Optimization of Reactor Temperature for Continuous Anaerobic Digestion of Cow Manure: Bangladesh Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
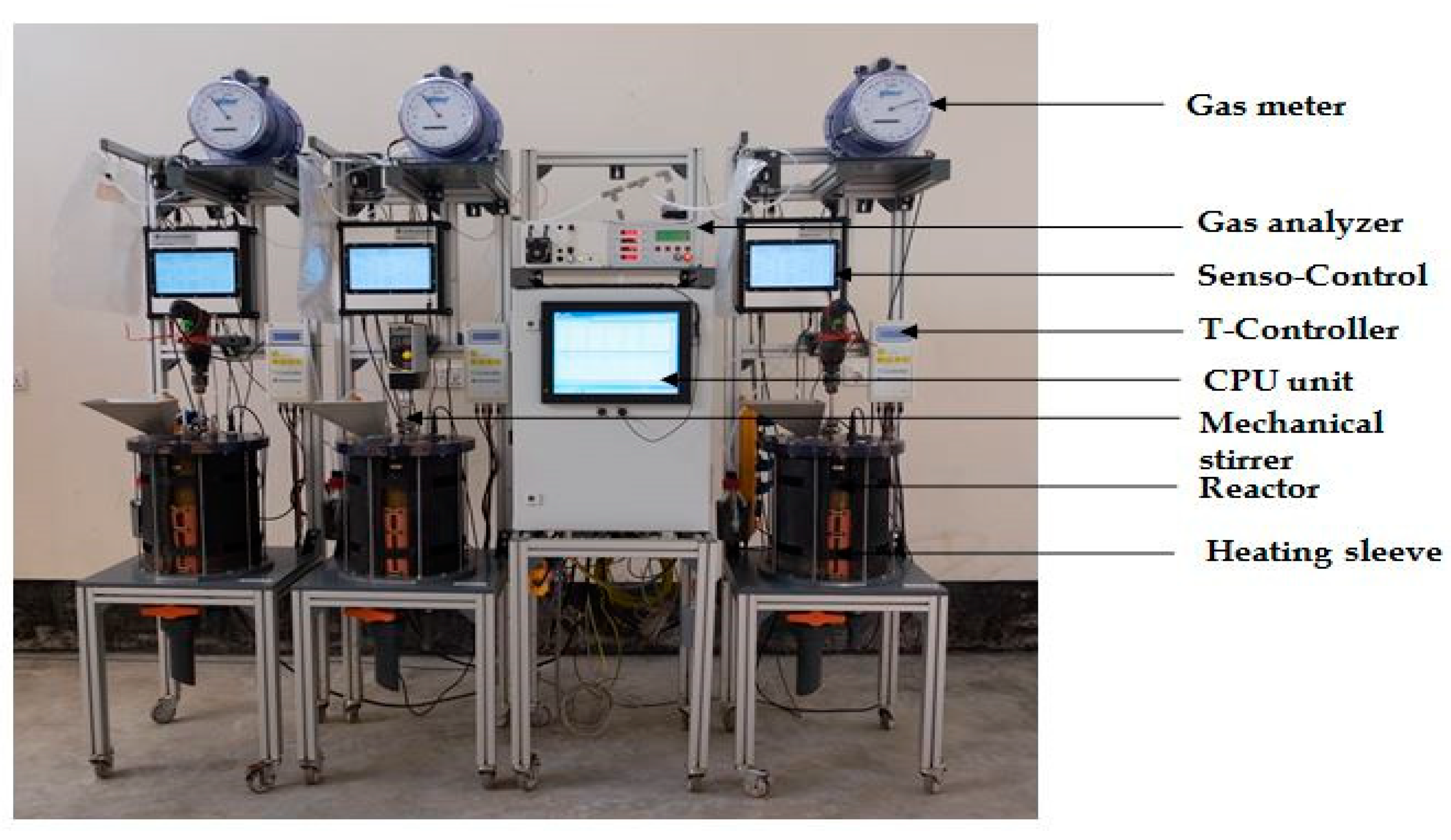
2.2. Experimental Setup
2.3. Experimental Procedure
2.4. Analytical Methods
2.5. Calculation and Statistical Analysis
2.6. Optimization
3. Results and Discussions
3.1. Substrate Characteristics
3.2. Effect of Temperature on pH
3.3. Effect of Temperature on VFA
3.4. Effect of Temperature on TS and VS Degradation
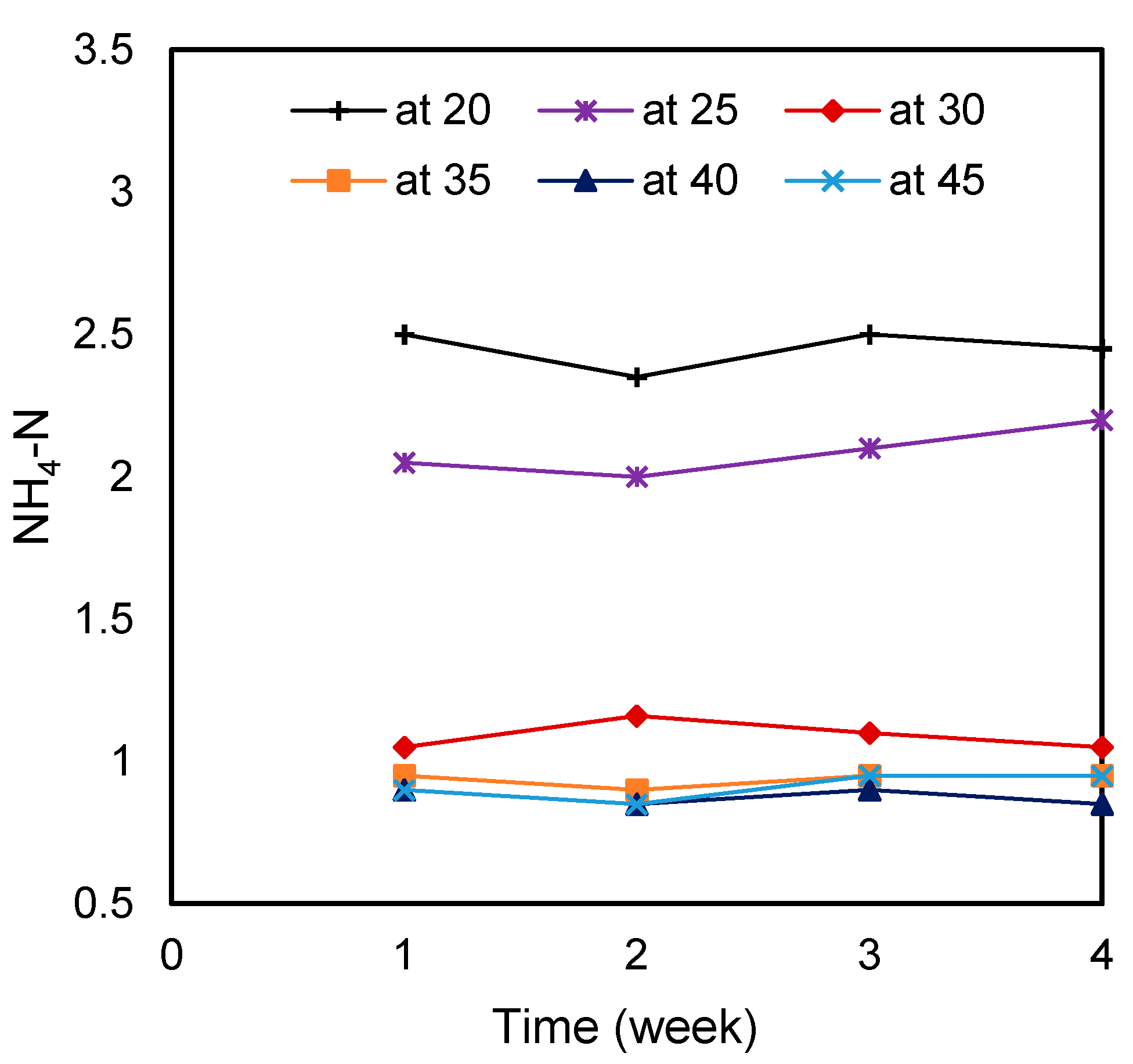
3.5. Effect of Temperature on Ammonia-Nitrogen
