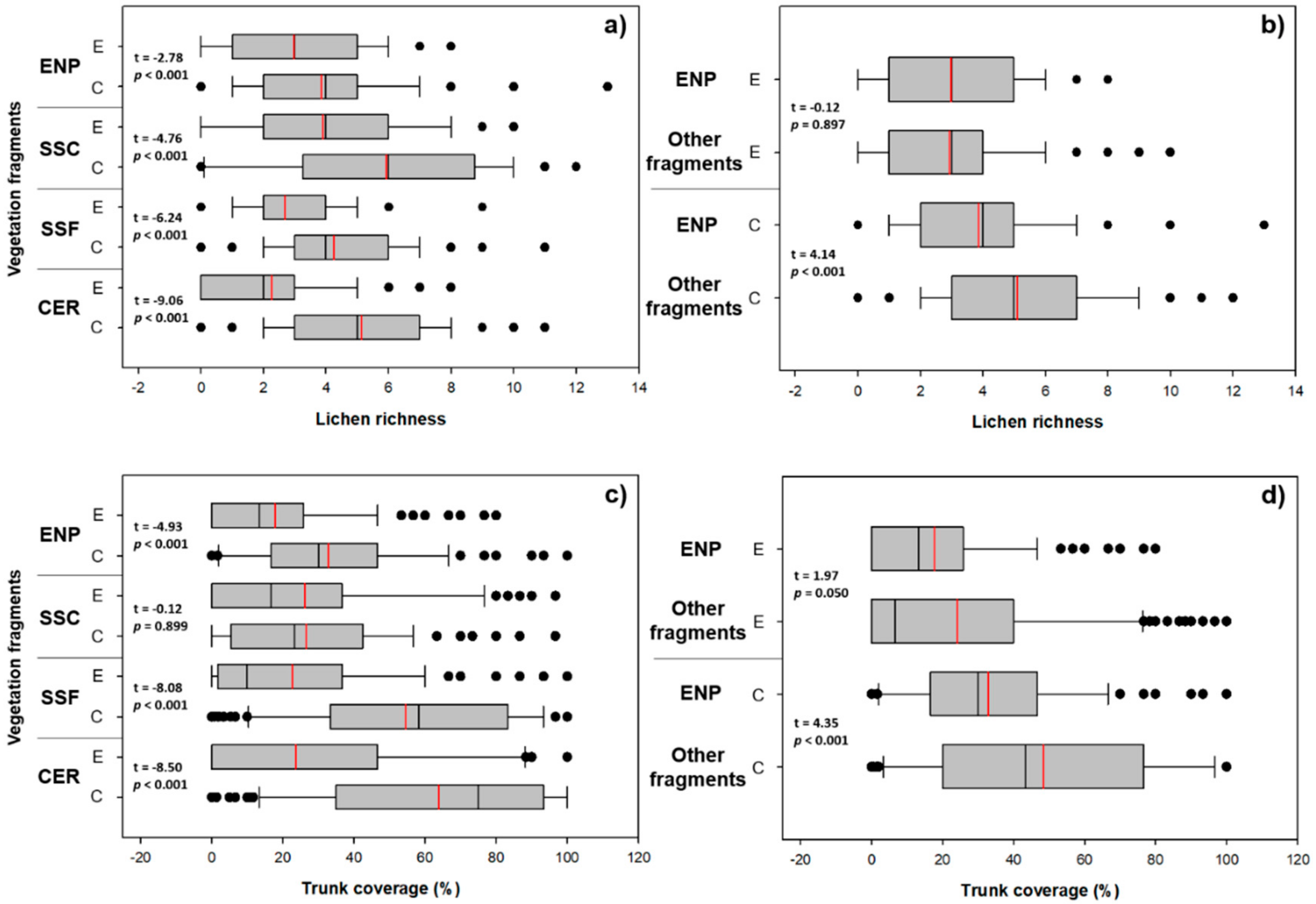
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rubio-Salcedo, M.; Psomas, A.; Prieto, M.; Zimmermann, N.E.; Martínez, I. Case study of the implications of climate change for lichen diversity and distributions. Biodivers. Conserv. 2016, 26, 1121–1141. [Google Scholar] [CrossRef]
- McMurray, J.A.; Roberts, D.W.; Geiser, L.H. Epiphytic lichen indication of nitrogen deposition and climate in the northern rocky mountains, USA. Ecol. Indic. 2015, 49, 154–161. [Google Scholar] [CrossRef]
- Root, H.T.; Geiser, L.H.; Jovan, S.; Neitlich, P. Epiphytic macrolichen indication of air quality and climate in interior forested mountains of the Pacific Northwest, USA. Ecol. Indic. 2015, 53, 95–105. [Google Scholar] [CrossRef]
- Matos, P.; Geiser, L.; Hardman, A.; Glavich, D.; Pinho, P.; Nunes, A.; Soares, A.M.V.M.; Branquinho, C. Tracking global change using lichen diversity: Towards a global-scale ecological indicator. Methods Ecol. Evol. 2017, 8, 788–798. [Google Scholar] [CrossRef] [Green Version]
- Sett, R.; Kundu, M. Epiphytic lichens: Their usefulness as bio-indicators of air pollution. Donnish J. Res. Environ. Stud. 2016, 3, 17–24. [Google Scholar]
- Koch, N.M.; Matos, P.; Branquinho, C.; Pinho, P.; Lucheta, F.; de Azevedo Martins, S.M.; Ferrão Vargas, V.M. Selecting lichen functional traits as ecological indicators of the effects of urban environment. Sci. Total Environ. 2019, 654, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Matos, P.; Vieira, J.; Rocha, B.; Branquinho, C.; Pinho, P. Modeling the provision of air-quality regulation ecosystem service provided by urban green spaces using lichens as ecological indicators. Sci. Total Environ. 2019, 665, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Cardós, J.L.H.; Martínez, I.; Calvo, V.; Aragón, G. Epiphyte communities in Mediterranean fragmented forests: Importance of the fragment size and the surrounding matrix. Landsc. Ecol. 2016, 31, 1975–1995. [Google Scholar] [CrossRef]
- Cardós, J.L.H.; Aragón, G.; Martínez, I. A species on a tightrope: Establishment limitations of an endangered lichen in a fragmented Mediterranean landscape. Am. J. Bot. 2017, 104, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.F.; Cromack, K., Jr.; Denison, W.; McKee, A.; Maser, C.; Sedetl, J.; Swanson, F.; Juday, G. Ecological Characteristics of Old-Growth Douglas-Fir Forests; General Technical Report PNW-GTR-118; U.S. Department of Agriculture, Forest Service: Portland, OR, USA, 1981; pp. 1–48. [CrossRef]
- Couvet, D. Deleterious effects of restricted gene flow in fragmented populations. Conserv. Biol. 2002, 16, 369–376. [Google Scholar] [CrossRef]
- Murphy, H.T.; Lovett-Doust, J. Context and connectivity in plant metapopulations and landscape mosaics: Does the matrix matter? Oikos 2004, 105, 3–14. [Google Scholar] [CrossRef]
- Driscoll, D.A.; Banks, S.C.; Barton, P.S.; Lindenmayer, D.B.; Smith, A.L. Conceptual domain of the matrix in fragmented landscapes. Trends Ecol. Evol. 2013, 28, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. How much habitat is enough? Biol. Conserv. 2001, 100, 65–74. [Google Scholar] [CrossRef]
- Liepa, L.; Straupe, I. Edge effects on epiphytic lichens in unmanaged black alder stands in Southern Latvia. Res. Rural Dev. 2015, 2, 44–49. [Google Scholar]
