Plant Pellets: A Compatible Vegan Feedstock for Preparation of Plant-Based Culture Media and Production of Value-Added Biomass of Rhizobia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Rhizobia Strain
2.3. Plant Materials
2.4. Agro-Industrial Byproducts
2.5. Formulation of Plant Pellets (PPs) and Derived Culture Media
2.6. Experimental Units
2.7. Biomass Production of Rhizobium Leguminosarum on Tested Culture Media
3. Statistical Analyses
4. Results
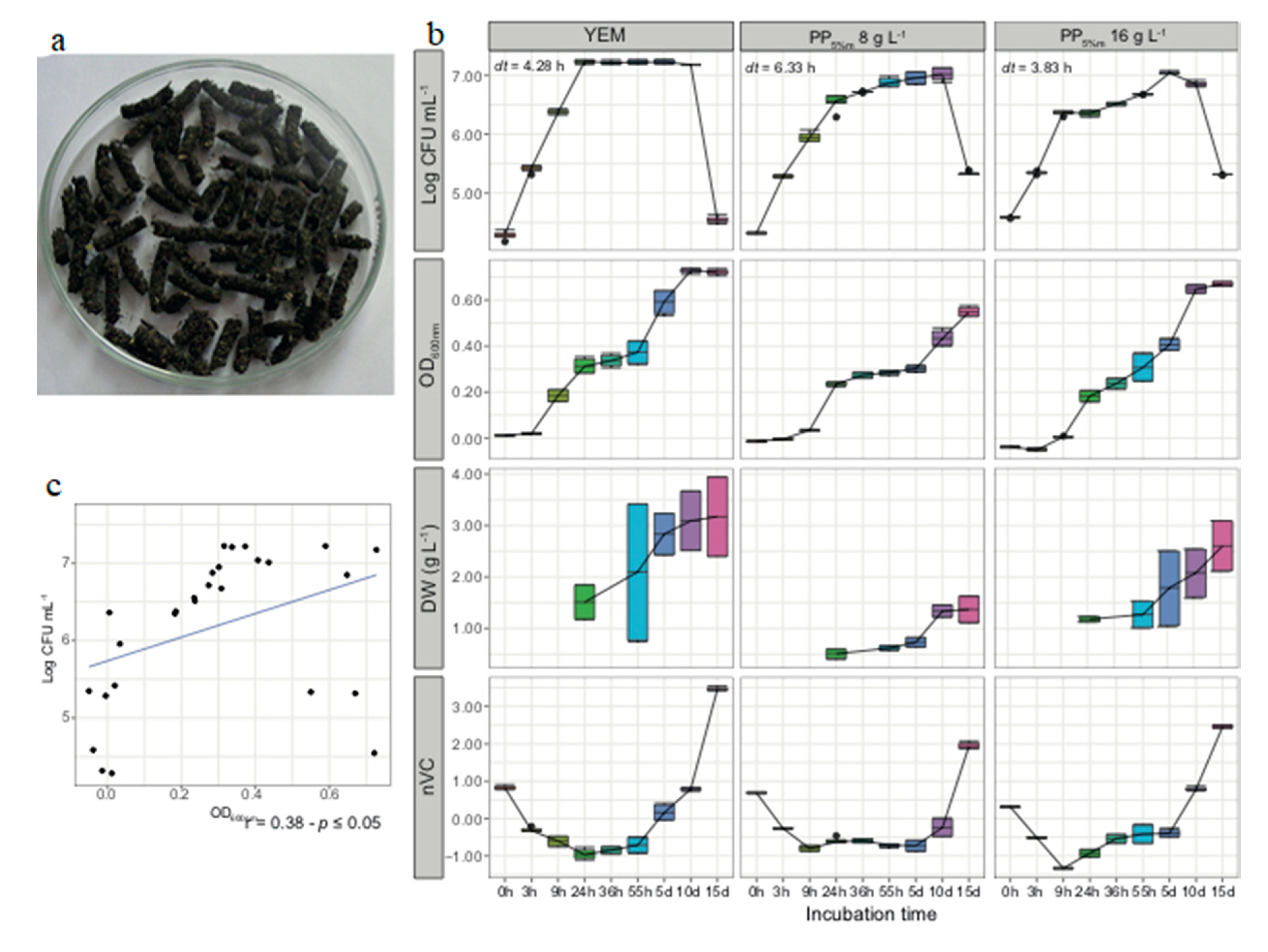
4.1. Glycerol and Molasses Stimulate Biomass Production of Rhizobia and Facilitate Processing of Plant Pellets
4.2. Culture Media Prepared from Plant Pellets Contained in Teabags Supported Good Growth and Biomass Production of Rhizobium Leguminosarum
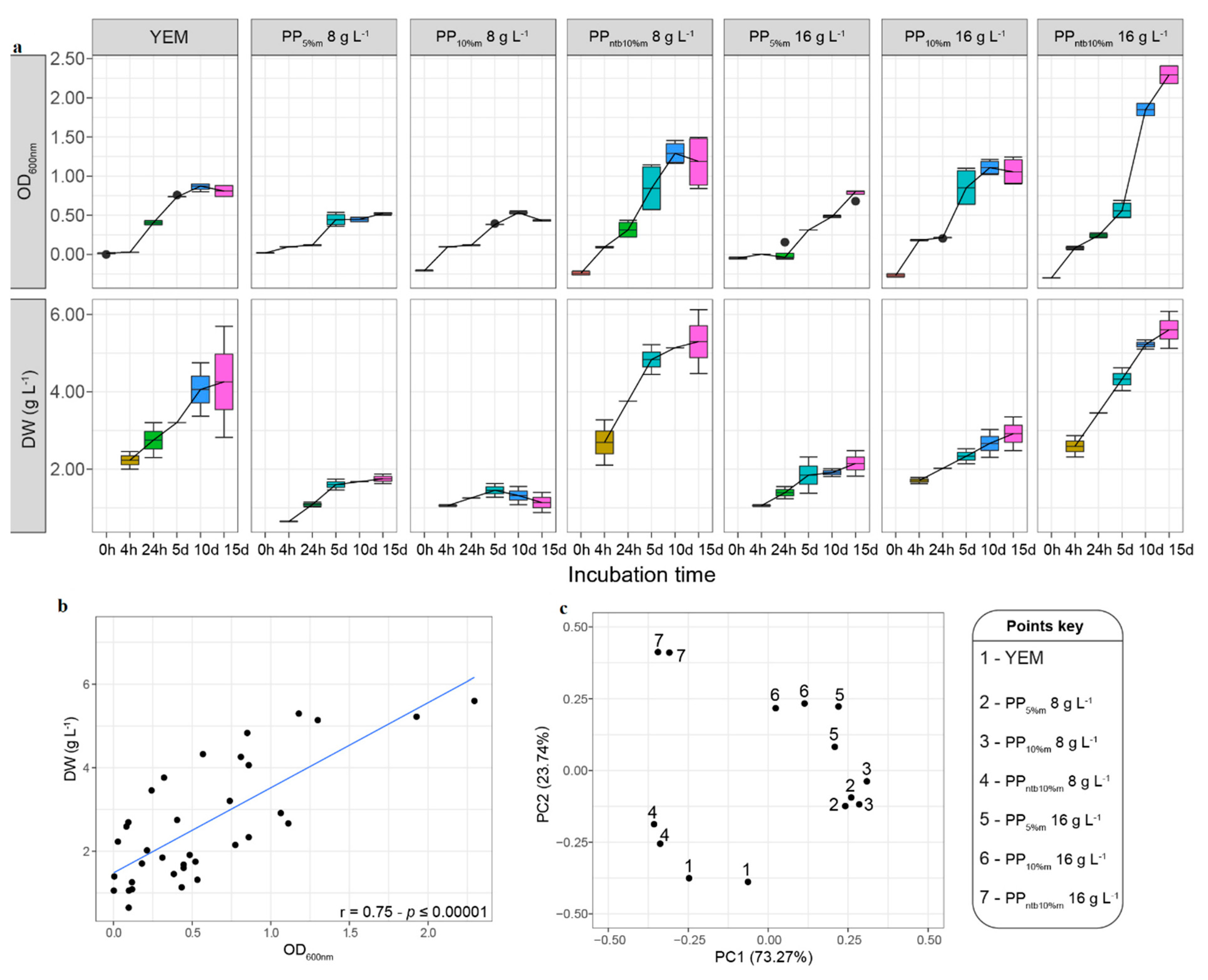
4.3. Fortified PP Preparations Improved Growth and Biomass Production of Tested Rhizobia
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Term | Abbreviation |
| 1-aminocyclopropane-1-carboxylate | ACC deaminase |
| Analysis of variance | ANOVA |
| Bio-fertilizers and bio-pesticides | Agro-biopreparates |
| Byproducts of agro-industries | Agro-byproducts |
| Coefficients of variation | CV |
| Colony forming units | CFUs |
| Dry weight | DW |
| Electric conductivity | EC |
| Estimates of nonviable cells | nVCs |
| Exopolysaccharide | EPS |
| Good agricultural practices | GAPs |
| Optical density measured at 600 nanometers | OD600nm |
| Plant-growth-promoting rhizobacteria | PGPRs |
| Plant pellets | PPs |
| Potential of hydrogen | pH |
| Principal components analysis | PCA |
| Root zone | Rhizosphere |
| VC | Viable cells |
| Yeast extract mannitol | YEM |
References
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, P.R.; Mauchline, T.H. Who’s who in the plant root microbiome? Nat. Biotechnol. 2012, 30, 961–962. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities 1. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica (Cairo) 2012, 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer: Heidelberg, Germany, 2015; ISBN 9783319139210. [Google Scholar]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G. Proposal for the division of plant growth-promoting rhizobacteria into two classifications: Biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol. Biochem. 1998, 30, 1225–1228. [Google Scholar] [CrossRef]
