Statistical Approach for Assessing the Suitability of Substrates for a Biogas Plant
Abstract
:1. Introduction
2. Methods and Methodology
3. Results
3.1. Analysis of Variability of CH4 and H2S Production from Individual Substrates
3.2. Selection of Substrates with Stable Production
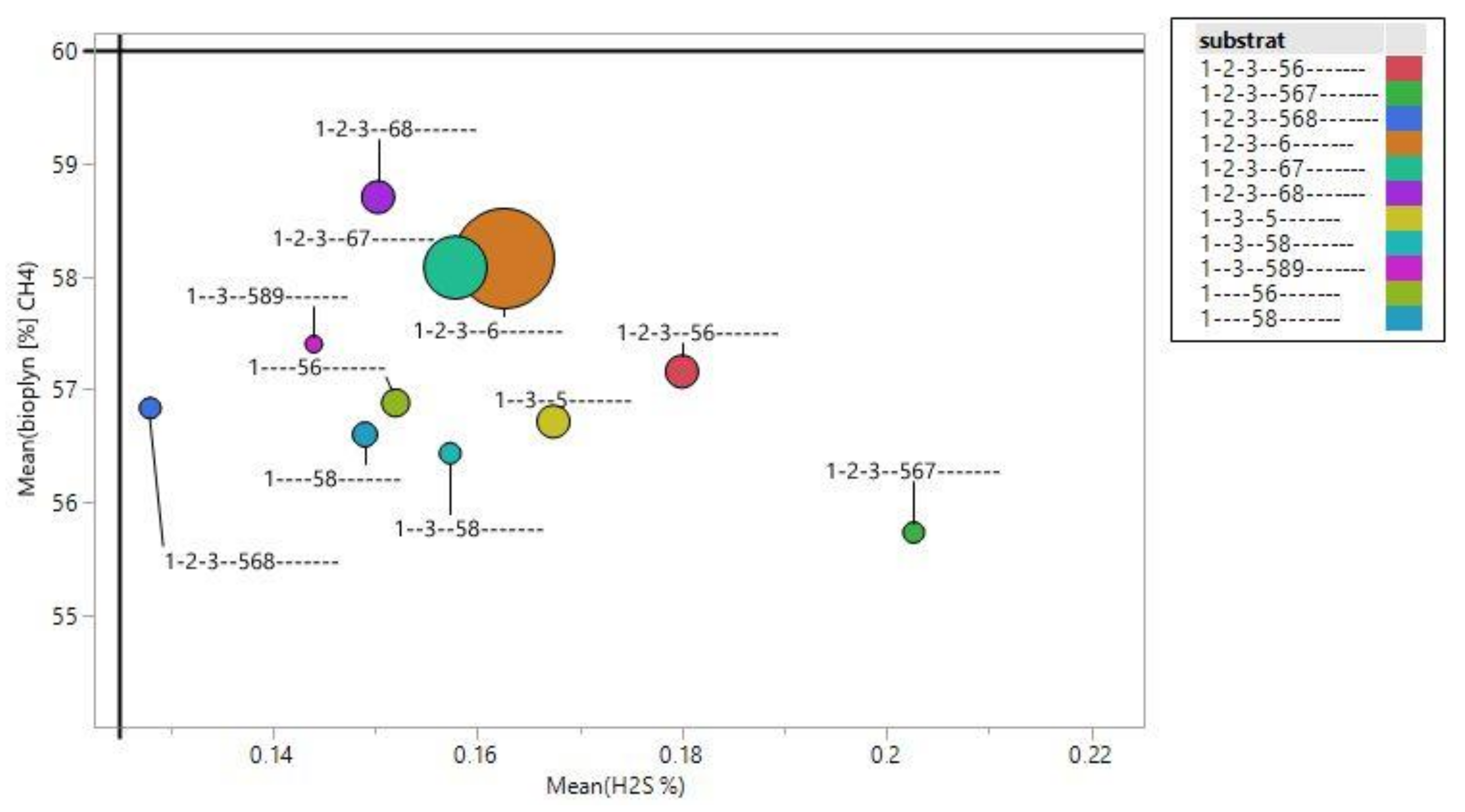
3.3. Assessment of Substrate Quality
- I.