- Barry, M.; McMullin, R.T.; Horn, A. Edge effects on the lichen genus Lobaria in Atlantic Canadian Forests. Forest. Chron. 2015, 91, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Aragón, G.; Abuja, L.; Belinchón, R.; Martínez, I. Edge type determines the intensity of forest edge effect on epiphytic communities. Eur. J. For. Res. 2015, 134, 443–451. [Google Scholar] [CrossRef]
- Ratter, J.A.; Ribeiro, J.F.; Bridgewater, S. The Brazilian Cerrado vegetation and threats to its biodiversity. Ann. Bot. 1997, 80, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Eaten, G. The cerrado vegetation of Brazil. Bot. Rev. 1972, 38, 201–341. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, X.; Wang, B.; Nie, P. Agricultural pollution and regulation: How to subsidize agriculture? J. Clean. Prod. 2017, 164, 258–264. [Google Scholar] [CrossRef]
- Roseiro, M.N.V.; Takanavanagui, A.M.M. Meio ambiente e poluição atmosférica: O caso da cana-de-açúcar. Rev. Do Cent. De Ciências Da Saúde 2004, 30, 73–86. [Google Scholar] [CrossRef]
- Langenbach, T.; Mano, D.; Campos, M.M.; Cunha, A.L.M.C.; De Campos, T.M.P. Pesticide dispersion by spraying under tropical conditions. J. Environ. Sci. Health B 2017, 52, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.G.F.; da Silva, A.C.; dos Santos Araújo, R.; Rigotto, R.M. Evaluation of the atmospheric contamination level for the use of herbicide glyphosate in the northeast region of Brazil. Environ. Monit. Assess. 2019, 191, 604. [Google Scholar] [CrossRef] [PubMed]
- Di Martos, L.M.; Jost, C.L. Sequential determination of five heavy metal ions in Brazilian phosphate fertilizers and surface waters by stripping voltammetry. Int. J. Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Rangel, M.G.L.; Henríquez, J.R.; Costa, J.A.P.; de Lira Junior, J.C. An assessment of dispersing pollutants from the pre-harvest burning of sugarcane in rural areas in the northeast of Brazil. Atmos. Environ. 2018, 178, 265–281. [Google Scholar] [CrossRef]
- Nacke, H.; Gonçalves, A.C.; Schwantes, D.; Nava, I.A.; Strey, L.; Coelho, G.F. Availability of heavy metals (Cd, Pb, and Cr) in agriculture from commercial fertilizers. Arch. Environ. Contam. Toxicol. 2013, 64, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Zoffoli, H.J.O.; do Amaral-Sobrinho, N.M.B.; Zonta, E.; Luisi, M.V.; Marcon, G.; Tolón-Becerra, A. Inputs of heavy metals due to agrochemical use in tobacco fields in Brazil’s Southern Region. Environ. Monit. Assess. 2013, 185, 2423–2437. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, M.E.S.; Aptroot, A.; Lücking, R. Lichen fungi in the Atlantic rain forest of Northeast Brazil: The relationship of species richness with habitat diversity and conservation status. Braz. J. Bot. 2016, 40, 145–156. [Google Scholar] [CrossRef]
- López, L.G.C.; Medina, E.A.S.; Peña, A.M. Effects of microclimate on species diversity and functional traits of corticolous lichens in the Popayan Botanical Garden (Cauca, Colombia). Cryptogam. Mycol. 2016, 37, 205–215. [Google Scholar] [CrossRef]
- Käffer, M.; Koch, N.; Martins, S.; Vargas, V. Lichen community versus host tree bark texture in an urban environment in southern Brazil. Iheringia Ser. Bot. 2016, 71, 49–54. [Google Scholar]
- Abas, A.; Nizam, M.S. Elevated CO2 effects on corticolous lichen and bark pH in free-air CO2 enrichments (FACE) station. Adv. Environ. Biol. 2017, 11, 68–72. [Google Scholar]