- Udvardi, M.; Poole, P.S. Transport and Metabolism in Legume-Rhizobia Symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2014, 5, 355–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability-A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Shameer, S.; Prasad, T.N. Plant growth promoting rhizobacteria for sustainable agricultural practices with special reference to biotic and abiotic stresses. Plant Growth Regul. 2018, 84, 603–615. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Mahdi, S.S.; Hassan, G.I.; Samoon, S.A.; Rather, H.A.; Dar, S.A.; Zehra, B. Bio-Fertilizers in Organic Agriculture. J. Phytol. 2010, 2, 42–54. [Google Scholar]
- Parekh, S.; Vinci, V.A.; Strobel, R.J. Improvement of microbial strains and fermentation processes. Appl. Microbiol. Biotechnol. 2000, 54, 287–301. [Google Scholar] [CrossRef]
- Okafor, N. Modern Industrial Microbiology and Biotechnology; Science Publishers: Enfield, NH, USA, 2007; ISBN 9781578084340. [Google Scholar]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Ali, S.M.; Hamza, M.A.; Amin, G.; Fayez, M.; El-Tahan, M.; Monib, M.; Hegazi, N.A. Production of biofertilizers using baker’s yeast effluent and their application to wheat and barley grown in north Sinai deserts. Arch. Agron. Soil Sci. 2005, 51, 589–604. [Google Scholar] [CrossRef]
- Essien, J.P.; Akpan, E.J.; Essien, E.P. Studies on mould growth and biomass production using waste banana peel. Bioresour. Technol. 2005, 96, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kapoor, V.; Kumar, V. Utilization of agro-industrial wastes for the simultaneous production of amylase and xylanase by thermophilic actinomycetes. Braz. J. Microbiol. 2012, 43, 1545–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ire, F.S.; Ezebuiro, V.; Ogugbue, C.J. Production of bioethanol by bacterial co-culture from agro-waste-impacted soil through simultaneous saccharification and co-fermentation of steam-exploded bagasse. Bioresour. Bioprocess. 2016, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Geddes, B.A.; Oresnik, I.J. Physiology, genetics, and biochemistry of carbon metabolism in the alphaproteobacterium Sinorhizobium meliloti. Can. J. Microbiol. 2014, 60, 491–507. [Google Scholar] [CrossRef] [Green Version]