3.6. Effect of Temperature on Biogas Yield and Composition
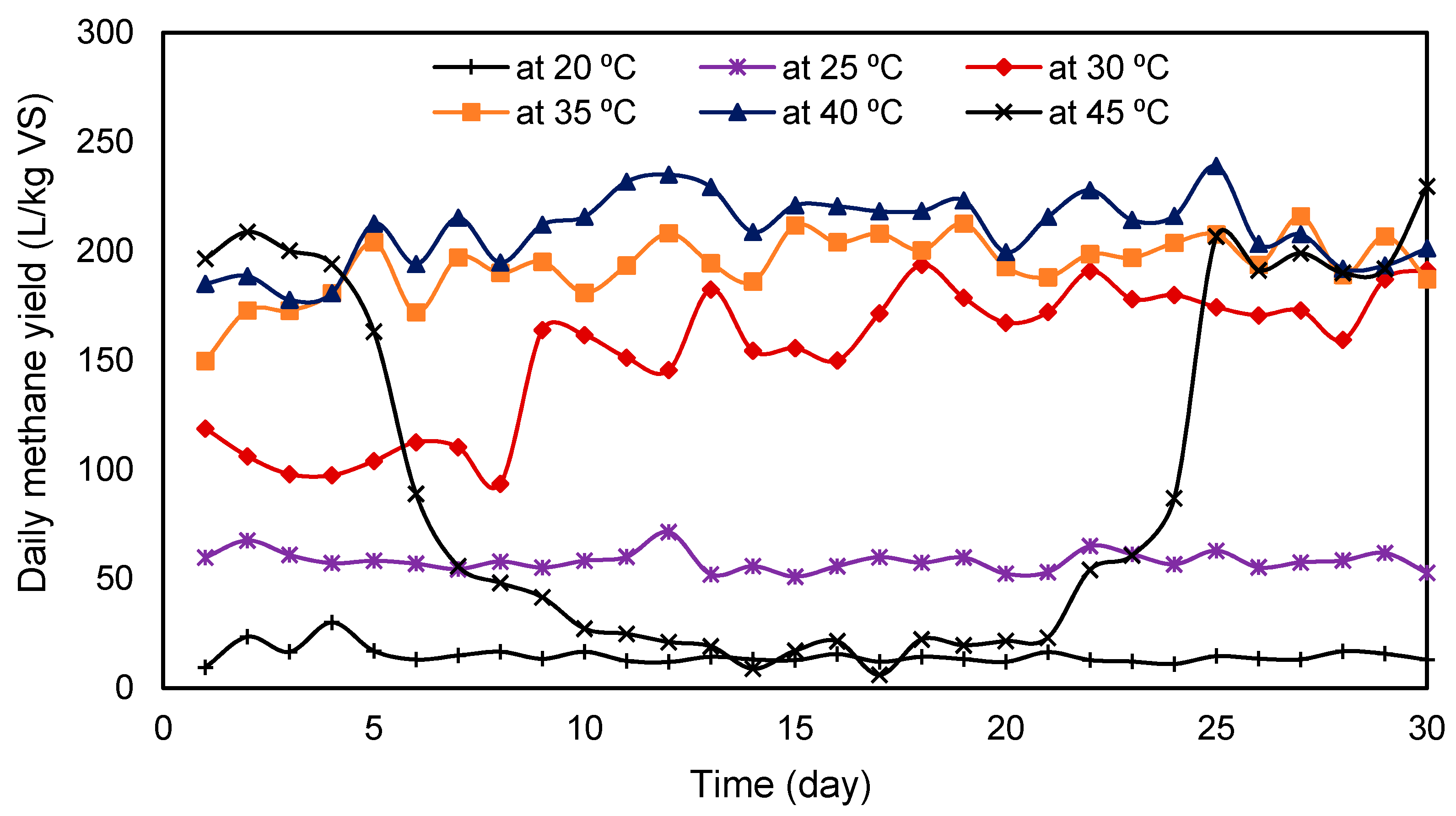
3.7. Effect of Temperature on Methane Yield
3.8. Model Fitting and Optimization
3.9. Model Validation
4. Conclusions
- ▪
- The pH of the reactors at 30 °C, 35 °C and 40 °C was stable and favorable for biogas production. However, at 45 °C the pH was low and varied significantly. At lower (20 °C and 25 °C) temperatures, pH values were stable for a majority of the time, but the average levels were lower than those of at 30, 35 and 40 °C. Unstable and lower pH values at digester temperatures of 20, 25 ad 45 °C were found to be correlated with the higher levels of VFA accumulation, which in turn was correlated with the decreased levels of biogas production
- ▪
- Total methane production increased with the temperature from 20 to 40 °C. At 45 °C, VFA accumulation escalated and caused a process imbalance and as a result reduced the biogas yield. Among the six temperatures used, the reactor at 40 °C produced the highest amount of average methane, while the reactor at 35 °C produced only 7.59% less methane compared to that of at 40 °C.