- Rasmussen, H.N.; Nord-Larsen, T.; Hansen, E.S.; Hoareau, G. Estimation of life history in corticolous lichens by zonation. Lichenologist 2018, 50, 697–704. [Google Scholar] [CrossRef]
- Simijaca, D.; Moncada, B.; Lücking, R. Oak forest or conifer plantation, what do epiphytic lichens prefer? Colomb. For. 2018, 21, 123–141. [Google Scholar] [CrossRef]
- Yatawara, M.; Dayananda, N. Use of corticolous lichens for the assessment of ambient air quality along rural–urban ecosystems of tropics: A study in Sri Lanka. Environ. Monit. Assess. 2019, 191, 179. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, G.B.; Blank, G.B.; Wentworth, T.R.; Lowman, M.D.; Neufeld, H.S.; Plata, E.R. Highway pollution effects on microhabitat community structure of corticolous lichens. Bryologist 2018, 121, 1–13. [Google Scholar] [CrossRef]
- Millar, R.B.; Anderson, M.J. Remedies for pseudoreplication. Fish. Res. 2004, 70, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.D.; Keitt, T.H. Species’ borders: A unifying theme in ecology. Oikos 2005, 108, 3–6. [Google Scholar] [CrossRef]
- Ribeiro, J.F.; Walter, B.M. Tdo bioma Cer. Fitofisionomias rado. In Cerrado: Ambiente e Flora. Planaltina; Sano, S.M., Almeida, S.P., Eds.; Embrapa-CPAC: Brasília, Brazil, 1998; pp. 87–166. [Google Scholar]
- Kilca, R.V.; Schiavini, I.; Monteiro, G.A. Floristic patterns on two seasonal forests types in the Cerrado. Biosci. J. 2014, 30, 903–913. [Google Scholar]
- Schlichting, A.F.; Bonfim-Silva, E.M.; Silva, M.C.; Pietro-Souza, W.; da Silva, T.J.A.; Farias, L.N. Efficiency of portable chlorophyll meters in assessing the nutritional status of wheat plants. Rev. Bras. Eng. Agríc. Ambient 2015, 19, 1148–1151. [Google Scholar] [CrossRef] [Green Version]
- Silva Júnior, M.C. 100 Árvores do Cerrado Sentido Restrito: Guia de Campo; Rede de Sementes do Cerrado: Brasília, Brazil, 2012; pp. 1–360. [Google Scholar]
- Silva Júnior, M.C.; Pereira, B.D.S. 100 Árvores do Cerrado-Matas de Galeria: Guia de Campo; Rede de Sementes do Cerrado: Brasília, Brazil, 2009; pp. 1–288. [Google Scholar]
- Kuhlmann, M. Frutos e Sementes do Cerrado: Espécies Atrativas Para A Fauna; Ipsis Gráfica e Editora: Brasília, Brazil, 2018; pp. 1–464. [Google Scholar]
- APG IV. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Kricke, R. Measuring Bark pH. In Monitoring with Lichens-Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 333–336. [Google Scholar] [CrossRef]
- McDonald, L.; Van Woudenberg, M.; Dorin, B.; Adcock, A.M.; McMullin, R.T.; Cottenie, K. The effects of bark quality on corticolous lichen community composition in urban parks of southern Ontario. Botany 2017, 95, 1141–1149. [Google Scholar] [CrossRef]
- Ferreira, T.; Rasband, W. O ImageJ Guia do Usuário-Version 1.43. 2010. Available online: http://rsbweb.nih.gov/ij/docs/user-guide.pdf (accessed on 1 February 2020).
- Benítez, A.; Aragón, G.; González, Y.; Prieto, M. Functional traits of epiphytic lichens in response to forest disturbance and as predictors of total richness and diversity. Ecol. Indic. 2018, 86, 18–26. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Nagendra, H. Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl. Geogr. 2002, 22, 175–186. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 30 January 2020).
- Hadfield, J.D. Package ‘MCMCglmm’, MCMC Generalised Linear Mixed Models, Version 2.29. 2019. Available online: https://cran.r-project.org/web/packages/MCMCglmm/MCMCglmm.pdf (accessed on 12 February 2020).