- Janczarek, M.; Rachwał, K.; Cieśla, J.; Ginalska, G.; Bieganowski, A. Production of exopolysaccharide by Rhizobium leguminosarum bv. trifolii and its role in bacterial attachment and surface properties. Plant Soil 2015, 388, 211–227. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, N.; Malusa, E.; Requena, A.R.; Martos, V.; López, A.; Maksimovic, I.; Vassileva, M. Potential application of glycerol in the production of plant beneficial microorganisms. J. Ind. Microbiol. Biotechnol. 2017, 44, 735–743. [Google Scholar] [CrossRef]
- Rane, A.N.; Baikar, V.V.; Ravi Kumar, D.V.; Deopurkar, R.L. Agro-Industrial Wastes for Production of Biosurfactant by Bacillus subtilis ANR 88 and Its Application in Synthesis of Silver and Gold Nanoparticles. Front. Microbiol. 2017, 8, 492. [Google Scholar] [CrossRef] [Green Version]
- Nour, E.H.; Hamza, M.A.; Fayez, M.; Monib, M.; Ruppel, S.; Hegazi, N.A. The crude plant juices of desert plants as appropriate culture media for the cultivation of rhizospheric microorganisms. J. Adv. Res. 2012, 3, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Khalil, S.; Ali, T.A.; Skory, C.; Slininger, P.; Schisler, D.A.; Khalil, S. Evaluation of economically feasible, natural plant extract-based microbiological media for producing biomass of the dry rot biocontrol strain Pseudomonas fluorescens P22Y05 in liquid culture. World J. Microbiol. Biotechnol. 2016, 32, 1–11. [Google Scholar] [CrossRef]
- Youssef, H.H.; Hamza, M.A.; Fayez, M.; Mourad, E.F.; Saleh, M.Y.; Sarhan, M.S.; Suker, R.M.; Eltahlawy, A.A.; Nemr, R.A.; El-Tahan, M.; et al. Plant-based culture media: Efficiently support culturing rhizobacteria and correctly mirror their in-situ diversity. J. Adv. Res. 2016, 7, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Sarhan, M.S.; Mourad, E.F.; Hamza, M.A.; Youssef, H.H.; Scherwinski, A.C.; El-Tahan, M.; Fayez, M.; Ruppel, S.; Hegazi, N.A. Plant powder teabags: A novel and practical approach to resolve culturability and diversity of rhizobacteria. Physiol. Plant. 2016, 157, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, N.A.; Sarhan, M.S.; Fayez, M.; Patz, S.; Murphy, B.R.; Ruppel, S. Plant-fed versus chemicals-fed rhizobacteria of Lucerne: Plant-only teabags culture media not only increase culturability of rhizobacteria but also recover a previously uncultured Lysobacter sp., Novosphingobium sp. and Pedobacter sp. PLoS ONE 2017, 12, e0180424. [Google Scholar] [CrossRef] [PubMed]