- ▪
- Statistical analysis results from the Minitab® showed the reactor temperature of 35.82 °C as the optimum for maximizing biogas production from the cow-dung.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasanuzzaman, M.; Islam, M.A.; Rahim, N.A.; Yanping, Y. Energy demand. Energy Sustain. Dev. 2020, 41–87. [Google Scholar] [CrossRef]
- Gómez-Camacho, C.E.; Ruggeri, B. Energy Sustainability Analysis (ESA) of Energy-Producing Processes: A Case Study on Distributed H2 Production. Sustainability 2019, 11, 4911. [Google Scholar] [CrossRef] [Green Version]
- Jeung, J.H.; Chung, W.J.; Chang, S.W. Evaluation of Anaerobic Co-Digestion to Enhance the Efficiency of Livestock Manure Anaerobic Digestion. Sustainability 2019, 11, 7170. [Google Scholar] [CrossRef] [Green Version]
- BPDB (Bangladesh Power Development Board). Available online: https://www.bpdb.gov.bd/bpdb_new/resourcefile/annualreports/annualreport_1574325376_Annual_Report_2018-19.pdf (accessed on 10 July 2020).
- Hasan, A.S.M.M.; Ammenberg, J. Biogas potential from municipal and agricultural residual biomass for power generation in Hazaribagh, Bangladesh—A strategy to improve the energy system. Renew. Energy Focus 2019, 29, 14–23. [Google Scholar] [CrossRef]
- Masud, M.H.; Nuruzzaman, M.; Ahamed, R.; Ananno, A.A.; Tomal, A.N.M.A. Renewable energy in Bangladesh: Current situation and future prospect. Int. J. Sustain. Energy 2019, 1–44. [Google Scholar] [CrossRef]
- Rahman, A. Green Banking and Sustainable Development: The Case of Bangladesh. Available online: Bb.org.bd/governor/speech/feb022013gse.pdf (accessed on 10 July 2020).
- BBS (Bangladesh Bureau of Statistics). Available online: https://bbs.portal.gov.bd/sites/default/files/files/bbs.portal.gov.bd/page/86b66335_4fa7_4143_a034_9abe3c537553/2020-05-15-16-25-34428631989cd04e5d8609c8ae6aed5c.pdf (accessed on 10 July 2020).