- Hadfield, J.D. MCMC methods for Multi–response Generalised Linear Mixed Models: The MCMCglmm R Package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Burnham, K.P.; Anderson, D.R. Model. Selection and Multi-Model Inference; Springer: Detroit, MI, USA, 2002; pp. 1–487. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. The comparison of dendrograms by objective methods. Taxon 1962, 11, 33. [Google Scholar] [CrossRef]
- Garcia-Vallve, S.; Palau, J.; Romeu, A. Horizontal gene transfer in glycosyl hydrolases inferred from codon usage in Escherichia coli and Bacillus subtilis. Mol. Biol. Evol. 1999, 16, 1125–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geldmann, J.; Barnes, M.; Coad, L.; Craigie, I.D.; Hockings, M.; Burgess, N.D. Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol. Conserv. 2013, 161, 230–238. [Google Scholar] [CrossRef]
- Coetzee, B.W.T.; Gaston, K.J.; Chown, S.L. Local scale comparisons of biodiversity as a test for global protected area ecological performance: A meta-analysis. PLoS ONE 2014, 9, e105824. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.L.; Hill, S.L.L.; Newbold, T.; Hudson, L.N.; Börger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J.P.W. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 2016, 7, 12306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayner, L.; Lindenmayer, D.B.; Wood, J.T.; Gibbons, P.; Manning, A.D. Are protected areas maintaining bird diversity? Ecography 2013, 37, 43–53. [Google Scholar] [CrossRef]
- Brown, J.A.; Lockwood, J.L.; Avery, J.D.; Curtis Burkhalter, J.; Aagaard, K.; Fenn, K.H. Evaluating the long-term effectiveness of terrestrial protected areas: A 40-year look at forest bird diversity. Biodivers. Conserv. 2019. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Novaes, P.C.; Ferreira, L.G.; Dias, R. Identificação de áreas prioritárias para conservação da biodiversidade do estado de Goiás. Bol. Goiano De Geogr. 2003, 23, 41–58. [Google Scholar] [CrossRef]
- Mantovani, J.E.; Pereira, A. Estimativa da integridade da cobertura de vegetação do cerrado através de dados Landsat-TM. In Proceedings of the Simpósio Brasileiro de Sensoriamento Remoto 9, Santos, Brazil, 11–18 September 1998; pp. 1455–1466. [Google Scholar]
- Sano, E.E.; Barcellos, A.O.; Bezerra, H.S. Assessing the spatial distribution of cultivated pastures in the Brazilian savanna. Pasturas Trop. 2001, 22, 2–15. [Google Scholar]
- Latrubesse, E.M.; Arima, E.; Ferreira, M.E.; Nogueira, S.H.; Wittmann, F.; Dias, M.S.; Dagosta, F.; Bayer, M. Fostering water resource governance and conservation in the Brazilian Cerrado biome. Conserv. Sci. Pract. 2019, 1, e77. [Google Scholar] [CrossRef] [Green Version]
- França, H.; Ramos Neto, M.B.; Setzer, A. O Fogo no Parque Nacional das Emas; MMA, Ministério do Meio Ambiente: Brasília, Brazil, 2007; pp. 1–140.
- Souchie, F.F.; Pinto, J.R.R.; Lenza, E.; Gomes, L.; Maracahipes-Santos, L.; Silvério, D.V. Post-fire resprouting strategies of woody vegetation in the Brazilian savanna. Acta Bot. Bras. 2017, 31, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Lenza, E.; Abadia, A.C.; Menegat, H.; Lúcio, N.W.; Marachipes-Santos, L.; Mews, H.A.; Santos, J.O.; Martins, J. Does fire determine distinct floristic composition of two Cerrado savanna communities on different substrates? Acta Bot. Bras. 2017, 31, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Mistry, J. Corticolous lichens as potential bioindicators of fire history: A study in the cerrado of the Distrito Federal, central Brazil. J. Biogeogr. 1998, 25, 409–441. [Google Scholar] [CrossRef] [Green Version]
- Mistry, J. A preliminary lichen fire history (LFH) key for the Cerrado of the Distrito Federal, central Brazil. J. Biogeogr. 1998, 25, 443–452. [Google Scholar] [CrossRef]