- Mourad, E.F.; Sarhan, M.S.; Daanaa, H.-S.A.; Abdou, M.; Morsi, A.T.; Abdelfadeel, M.R.; Elsawey, H.; Nemr, R.; El-Tahan, M.; Hamza, M.A.; et al. Plant Materials are Sustainable Substrates Supporting New Technologies of Plant-Only-Based Culture Media for in vitro Culturing of the Plant Microbiota. Microbes Environ. 2018, 33, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarhan, M.S.; Hamza, M.A.; Youssef, H.H.; Patz, S.; Becker, M.; ElSawey, H.; Nemr, R.; Daanaa, H.S.A.; Mourad, E.F.; Morsi, A.T.; et al. Culturomics of the plant prokaryotic microbiome and the dawn of plant-based culture media—A review. J. Adv. Res. 2019, 19, 15–27. [Google Scholar] [CrossRef]
- Saleh, M.Y.; Sarhan, M.S.; Mourad, E.F.; Hamza, M.A.; Abbas, M.T.; Othman, A.A.; Youssef, H.H.; Morsi, A.T.; Youssef, G.H.; El-Tahan, M.; et al. A novel plant-based-sea water culture media for in vitro cultivation and in situ recovery of the halophyte microbiome. J. Adv. Res. 2017, 8, 577–590. [Google Scholar] [CrossRef]
- Hashem, F.; Kuykendall, D. Plasmid DNA content of several agronomically important Rhizobium species that nodulate alfalfa, berseem clover, or Leucaena. Symbiotic Nitrogen Fixat. SE-22 1994, 57, 181–188. [Google Scholar] [CrossRef]
- Kuykendall, L.D.; Abdel-Wahab, S.M.; Hashem, F.M.; van Berkum, P. Symbiotic competence and genetic diversity of Rhizobium strains used as inoculants for alfalfa and berseem clover. Lett. Appl. Microbiol. 1994, 19, 477–482. [Google Scholar] [CrossRef]
- da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root-nodule Bacteria. In I.B.P. Handbook; Blackwell Scientific Publications: Oxford, UK, 1970; Volume 8, pp. 8–9. [Google Scholar]
- Bashan, Y.; Trejo, A.; De-Bashan, L.E. Development of two culture media for mass cultivation of Azospirillum spp. and for production of inoculants to enhance plant growth. Biol. Fertil. Soils 2011, 47, 963–969. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Microbial production of hyaluronic acid from agro-industrial by-products: Molasses and corn steep liquor. Biochem. Eng. J. 2017, 117, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Pirt, S.J. Principles of Microbe and Cell Cultivation; Blackwell Scientific Publications: Oxford, UK, 1975; ISBN 0632081503. [Google Scholar]