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. Oxf. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef] [Green Version]
- GEKH (Green Energy Knowledge Hub). Survey Report on Biogas Plans in Bangladesh by Green Energy Knowledge Hub; Bangladesh Agricultural University: Mymensingh, Bangladesh, 2015. [Google Scholar]
- Hasan, A.B.M.S.; Rahman, M.Z. Change in Temperature over Bangladesh Associated with Degrees of Global Warming. Asian J. Appl. Sci. Eng. 2013, 2, 62–75. [Google Scholar]
- Wang, S.; Ma, F.; Ma, W.; Wang, P.; Zhao, G.; Lu, X. Influence of Temperature on Biogas Production Efficiency and Microbial Community in a Two-Phase Anaerobic Digestion System. Water 2019, 11, 133. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; De Francisci, D.; Kougias, P.G.; Laura, T.; Zhu, X.; Angelidaki, I. New steady-state microbial community compositions and process performances in biogas reactors induced by temperature disturbances. Biotechnol. Biofuels 2015, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Garba, B. Effect of temperature and retention period on biogas production from ligrocellulosic material. Renew. Energy 1996, 9, 938–941. [Google Scholar] [CrossRef]
- Leven, L.; Eriksson, A.R.B.; Schnürer, A. Effect of process temperature on bacterial and archael communities in two methanogenic bioreactors treating organic household waste. FEMS Microbiol. Ecol. 2007, 59, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Chattopadhyay, P.; Shome, A.; Banerjee, S.N.; Chakraborty, A.K.; Mathew, A.K.; Chaudhury, S. An overview of physico-chemical mechanisms of biogas production by microbial communities: A step towards sustainable waste management. Biotech. 2016, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C. Effect of Temperature on Biogas Production in Anaerobic Treatment of Domestic Wastewater UASB System in Hammarby Sjöstadsverk. Master’s Thesis, Royal Institute of Technology (KTH), Stockholm, Sweden, 2011. [Google Scholar]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Dobre, P.; Matei, F.; Nicolae, F. Main factors affecting biogas production-an overview. Rom. Biotechnol. Lett. Romenia 2014, 19, 9283–9296. [Google Scholar]
- Alvarez, J.A.; Zapico, C.A.; Gomez, M.; Ruiz, I.; Soto, M. Anaerobic treatment and pre-treatment of municipal wastewater at low ambient temperature. In Proceedings of the 9th World Congress Anaerobic Digestion, Antwerp, Belgium, 2–6 September 2001. [Google Scholar]
- Zhou, S.; Zhang, J.; Zou, G.; Riya, S.; Hosomi, M. Mass and Energy Balances of Dry Thermophilic Anaerobic Digestion Treating Swine Manure Mixed with Rice Straw. Biotechnol. Res. Int. 2015, 895015. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, S.A.; Rahaman, S.; Rahman, M.; Yousuf, A. Anaerobic digestion of kitchen waste to produce biogas. Procedia. Eng. 2014, 90, 657–662. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Saha, C.K.; Feng, L.; Møller, H.B.; Alam, M.M. Anaerobic digestion of agro-industrial wastes of Bangladesh: Influence of total solids content. Eng. Agric. Environ. Food 2019. [Google Scholar] [CrossRef]
- Akter, S.; Tasnim, S.M.; Saha, C.K.; Nandi, R.; Huda, M.S. Bio-energy potential of different food wastes. J. Bangladesh Agric. Univ. 2020, 18, 138–144. [Google Scholar] [CrossRef]
- Tasnim, T.; Behera, S.K.; Zafar, M.; Park, H. Batch anaerobic digestion of simulated Bangladeshi food waste: Methane production at different inoculum-to-substrate ratios and kinetic analysis. Int. J. Glob. Warm. 2016, 9, 95–101. [Google Scholar] [CrossRef]