- Mistry, J.; Berardi, A. Effects of phorophyte determinants on lichen abundance in the cerrado of central Brazil. Plant. Ecol. 2005, 178, 61–76. [Google Scholar] [CrossRef]
- Donoso, D.S.; Grez, A.A.; Simonetti, J.A. Effects of forest fragmentation on the granivory of differently sized seeds. Biol. Conserv. 2004, 115, 63–70. [Google Scholar] [CrossRef]
- Alexander, H.M.; Price, S.; Houser, R.; Finch, D.; Tourtellot, M. Is there reduction in disease and pre-dispersal seed predation at the border of a host plant’s range? Field and herbarium studies of Carex blanda. J. Ecol. 2007, 95, 446–457. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhou, H.; Wang, B.; Jian, Z.; Wang, F.; Huang, J.; Nie, L.; Cui, K.; Peng, S. Quantification of border effect on grain yield measurement of hybrid rice. Field Crops Res. 2013, 141, 47–54. [Google Scholar] [CrossRef]
- Hauck, M. Edge effects on epiphytic lichen diversity in the forest-steppe of the Kazakh Altai. Plant Ecol. Divers. 2013, 7, 473–483. [Google Scholar] [CrossRef]
- Will-Wolf, S.; Esseen, P.-A.; Neitlich, P. Monitoring biodiversity and ecosystem function: Forests. In Monitoring with Lichens–Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 203–222. [Google Scholar] [CrossRef]
- Jüriado, I.; Paal, J.; Liira, J. Epiphytic and epixylic lichen species diversity in Estonian natural forests. Biodivers. Conserv. 2003, 12, 1587–1607. [Google Scholar] [CrossRef]
- Brunialti, G.; Giordani, P. Variability of lichen diversity in a climatically heterogeneous area (Liguria, NW Italy). Lichenologist 2003, 35, 55–69. [Google Scholar] [CrossRef]
- Tripathi, M.; Joshi, Y. (Eds.) What are Lichenized Fungi? In Endolichenic Fungi: Present and Future Trends; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–26. [Google Scholar] [CrossRef]
- Costa, W.R.; Mineo, M.F. Os líquens como bioindicadores de poluição atmosférica no município de Uberaba, Minas Gerais. Rev. Eletrônica Em Gestão Educ. E Tecnol. Ambient. 2013, 13, 2690–2700. [Google Scholar] [CrossRef] [Green Version]
- Käffer, M.I.; Marcelli, M.P.; Ganade, G. Distribution and composition of the lichenized mycota on a landscape mosaic from southern Brazil. Acta Bot. Bras. 2010, 24, 790–802. [Google Scholar] [CrossRef]
- Käffer, M.I.; Ganade, G.; Marcelli, M.P. Lichen diversity and composition in Araucaria forests and tree monocultures in southern Brazil. Biodivers. Conserv. 2009, 18, 3543–3561. [Google Scholar] [CrossRef]
- Lemos, A.; Käffer, M.I.; Martins, S.A. Composição e diversidade de liquens corticícolas em três diferentes ambientes: Florestal, Urbano e Industrial. Rev. Bras. De Biociências 2007, 5, 228–230. [Google Scholar]
- Armstrong, R.A.; Welch, A.R. Competition in lichen communities. Symbiosis 2007, 43, 1–12. [Google Scholar]
- Goga, M.; Elečko, J.; Marcinčinová, M.; Ručová, D.; Bačkorová, M.; Bačkor, M. Lichen metabolites: An overview of some secondary metabolites and their biological potential. Co-Evol. Second. Metab. 2020, 1–36. [Google Scholar] [CrossRef]
- Torzilli, A.P.; Mikelson, P.A.; Lawrey, J.D. Physiological effect of lichen secondary metabolites on the lichen parasite Marchandiomyces corallinus. Lichenologist 1999, 31, 307–314. [Google Scholar] [CrossRef]
- Lawrey, J.D. Lichen Allelopathy: A Review. In Allelopathy-Organisms, Processes, and Applications; Inderjit Dakshini, K.M.M., Einhellig, F.A., Eds.; American Chemical Society: Washington, DC, USA, 1994; pp. 26–38. [Google Scholar] [CrossRef]
- Goga, M.; Antreich, S.J.; Bačkor, M.; Weckwerth, W.; Lang, I. Lichen secondary metabolites affect growth of Physcomitrella patens by allelopathy. Protoplasma 2016, 254, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Ručová, D.; Goga, M.; Sabovljević, M.; Vilková, M.; Petruľová, V.; Bačkor, M. Insights into physiological responses of mosses Physcomitrella patens and Pohlia drummondii to lichen secondary metabolites. Protoplasma 2019. [Google Scholar] [CrossRef] [PubMed]