- Quadrant with CH4 value greater than 60% and H2S value less than 0.125%.
- II.
- Quadrant with CH4 greater than 60% and H2S greater than 0.125%.
- III.
- Quadrant with CH4 less than 60% and H2S less than 0.125%.
- IV.
- Quadrant with CH4 less than 60% and H2S greater than 0.125%.
3.4. Suitable Substrates
3.5. Unsuitable Substrates
- 5-Rye [kg]
- 7-Oil [kg]
- 12-Other 4 [kg]
- 14-Corn chips silage [kg]
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Banja, M.; Jégard, M.; Motola, V.; Sikkema, R. Support for biogas in the EU electricity sector—A comparative analysis. Biomass Bioenergy 2019, 128. [Google Scholar] [CrossRef]
- CBA (Česká Bioplynová Asociace). National Technology Platform for Biogas. 2019. Available online: https://www.czba.cz/ (accessed on 1 March 2020).
- EBA (European Biogas Association). EBA Statistical Report 2018. Renewable Energy House. 2019. Available online: www.europeanbiogas.eu (accessed on 1 April 2019).
- Energie Portal. Biogas Station in Slovakia. 2017. Available online: https://www.energie-portal.sk/Dokument/bioplynove-stanice-v-sr-100191.aspx (accessed on 3 January 2017).
- Energie Portal. Additional Subsidies from Eurofunds go to Biogas Plants. 2019. Available online: https://www.energie-portal.sk/Dokument/na-bioplynove-stanice-smeruju-dalsie-dotacie-z-eurofondov-105302.aspx (accessed on 25 July 2017).
- Appunn, K. Bioenergy—The Troubled Pillar of the Energiewende. 2016. Available online: https://www.cleanenergywire.org/dossiers/bioenergy-germany (accessed on 30 September 2016).
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-Santana, P.I.; Colón, J.; Ramírez, M.; Lafuente, F.J.; Gabriel, D.; Cantero, D. Life cycle assessment of different physical-chemical and biological technologies for biogas desulfurization in sewage treatment plants. J. Clean. Prod. 2018, 181, 663–674. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Bolvin, S. A review of biegas purification processes. Biofuels Bioprod. Biorefin. 2009, 3, 42–47. [Google Scholar] [CrossRef]
- Salehi, R.; Chaiprapat, S. Single-/triple-stage biotrickling filter treating a H2S-rich biogas stream: Statistical analysis of the effect of empty bed retention time and liquid recirculation velocity. J. Air Waste Manag. Assoc. 2019, 69, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Andriani, D.; Rajani, A.; Santosa, A.; Saepudin, A.; Wresta, A.; Atmaja, T.D. A review on biogas purification through hydrogen sulphide removal. IOP Conf. Ser. Earth Environ. Sci. 2020, 483, 1–11. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/483/1/012034/pdf (accessed on 1 June 2020). [CrossRef]
- Afridi, Z.U.R.; Qammar, N.W. Technical Challenges and Optimization of Biogas Plants. CBEN 2020, 7, 119–129. [Google Scholar] [CrossRef]
- Petersson, A. Biogas cleaning. In The Biogas Handbook; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 329–341. [Google Scholar] [CrossRef]
- Smatanova, M.; Biogas Station. Central Institute for Supervising and Testing in Agriculture. Available online: http://eagri.cz/public/web/file/214721/_2_BPS.pdf (accessed on 28 September 2020).