- Colombié, S.; Latrille, E.; Sablayrolles, J.-M. Interest of on-line monitoring electrical conductivity during wine fermentation. Eur. Food Res. Technol. 2008, 226, 1553–1557. [Google Scholar] [CrossRef]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of fermentation technology. J. Chem. Inf. Model. 1984, 53, 1689–1699. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Pyruvate Dehydrogenase Multienzyme Complex. Biochemistry 1995, 269, 541. [Google Scholar]
- Hartwig, U.A.; Joseph, C.M.; Phillips, D.A. Flavonoids Released Naturally from Alfalfa Seeds Enhance Growth Rate of Rhizobium meliloti. Plant Physiol. 1991, 95, 797–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maj, D.; Wielbo, J.; Marek-Kozaczuk, M.; Skorupska, A. Response to flavonoids as a factor influencing competitiveness and symbiotic activity of Rhizobium leguminosarum. Microbiol. Res. 2010, 165, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-gendy, N.S.; Madian, H.R.; Amr, S.S.A. Design and Optimization of a Process for Sugarcane Molasses Fermentation by Saccharomyces cerevisiae Using Response Surface Methodology. Int. J. Microbiol. 2013, 2013, 2013–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares, J.; Bedmar, E.J.; Sanjuán, J. Biological nitrogen fixation in the context of global change. Mol. Plant. Microbe. Interact. 2013, 26, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Dunn, M.F. Key roles of microsymbiont amino acid metabolism in rhizobia-legume interactions. Crit. Rev. Microbiol. 2014, 7828, 1–41. [Google Scholar] [CrossRef]
- Sonnleitner, B.; Locher, G.; Fiechter, A. Biomass determination. J. Biotechnol. 1992, 25, 5–22. [Google Scholar] [CrossRef]
- Wechselberger, P.; Sagmeister, P.; Herwig, C. Real-time estimation of biomass and specific growth rate in physiologically variable recombinant fed-batch processes. Bioprocess Biosyst. Eng. 2013, 36, 1205–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, K.R.; Wright, S.W. Microbial Biomass Measurement Methods; Taylor & Francis: New York, NY, USA, 2004. [Google Scholar]