- Miah, M.R.; Rahman, A.K.M.L.; Akanda, M.R.; Pulak, A.; Rouf, M.A. Production of biogas from poultry litter mixed with the co-substrate cow dung. J. Taibah Univ. Sci. 2016, 10, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Hinks, J.; Edwards, S.; Sallis, P.J.; Caldwell, G.S. The steady state anaerobic digestion of Laminaria hyperborean—Effect of hydraulic residence on biogas production and bacterial community composition. Bioresour. Technol. 2013, 143, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Feng, L.; Moset, V.; Moller, H.B. Estimation of Methane Yields in Continuous Biogas Reactors Using Kinetic and Mass Flow Models. Chem. Eng. Technol. 2018, 41, 761–767. [Google Scholar] [CrossRef]
- Nandi, R.; Saha, C.K.; Huda, M.S.; Alam, M.M. Effect of Mixing on Biogas Production from Cowdung. Eco Friendly Agric. J. 2017, 10, 7–13. [Google Scholar]
- APHA. Standard Methods for the Examination of Waste Water, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Møller, H.B.; Ward, A.J. Modeling Volatile Fatty Acid Concentration in Livestock Manure–Based Anaerobic Digesters by Simple Titration. Environ. Eng. Sci. 2011, 28, 507–513. [Google Scholar] [CrossRef]
- Waskman, S.A. Chemical nature of organic matter or humus in sols, peat bogs and composts. J. Chem. Educ. 1935, 12, 511–519. [Google Scholar] [CrossRef]
- Abubakar, B.S.U.I.; Ismail, N. Anaerobic digestion of cow dung for biogas production. ARPN J. Eng. Appl. Sci. 2012, 7, 169–172. [Google Scholar]
- Rahman, M.A.; Moller, H.B.; Saha, C.K.; Alam, M.M.; Wahid, R.; Feng, L. Optimal ratio for anaerobic co-digestion of poultry droppings and lignocellulosic-rich substrates for enhanced biogas production. Energy Sustain. Dev. 2017, 39, 59–66. [Google Scholar] [CrossRef]
- Giovanna, G.; Claudia, C.; Filomena, D.; Stefania, P.; Biagio, M.; Mario, M. Does the C/N ratio really affect the bio-methane yield? A three years investigation of Buffalo manure digestion. Chem. Eng. Trans. 2016, 49, 463–468. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Wang, X.; Cheng, S.; Yin, F. Study on anaerobic co-digestion of cow manure, maize straw and vegetable waste. In Proceedings of the 2015 International Conference on Applied Science and Engineering Innovation, Henan, China, 30–31 August 2015; pp. 357–363. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Gupta, R.; Garg, V. Organic manure production from cow dung and biogas plant slurry by vermicomposting under field conditions. Int. J. Recycl. Org. Waste Agric. 2013, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Komemoto, K.; Lim, Y.G.; Nagao, N.; Onoue, Y.; Niwa, C.; Toda, C. Effect of temperature on VFA’s and biogas production in anaerobic solubilisation of food waste. Waste Manag. 2009, 29, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Møller, H.B.; Saha, C.K.; Alam, M.M. The effect of temperature on the anaerobic co-digestion of poultry droppings and sugar mill press mud. Biofuels 2019. [Google Scholar] [CrossRef]
- Karakashev, D.; Bastone, D.; Angelidaki, I. Influence of environmental conditions on methanogenic compositions in anaerobic biogas reactors. Appl. Environ. Microbiol. 2005, 71, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Vanegas, C.; Bartlett, J. Anaerobic Digestion of Laminaria digitata: The Effect of Temperature on Biogas Production and Composition. Waste Biomass Valorization 2013, 4, 509–515. [Google Scholar] [CrossRef]