- Frahm, J.P.; Specht, A.; Reifenrath, K.; Vargas, Y.L. Allelopathic effect of crustaceous lichens on epiphytic bryophytes and vascular plants. Nova Hedwig. 2000, 70, 245–254. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Piervittori, R. Lichen-plant interactions. J. Plant. Interact. 2010, 5, 163–177. [Google Scholar] [CrossRef]
- Pizňak, M.; Kolarčik, V.; Goga, M.; Bačkor, M. Allelopathic effects of lichen metabolite usnic acid on growth and physiological responses of Norway spruce and Scots pine seedlings. S. Afr. J. Bot. 2019, 124, 14–19. [Google Scholar] [CrossRef]
- Degtjarenko, P.; Matos, P.; Marmor, L.; Branquinho, C.; Randlane, T. Functional traits of epiphytic lichens respond to alkaline dust pollution. Fungal Ecol. 2018, 36, 81–88. [Google Scholar] [CrossRef]
- Fritz, O.; Brunet, J.; Caldiz, M. Interacting effects of tree charactersictics on the occurrence of rare epiphytes in a Swedish boreal forest. Bryologist 2009, 112, 488–505. [Google Scholar] [CrossRef]
- Ranius, T.; Johansson, P.; Berg, N.; Niklasson, M. The influence of tree age and microhabitat quality on the occurrence of crustose lichens associated with old oaks. J. Veg. Sci. 2008, 19, 653–662. [Google Scholar] [CrossRef]
- Spier, L.; van Dobben, H.; van Dort, K. Is bark pH more important than tree species in determining the composition of nitrophytic or acidophytic lichen floras? Environ. Pollut. 2010, 158, 3607–3611. [Google Scholar] [CrossRef] [PubMed]
- Uliczka, H.; Angelstam, P. Occurrence of epiphytic macrolichens in relation to tree species and age in managed boreal forest. Ecography 1999, 22, 396–405. [Google Scholar] [CrossRef]
- Lamit, L.J.; Bowker, M.A.; Holeski, L.M.; Næsborg, R.R.; Wooley, S.C.; Zinkgraf, M.; Lindroth, R.L.; Whitham, T.G.; Gehring, C.A. Genetically-based trait variation within a foundation tree species influences a dominant bark lichen. Fungal Ecol. 2011, 4, 103–109. [Google Scholar] [CrossRef]




| Abbreviation | Definition |
|---|---|
| AIC | Akaike Information Criterion |
| ΔAIC | Delta Akaike Information Criterion |
| wAIC | Weight Akaike Information Criterion |
| ANOVA | Analysis of Variance |
| APG IV | Angiosperm Phylogeny Group |
| BF | Bark fissuring |
| C | Center |
| CBH | Circumference of the stem at breast height |
| CER | Cerradão |
| E | Edge |
| ENP | Emas National Park |
| FCI | Falker Chlorophyll Index |
| GLMs | Generalized Linear Models |
| GLMMs | Generalized Linear Mixed Models |
| H | height of the host plant |
| H′ | Shannon-Wiener diversity index |
| Lum | Illuminance |
| r | Pearson correlation coefficient |
| SSC | Cerrado sensu stricto |
| SSF | Seasonal Semi-deciduous Forest |
| TC | Total chlorophyll |
| UPGMA | Unweighted Pair Group Method using Arithmetic averages |
| Model | Center | Edge | Total | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| CBH vs. Richness | 0.142 | 0.012 * | 0.175 | 0.003 ** | 0.068 | 0.097 |
| H vs. Richness | 0.171 | 0.002 ** | 0.037 | 0.537 | 0.077 | 0.061 |
| pH vs. Richness | 0.093 | 0.100 | −0.179 | 0.003 ** | −0.029 | 0.488 |
| TC vs. Richness | −0.183 | 0.001 ** | −0.030 | 0.621 | −0.128 | 0.002 ** |
| BF vs. Richness | −0.152 | 0.007 ** | −0.170 | 0.004 ** | −0.055 | 0.178 |
| Lum vs. Richness | −0.476 | 0.034 * | −0.380 | 0.098 | −0.598 | 0.005 ** |
| CBH vs. Cover | −0.013 | 0.821 | 0.127 | 0.033 * | −0.009 | 0.825 |
| H vs. Cover | −0.012 | 0.838 | 0.060 | 0.318 | −0.010 | 0.809 |
| pH vs. Cover | −0.115 | 0.043 * | −0.158 | 0.008 ** | −0.140 | 0.001 ** |
| TC vs. Cover | −0.187 | 0.001 ** | −0.078 | 0.192 | −0.148 | 0.001 ** |