- Stowers, M.D. Carbon metabolism in Rhizobium species. Metab. Clin. Exp. 1985, 39, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.E.; Darby, B.A.; Giasson, M.A.; Kramer, M.A.; Phillips, R.P.; Finzi, A.C. Stoichiometry constrains microbial response to root exudation-insights from a model and a field experiment in a temperate forest. Biogeosciences 2013, 10, 821–838. [Google Scholar] [CrossRef] [Green Version]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaudi, L.V.; Giordano, W. An integrated view of biofilm formation in rhizobia. FEMS Microbiol. Lett. 2010, 304, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nocelli, N.; Bogino, P.C.; Banchio, E.; Giordano, W. Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of rhizobia. Materials 2016, 9, 418. [Google Scholar] [CrossRef] [Green Version]
- Souri, M.K.; Rashidi, M.; Kianmehr, M.H. Effects of manure-based urea pellets on growth, yield, and nitrate content in coriander, garden cress, and parsley plants. J. Plant Nutr. 2018, 41, 1405–1413. [Google Scholar] [CrossRef]
- Owens, B.J.D. Formulation of Culture Media for Conductimetric Assays: Theoretical Considerations. Extrapolation 1985, 131, 3055–3076. [Google Scholar] [CrossRef] [Green Version]
- Cieśla, J.; Bieganowski, A.; Janczarek, M.; Urbanik-Sypniewska, T. Determination of the electrokinetic potential of Rhizobium leguminosarum bv trifolii Rt24.2 using Laser Doppler Velocimetry—A methodological study. J. Microbiol. Methods 2011, 85, 199–205. [Google Scholar] [CrossRef]

| PPs Formulations | Application Method | Culture Medium Abbreviation * |
|---|---|---|
| 8 g of plant pellets formulated with 5% molasses | Within teabags | PP5%m 8 g L−1 |
| 8 g of plant pellets formulated with 10% molasses | PP10%m 8 g L−1 | |
| 16 g of plant pellets formulated with 5% molasses | PP5%m 16 g L−1 | |
| 16 g of plant pellets formulated with 10% molasses | PP10%m 16 g L−1 | |
| 8 g of plant pellets formulated with 10% molasses | Without teabags | PPntb10%m 8 g L−1 |
| 16 g of plant pellets formulated with 10% molasses | PPntb10%m 16 g L−1 | |
| Yeast extract mannitol | YEM |
| ANOVA: Single Effect (Culture Media) | OD600nm | DW (g L−1) | Log CFU (mL−1) | nVCs | |
|---|---|---|---|---|---|
| YEM | 0.36 a | 2.54 a | 6.29 a | 0.20 a | |
| PP5%m 8 g L−1 | 0.23 c | 0.91 c | 6.11 a | −0.14 a | |
| PP5%m 16 g L−1 | 0.26 b | 1.78 b | 6.11 a | −0.06 a | |