- Mao, C.; Zhang, T.; Wang, X.; Feng, Y.; Ren, G.; Yang, G. Process performance and methane production optimizing of anaerobic co-digestion of swine manure and corn straw. Sci. Rep. 2017, 7, 9379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sneath, R.W.; Burton, C.H.; Williams, A.G. Continuous aerobic treatment of piggery slurry for odor control scaled up to a farm-size unit. J. Agric. Eng. Res. 1992, 53, 81–92. [Google Scholar] [CrossRef]
- Hansson, G. Methane production from marine, green macro algae. Resour. Conserv. 1983, 8, 185–194. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Effect of temperature on fermentative hydrogen production by mixed cultures. Int. J. Hydrog. Energy 2008, 33, 5392–5397. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Ramaraj, R.; Unpaprom, Y. Effect of temperature on the performance of biogas production from Duckweed. Chem. Sci. 2016, 1, 58–66. [Google Scholar]
- Liu, Z.; Lv, J. The effect of total solids concentration and temperature on biogas production by anaerobic digestion. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3534–3541. [Google Scholar] [CrossRef]
- Obiukwu, O.O.; Grema, L.U. The Optimum Mesophilic Temperature of Batch Process Biogas Production from Animal-based Wastes. Res. J. Appl. Sci. Eng. Technol. 2014, 16, 1772–1776. [Google Scholar] [CrossRef]
- Pfeffer, J.T. Temperature effects on anaerobic fermentation of domestic refuse. Biotechnol. Bioeng. 1974, 16, 771–787. [Google Scholar] [CrossRef]
- Franqueto, R.; da Silva, J.D.; Konig, M. Effect of Temperature Variation on Codigestion of Animal Waste and Agricultural Residue for Biogas Production. Bioenergy Res. 2020, 13, 630–642. [Google Scholar] [CrossRef]
- Membere, E.; Sallis, P. Effect of temperature on kinetics of biogas production from macroalgae. Bioresour. Technol. 2018, 263, 410–417. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of methane production in anaerobic digestion process: A review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Zhou, X.; Wang, Q.; Wu, Y.; Saino, M.; Bai, X. Study on the bio-methane yield and microbial community structure in enzyme enhanced anaerobic co-digestion of cow manure and corn straw. Bioresour. Technol. 2016, 219, 150–157. [Google Scholar] [CrossRef]
- Connelly, R. Second-Generation Biofuel from High-Efficiency Algal-Derived Biocrude. In Bioenergy Research: Advances and Applications, 1st ed.; Gupta, V., Touhy, M., Kubicek, C., Saddler, J., Xu, F., Eds.; Elsevier: Waltham, MA, USA, 2014; pp. 153–170. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Chynoweth, D.; Isaacson, R. Anaerobic Digestion of Biomass; Elsevier Applied Science: New York, NY, USA, 1987. [Google Scholar]
- Chen, P.H. Factors Influencing Methane Fermentation of Micro-algae. Ph.D. Thesis, University of California, Berkeley, CA, USA, 1987. [Google Scholar]
- Artsupho, L.; Jutakridsada, P.; Laungphairojana, A.; Rodriguez, J.F.; Kamwilaisak, K. Effect of Temperature on Increasing Biogas Production from Sugar Industrial Wastewater Treatment by UASB Process in Pilot Scale. Energy Procedia. 2016, 100, 30–33. [Google Scholar] [CrossRef] [Green Version]
- Hobson, P.N.; Bousfield, S.; Summers, R.; Mills, P.J. Anaerobic digestion of piggery and poultry wastes. In Anaerobic Digestion; Stafford, B.E., Wheatley, B.I., Hughes, D.E., Eds.; Applied Science Publishers: London, UK, 1980; pp. 237–253. [Google Scholar]