| BF vs. Cover | −0.062 | 0.278 | −0.025 | 0.670 | 0.038 | 0.361 |
| Lum vs. Cover | −0.291 | 0.214 | −0.414 | 0.069 | −0.519 | 0.001 ** |
| Model | ∆AICc | wAIC | k | β | p |
|---|---|---|---|---|---|
| Richness Total | |||||
| Species | 0 | 0.1517 | 4 | 0.1061 | - |
| Species + BF | 0.2 | 0.1352 | 5 | 0.1185 | 0.0550 |
| Species + pH | 0.7 | 0.1072 | 5 | 0.1060 | 0.8900 |
| Species + pH + BF | 0.9 | 0.0954 | 6 | 0.1185 | 0.1574 |
| Species + TC | 1.2 | 0.0845 | 5 | 0.0927 | 0.2818 |
| Species + H + BF | 1.6 | 0.0691 | 6 | 0.0988 | 0.0548 |
| Species + TC + BF | 1.8 | 0.0629 | 6 | 0.1070 | 0.1068 |
| Species + CBH + BF | 1.8 | 0.0622 | 6 | 0.0816 | 0.0531 |
| Species + TC + pH | 1.9 | 0.0595 | 6 | 0.0927 | 0.5564 |
| Species + H | 2.3 | 0.0484 | 5 | 0.0903 | 0.2713 |
| Species + H + pH | 2.9 | 0.0351 | 6 | 0.0899 | 0.5300 |
| Species + CBH | 3.1 | 0.0319 | 5 | 0.0844 | 0.4011 |
| Species + CBH + pH | 3.8 | 0.0225 | 6 | 0.0844 | 0.6982 |
| Species + TC + CBH | 4.2 | 0.0184 | 6 | 0.0696 | 0.3805 |
| Species + CBH + H | 5.5 | 0.0099 | 6 | 0.0877 | 0.5425 |
| Species + TC + CBH + H + pH + BF | 6.4 | 0.0062 | 9 | 0.0728 | 0.2121 |
| Species + TC + H | 3679.1 | <0.001 | 6 | −4.6210 | 1.0000 |
| Center | |||||
| Species + pH | 0 | 0.1934 | 5 | 0.3165 | 0.3194 |
| Species + TC + pH | 0.7 | 0.1350 | 6 | 0.2984 | 0.4002 |
| Species | 0.8 | 0.1272 | 4 | 0.3349 | - |
| Species + TC | 1.6 | 0.0882 | 5 | 0.3172 | 0.3656 |
| Species + H + pH | 1.6 | 0.0865 | 6 | 0.3418 | 0.3232 |
| Species + CBH + pH | 1.9 | 0.0737 | 6 | 0.3274 | 0.3501 |
| Species + H | 2.2 | 0.0651 | 5 | 0.3599 | 0.2120 |
| Species + CBH | 2.8 | 0.0475 | 5 | 0.3458 | 0.3022 |
| Species + pH + BF | 2.9 | 0.0444 | 6 | 0.3131 | 0.6019 |
| Species + TC + CBH | 3.5 | 0.0332 | 6 | 0.3284 | 0.3869 |
| Species + BF | 3.8 | 0.0293 | 5 | 0.3310 | 0.8638 |
| Species + CBH + H | 4.5 | 0.0208 | 6 | 0.3586 | 0.4497 |
| Species + TC + BF | 4.5 | 0.0205 | 6 | 0.3121 | 0.6476 |
| Species + H + BF | 5.0 | 0.0162 | 6 | 0.3524 | 0.4235 |
| Species + CBH + F | 5.4 | 0.0131 | 6 | 0.3354 | 0.5080 |
| Species + TC + CBH + H + pH + BF | 7.0 | 0.0059 | 9 | 0.3097 | 0.6081 |
| Species + TC + H | 1874.6 | <0.001 | 6 | 4.0750 | 1.0000 |
| Edge | |||||
| Species + CBH + pH | 0.0 | 0.3218 | 6 | −0.3532 | <0.001 *** |
| Species + CBH | 0.2 | 0.2928 | 5 | −0.3514 | <0.001 *** |
| Species + CBH + BF | 1.8 | 0.1293 | 6 | −0.3218 | <0.001 *** |
| Species + TC + CBH | 1.9 | 0.1237 | 6 | −0.3599 | <0.001 *** |
| Species + CBH + H | 2.5 | 0.0930 | 6 | −0.3516 | <0.001 *** |
| Species + TC + CBH + H + pH + BF | 5.7 | 0.0189 | 9 | −0.3217 | 0.0049 ** |
| Species + H + pH | 7.7 | 0.0069 | 6 | −0.2886 | 0.0338 * |
| Species + H | 8.7 | 0.0042 | 5 | −0.2878 | 0.0176 * |
| Species + H + BF | 9.2 | 0.0033 | 6 | −0.2513 | 0.0143 * |
| Species + pH | 10.9 | 0.0014 | 5 | −0.3799 | 0.4000 |
| Species + pH + BF | 11.0 | 0.0013 | 6 | −0.3363 | 0.1000 |
| Species | 11.5 | 0.0010 | 4 | −0.3767 | - |
| Species + BF | 11.6 | <0.001 | 5 | −0.3331 | 0.0683 |
| Species + TC + PH | 12.6 | <0.001 | 6 | −0.3887 | 0.6000 |
| Species + TC | 13.2 | <0.001 | 5 | −0.3849 | 0.7000 |
| Species + TC + BF | 13.4 | <0.001 | 6 | −0.3343 | 0.2000 |
| Species + TC + H | 1792.1 | <0.001 | 6 | 2.1170 | 1.0000 |
| Model | ∆AICc | wAIC | k | β | p |
|---|---|---|---|---|---|
| Total Cover | |||||
| Species + TC + CBH + H + pH + BF | 0.0 | 1.0 | 9.0 | 0.1727 | <0.001 *** |
| Species + TC + BF | 12.1 | 0.0 | 6.0 | 0.1000 | <0.001 *** |
| Species + CBH + BF | 14.6 | <0.001 | 6.0 | 0.1014 | <0.001 *** |
| Species + pH + BF | 15.1 | <0.001 | 6.0 | −0.0166 | <0.001 *** |
| Species + H + BF | 17.6 | <0.001 | 6.0 | 0.0257 | <0.001 *** |
| Species + BF | 19.4 | <0.001 | 5.0 | −0.0264 | <0.001 *** |