| ANOVA: Two-way Interaction (Culture Media and Time) | OD600nm | ||||
| Time (day) | |||||
| 1 | 2 | 5 | 10 | 14 | |
| YEM | 0.3153 gh | 0.3722 fg | 0.5895 cd | 0.7272 a | 0.7218 a |
| PP5%m 8 g L−1 | 0.2345 ij | 0.2840 hi | 0.3015 hi | 0.4363 e | 0.5502 d |
| PP5%m 16 g L−1 | 0.1825 j | 0.3080 h | 0.4067 ef | 0.6480 bc | 0.6700 ab |
| LSD (0.05) | 0.06042 | ||||
| DW (g L−1) | |||||
| YEM | 1.510 cde | 2.089 bc | 2.832 a | 3.092 a | 3.171 a |
| PP5%m 8 g L−1 | 0.5047 h | 0.6193 gh | 0.7320 fgh | 1.337 efg | 1.368 def |
| PP5%m 16 g L−1 | 1.177 efgh | 1.271 efg | 1.777 cde | 2.070 bcd | 2.604 ab |
| LSD (0.05) | 0.7196 | ||||
| nVCs | |||||
| YEM | −0.9512 i | −0.7126 hi | 0.1715 e | 0.7847 d | 3.467 a |
| PP5%m 8 g L−1 | −0.5883 gh | −0.7225 hi | −0.7275 hi | −0.2375 f | 1.957 c |
| PP5%m 16 g L−1 | −0.9285 i | −0.4149 fg | −0.3875 fg | −0.7961 hi | 2.465 b |
| LSD (0.05) | 0.2834 | ||||
| ANOVA: Single Effect (Culture Media) | DW (g L−1) | |||||
| PPs Within Teabags | YEM | 3.29 b ± 0.35 | PPs Within Teabags | PPs Free, Without Teabags | ||
| PP5%m 8 g L−1 | 1.35 d ± 0.14 |  | ||||
| PP5%m 16 g L−1 | 1.67 d ± 0.15 | |||||
| PP10%m 8 g L−1 | 1.24 d ± 0.07 | |||||
| PP10%m 16 g L−1 | 2.32 c ± 0.17 | |||||
| PPs Free, Without Teabags | PPntb10%m 8 g L−1 | 4.34 a ± 0.36 | ||||
| PPntb10%m 16 g L−1 | 4.23 a ± 0.38 | |||||
| DW (g L−1) | ||||||
| ANOVA: Two-way Interaction (Culture Media and Time) | Culture Media | 4 h | 1 d | 5 d | 10 d | 15 d |
| YEM | 2.231 jklmn | 2.749 hijk | 3.205 fghi | 4.059 def | 4.256 cde | |
| PPs Within Teabags | PP5%m 8 g L−1 | 0.6473 r | 1.085 qr | 1.601 mnopq | 1.676 mnopq | 1.751 lmnopq |
| PP5%m 16 g L−1 | 1.058 qr | 1.392 nopqr | 1.846 lmnopq | 1.9100 klmnopq | 2.147 jklmno | |
| PP10%m 8 g L−1 | 1.058 qr | 1.2560 opqr | 1.454 mnopqr | 1.318 opqr | 1.137 pqr | |
| PP10%m 16 g L−1 | 1.7057 lmnopq | 2.0198 jklmnop | 2.3340 ijklm | 2.6660 hijk | 2.9160 ghij | |
| PPs Free, Without Teabags | PPntb10%m 8 g L−1 | 2.6900 hijk | 3.761 efg | 4.833 abcd | 5.140 abc | 5.295 a |
| PPntb10%m 16 g L−1 | 2.591 hijkl | 3.454 efgh | 4.326 bcde | 5.223 ab | 5.600 a | |
| LSD (0.05) | 0.9120 | |||||
| Culture Media | |||||||
|---|---|---|---|---|---|---|---|
| Variable | YEM | PP5%m 8 g L−1 | PP5%m 16 g L−1 | PP10%m 8 g L−1 | PP10%m 16 g L−1 | PPntb10%m 8 g L−1 | PPntb10%m 16 g L−1 |