- Varel, V.H.; Chen, T.H.; Hashimoto, A.G. Thermophilic and mesophilic methane production from Spirulina maxima. Resour. Conserv. Recycl. 1988, 1, 19–26. [Google Scholar] [CrossRef]
- Samson, R.; LeDuy, A. Detailed study of anaerobic digestion of Spirulina maxima algal biomass. Biotechnol. Bioeng. 1986, 28, 1014–1023. [Google Scholar] [CrossRef] [PubMed]








| Parameter | Value |
|---|---|
| TS (%) | 17.30 ± 0.08 |
| VS (%) | 14. 51 ± 0.09 |
| pH | 7.08 ± 0.03 |
| VFA (g/L) | 4.03 ± 0.05 |
| Alkalinity (g/L) | 0.04 ± 0.00 |
| NH4-N (g/L) | 1.66 ± 0.03 |
| Total Nitrogen (g/L) | 3.16 ± 0.01 |
| C/N ratio | 26.02 ± 0.22 |
| Parameter | 20 °C | 25 °C | 30 °C | 35 °C | 40 °C | 45 °C |
|---|---|---|---|---|---|---|
| TS (%) | 7.87 ± 0.18 | 7.19 ± 0.20 | 6.39 ± 0.19 | 6.06 ± 0.31 | 5.95 ± 0.27 | 6.80 ± 0.39 |
| VS (%) | 6.37 ± 0.10 | 5.84 ± 0.13 | 5.16 ± 0.80 | 4.89 ± 0.30 | 4.72 ± 0.23 | 5.58 ± 0.40 |
| VFA (g/L) | 3.61 ± 0.22 | 2.20 ± 0.16 | 1.33 ± 0.19 | 1.10 ± 0.12 | 1.03 ± 0.08 | 3.02 ± 1.75 |
| NH4-N (g/L) | 2.45 ± 0.07 | 2.09 ± 0.09 | 1.09 ± 0.05 | 0.94 ± 0.03 | 0.88 ± 0.03 | 0.91 ± 0.05 |
| Temperature (°C) | Biogas Volume (L/kg VS) (Mean ± SD) | CH4 (%) | CH4 (L/kg VS) | pH |
|---|---|---|---|---|
| 20 | 26.44 ± 7.01 a | 55.63 ± 0.87 a | 14.70 ± 3.88 a | 6.16 ± 0.04 a |
| 25 | 101.80 ± 7.62 b | 57.16 ± 0.94 b | 58.20 ± 4.55 b | 6.28 ± 0.04 b |
| 30 | 230.28 ± 47.03 c | 66.37 ± 1.40 c | 153.05 ± 32.11 c | 6.44 ± 0.03 a |
| 35 | 289.09 ± 22.59 d | 67.05 ± 0.8 c | 193.78 ± 14.54 d | 6.45 ± 0.03 a |
| 40 | 312.43 ± 25.41 d | 67.16 ± 1.55 c | 209.70 ± 16.23 d | 6.45 ± 0.02 a |
| 45 | 142.4 ± 116.8 e | 61.83 ± 6.74 d | 94.6 ± 82.3 e | 6.13 ± 0.30 b |
| p-value (temperature) | 0.000 | 0.000 | 0.000 | 0.000 |
| p-value(time) | 0.872 | 0.513 | 0.848 | 0.398 |
| Methane Yield (L/kg VS) | VSD (%) | |||
|---|---|---|---|---|
| Model | Standard Error of Regression (S) | Regression Coefficient (R2) | Standard Error of Regression (S) | Regression Coefficient (R2) |
| Linear | 68.36 | 37.97% | 7.37 | 42.49% |
| Quadratic | 36.79 | 86.53% | 3.26 | 91.55% |
| Cubic | 12.04 | 99.04% | 1.48 | 98.84% |
| Predictor | Response Variable | Regression Equation |
|---|---|---|
| Temperature (T) | Methane yield (Y) | Y = 1020 − 129.1 T + 5.158 T2 − 0.06102 T3 |
| VSD | VSD (%) = 75.62 − 9.473 T + 0.4195 T2 − 0.005216 T3 |
| Methane Yield (L/kg VS) | VSD (%) | |||
|---|---|---|---|---|
| Temperature (°C) | Actual | Predicted | Actual | Predicted |
| 20 | 14.70 | 13.04 | 12.16 | 12.23 |
| 25 | 58.20 | 62.81 | 19.37 | 19.48 |
| 30 | 153.05 | 141.66 | 28.96 | 28.15 |
| 35 | 193.78 | 203.82 | 32.74 | 34.32 |
| 40 | 209.70 | 203.52 | 35.12 | 34.08 |
| 45 | 94.63 | 95.00 | 23.15 | 23.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandi, R.; Saha, C.K.; Sarker, S.; Huda, M.S.; Alam, M.M. Optimization of Reactor Temperature for Continuous Anaerobic Digestion of Cow Manure: Bangladesh Perspective. Sustainability 2020, 12, 8772. https://doi.org/10.3390/su12218772
Nandi R, Saha CK, Sarker S, Huda MS, Alam MM. Optimization of Reactor Temperature for Continuous Anaerobic Digestion of Cow Manure: Bangladesh Perspective. Sustainability. 2020; 12(21):8772. https://doi.org/10.3390/su12218772
Chicago/Turabian StyleNandi, Rajesh, Chayan Kumer Saha, Shiplu Sarker, Md. Sanaul Huda, and Md. Monjurul Alam. 2020. "Optimization of Reactor Temperature for Continuous Anaerobic Digestion of Cow Manure: Bangladesh Perspective" Sustainability 12, no. 21: 8772. https://doi.org/10.3390/su12218772