| Species + TC + CBH | 45.6 | <0.001 | 6.0 | −0.0632 | <0.001 *** |
| Species + CBH + pH | 51.2 | <0.001 | 6.0 | −0.2577 | <0.001 *** |
| Species + CBH + H | 51.9 | <0.001 | 6.0 | −0.3173 | <0.001 *** |
| Species + CBH | 55.5 | <0.001 | 5.0 | −0.2529 | <0.001*** |
| Species + TC + H | 56.3 | <0.001 | 6.0 | −0.2043 | 0.0044 ** |
| Species + TC + pH | 56.4 | <0.001 | 6.0 | −0.3756 | 0.0197 * |
| Species + TC | 60.8 | <0.001 | 5.0 | −0.3798 | 0.0051 ** |
| Species + H + pH | 62.1 | <0.001 | 6.0 | −0.3817 | 0.1889 |
| Species + H | 66.5 | <0.001 | 5.0 | 0.0903 | 0.0692 |
| Species + pH | 66.9 | <0.001 | 5.0 | −0.5812 | 0.9855 |
| Species | 71.3 | <0.001 | 4.0 | −0.5831 | - |
| Center | |||||
| Species | 0.0 | 1.0 | 4.0 | 0.9667 | <0.001*** |
| Species + TC + CBH + H + pH + BF | 268.7 | <0.001 | 9.0 | −6.675 | <0.001*** |
| Species + TC + BF | 281.8 | <0.001 | 6.0 | −6.552 | <0.001*** |
| Species + pH + BF | 284.1 | <0.001 | 6.0 | −6.243 | <0.001*** |
| Species + CBH + BF | 285.7 | <0.001 | 6.0 | −6.077 | <0.001*** |
| Species + H + BF | 287.2 | <0.001 | 6.0 | −5.865 | <0.001*** |
| Species + BF | 290.1 | <0.001 | 5.0 | −6.153 | 0.0018 ** |
| Species + TC + CBH | 294.4 | <0.001 | 6.0 | −6.319 | 0.0087 ** |
| Species + CBH + pH | 296.1 | <0.001 | 6.0 | −5.999 | 0.0677 |
| Species + TC + pH | 298.7 | <0.001 | 6.0 | −6.511 | 0.0126 * |
| Species + CBH + H | 298.9 | <0.001 | 6.0 | −6.127 | 0.0320 * |
| Species + TC + H | 299.9 | <0.001 | 6.0 | −5.892 | 0.0994 |
| Species + H + pH | 300.7 | <0.001 | 6.0 | −5.537 | 0.0036 ** |
| Species + CBH | 301.9 | <0.001 | 5.0 | −5.909 | 0.0384 * |
| Species + TC | 304.6 | <0.001 | 5.0 | 1.015 | 0.2902 |
| Species + pH | 306.3 | <0.001 | 5.0 | −6.1 | 0.0819 |
| Species + H | 307.2 | <0.001 | 5.0 | −5.468 | - |
| Edge | |||||
| Species + TC + CBH + H + pH + BF | 0.0 | 0.9845 | 9.0 | 0.1119 | 0.1161 |
| Species + pH + BF | 10.8 | 0.0045 | 6.0 | 0.2575 | 0.0529 |
| Species + H + BF | 11.1 | 0.0038 | 6.0 | 0.07919 | 0.0161 * |
| Species + TC + BF | 12.5 | 0.0019 | 6.0 | 0.3047 | 0.0536 |
| Species + CBH + BF | 13.0 | 0.0015 | 6.0 | 0.2503 | 0.0343 * |
| Species + H + pH | 13.7 | 0.0011 | 6.0 | 0.8233 | 0.2949 |
| Species + TC + pH | 14.2 | <0.001 | 6.0 | 1.014 | 0.6127 |
| Species + TC + H | 15.1 | <0.001 | 6.0 | 0.8754 | 0.2484 |
| Species + CBH + pH | 15.9 | <0.001 | 6.0 | 0.9873 | 0.7215 |
| Species + BF | 16.0 | <0.001 | 5.0 | 0.2565 | 0.0177 * |
| Species + CBH + H | 16.6 | <0.001 | 6.0 | 0.7969 | 0.3417 |
| Species + TC + CBH | 17.1 | <0.001 | 6.0 | 1.036 | 0.5359 |
| Species + pH | 18.2 | <0.001 | 5.0 | 0.967 | 0.6318 |
| Species + H | 19.0 | <0.001 | 5.0 | 0.8278 | 0.1503 |
| Species + TC | 19.4 | <0.001 | 5.0 | 1.015 | 0.3770 |
| Species + CBH | 21.0 | <0.001 | 5.0 | 0.9874 | 0.4951 |
| Species | 23.3 | <0.001 | 4.0 | 0.9667 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palharini, K.M.Z.; Vitorino, L.C.; Menino, G.C.d.O.; Bessa, L.A. Edge Effects Reflect the Impact of the Agricultural Matrix on the Corticolous Lichens Found in Fragments of Cerrado Savanna in Central Brazil. Sustainability 2020, 12, 7149. https://doi.org/10.3390/su12177149
Palharini KMZ, Vitorino LC, Menino GCdO, Bessa LA. Edge Effects Reflect the Impact of the Agricultural Matrix on the Corticolous Lichens Found in Fragments of Cerrado Savanna in Central Brazil. Sustainability. 2020; 12(17):7149. https://doi.org/10.3390/su12177149
Chicago/Turabian StylePalharini, Kelly Maria Zanuzzi, Luciana Cristina Vitorino, Gisele Cristina de Oliveira Menino, and Layara Alexandre Bessa. 2020. "Edge Effects Reflect the Impact of the Agricultural Matrix on the Corticolous Lichens Found in Fragments of Cerrado Savanna in Central Brazil" Sustainability 12, no. 17: 7149. https://doi.org/10.3390/su12177149