| EC0 | 0.99 e * ± 0.00 | 1.70 c ± 0.00 | 2.28 b ± 0.02 | 1.40 d ± 0.00 | 2.45 a,b ± 0.00 | 1.20 d,e ± 0.00 | 2.68 a ± 0.00 |
| ECmin | 0.98 e ± 0.00 | 1.70 c ± 0.00 | 2.10 b ± 0.00 | 1.40 d ± 0.00 | 2.45 a,b ± 0.00 | 1.07 e ± 0.01 | 2.68 a ± 0.00 |
| ECfin | 1.04 e ± 0.01 | 1.93 c ± 0.03 | 2.45 b ± 0.30 | 1.97 c ± 0.10 | 2.75 b ± 0.06 | 1.46 d ± 0.12 | 3.47 a ± 0.04 |
| ECmin–EC0 | −0.01 | 0.00 | −0.18 | 0.00 | 0.00 | −0.13 | 0.00 |
| ECfin–ECmin | 0.06 | 0.23 | 0.35 | 0.57 | 0.30 | 0.39 | 0.80 |
| CV of EC | 3.39% | 5.46% | 10.79% | 16.03% | 5.55% | 12.67% | 11.50% |
| pH 0 | 7.28 a ± 0.02 | 7.20 a ± 0.10 | 7.25 a ± 0.05 | 7.00 a,b ± 0.00 | 6.50 b + 0.00 | 6.75 b ± 0.05 | 6.65 b ± 0.05 |
| pHmin | 5.10 d ± 0.1 | 5.50 c ± 0.00 | 6.00 b ± 0.00 | 6.40 a ± 0.10 | 6.30 a,b ± 0.00 | 5.68 c ± 0.09 | 5.47 c ± 0.04 |
| pHfin | 5.63 c ± 0.05 | 6.50 b ± 0.00 | 6.75 a,b ± 0.05 | 6.85 a ± 0.05 | 6.83 a ± 0.02 | 6.36 b ± 0.06 | 6.64 a,b ± 0.02 |
| pHmin–pH0 | −2.19 | −1.70 | −1.25 | −0.60 | −0.20 | −1.08 | −1.19 |
| pHfin–pHmin | 0.53 | 1.00 | 0.75 | 0.45 | 0.53 | 0.69 | 1.18 |
| CV of pH | 12.74% | 9.88% | 6.97% | 6.26% | 4.32% | 6.09% | 7.09% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Daanaa, H.-S.; Abdou, M.; Goda, H.A.; Abbas, M.T.; Hamza, M.A.; Sarhan, M.S.; Youssef, H.H.; Hamed, R.; El-Tahan, M.; Fayez, M.; et al. Plant Pellets: A Compatible Vegan Feedstock for Preparation of Plant-Based Culture Media and Production of Value-Added Biomass of Rhizobia. Sustainability 2020, 12, 8389. https://doi.org/10.3390/su12208389
A. Daanaa H-S, Abdou M, Goda HA, Abbas MT, Hamza MA, Sarhan MS, Youssef HH, Hamed R, El-Tahan M, Fayez M, et al. Plant Pellets: A Compatible Vegan Feedstock for Preparation of Plant-Based Culture Media and Production of Value-Added Biomass of Rhizobia. Sustainability. 2020; 12(20):8389. https://doi.org/10.3390/su12208389
Chicago/Turabian StyleA. Daanaa, Hassan-Sibroe, Mennatullah Abdou, Hanan A. Goda, Mohamed T. Abbas, Mervat A. Hamza, Mohamed S. Sarhan, Hanan H. Youssef, Reem Hamed, Mahmoud El-Tahan, Mohamed Fayez, and et al. 2020. "Plant Pellets: A Compatible Vegan Feedstock for Preparation of Plant-Based Culture Media and Production of Value-Added Biomass of Rhizobia" Sustainability 12, no. 20: 8389. https://doi.org/10.3390/su12208389