- Bernat, K.; Kulikowska, D.; Wojnowska-Baryła, I.; Zaborowska, M.; Pasieczna-Patkowska, S. Thermophilic and mesophilic biogas production from PLA-based materials: Possibilities and limitations. Waste Manag. 2021, 119, 295–305. [Google Scholar] [CrossRef]
- Zaefferer, M.; Gaida, D.; Bartz-Beielstein, T. Multi-fidelity modeling and optimization of biogas plants. Appl. Soft Comput. 2016, 48, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No. 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef]
- Olubunmi, O.A.; Abiodun, E.A.; Adesina, A.O.; Pourianejad, S.; Zentner, A.; Dornack, C. Stabilization of anaerobic co-digestion of biowaste using activated carbon of coffee ground biomass. Bioresour. Technol. 2021, 319. [Google Scholar] [CrossRef]
- Gaida, D.; Wolf, C.; Bongards, M.; Bäck, T. Matlab toolbox for biogas plant modelling and optimization. Prog. Biogas II 2011, 2, 67–70. [Google Scholar]
- Schoen, M.A.; Sperl, D.; Gadermaier, M.; Goberna, M.; Franke-Whittle, I.; Insam, H.; Ablinger, J.; Wett, B. Population dynamics at digester overload conditions. Bioresour. Technol. 2009, 100, 5648–5655. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; McLoone, S.; Bongards, M. Biogas plant optimization using genetic algorithms and particle swarm optimization. In Proceedings of the 16th IET Irish Signals and Systems Conference 2008, Galway, Ireland, 18–19 June 2008; pp. 244–249. [Google Scholar]
- Ziegenhirt, J.; Bartz-Beielstein, T.; Flasch, O.; Konen, W.; Zaefferer, M. Optimization of biogas production with computational intelligence a comparative study. In Proceedings of the IEEE Congres on Evolutionary Computation, Barcelona, Spain, 18–23 July 2010; pp. 1–8. [Google Scholar]
- Hansen, N.; Ostermeier, A. Adapting arbitrary normal mutation distributions in evolution strategies: The covariance matrix adaptation. In Proceedings of the 1996 IEEE International Conference on Evolutionary Computation, Nagoya, Japan, 20–22 May 1996; pp. 312–317. [Google Scholar]
- Hansen, N. The CMA evolution strategy: A comparing review. In Towards a New Evolutionary Computation, Studies in Fuzziness and Soft Computing; Lozano, J.A., Larranaga, P., Inza, I., Bengoetxea, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 192, pp. 75–102. [Google Scholar]
- Storn, R.; Price, K. Differential evolution—A simple and efficient heuristic for global optimization over continuous spaces. J. Glob. Optim. 1997, 11, 341–359. [Google Scholar] [CrossRef]
- Bartz-Beielstein, T.; Parsopoulos, K.E.; Vrahatis, M.N. Design and analysis of optimization algorithms using computational statistics. Appl. Numer. Anal. Comput. Math. 2004, 1, 413–433. [Google Scholar] [CrossRef]
- Forrester, A.I.; Sóbester, A.; Keane, A.J. Multi-fidelity optimization via surrogate modelling. Proc. R. Soc. A Math. Phys. Eng. Sci. 2007, 463, 3251–3269. [Google Scholar] [CrossRef]
- Cressie, N. The origins of kriging. Math. Geol. 1990, 22, 239–252. [Google Scholar] [CrossRef]
- Matheron, G. Principles of geostatistics. Econ. Geol. 1963, 58, 1246–1266. [Google Scholar] [CrossRef]
- Jones, D.; Schonlau, M.; Welch, W. Efficient global optimization of expensive black—Box functions. J. Glob. Optim. 1998, 13, 455–492. [Google Scholar] [CrossRef]
- Drucker, H.; Burges, C.J.C.; Kaufman, L.; Smola, A.J.; Vapnik, V. Support Vector Regression Machines; Mozer, M., Jordan, M.I., Petsche, T., Eds.; NIPS; MIT Press: Cambridge, MA, USA, 1996; pp. 155–161. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.H. Multivariate adaptive regression splines. Ann. Stat. 1991, 19, 1–141. [Google Scholar] [CrossRef]
- Allegue, L.B.; Hinge, J. Biogas upgrading Evaluation of methods for H2S removal. Available online: https://www.teknologisk.dk/_/media/60599_Biogas%20upgrading.%20Evaluation%20of%20methods%20for%20H2S%20removal.pdf (accessed on 1 December 2014).
- Kapoor, R.; Ghosh, P.; Kumar, M.; Vijay, V.K. Evaluation of biogas upgrading technologies and future perspectives: A review. Environ. Sci. Pollut. Res. 2019, 26, 11631–11661. [Google Scholar] [CrossRef]
- Agneessens, L.M.; Ottosen, L.D.M.; Voigt, N.V.; Nielsen, J.L.; de Jonge, N.; Fischer, C.H.; Kofoed, M.V.W. In-situ biogas upgrading with pulse H2 additions: The relevance of methanogen adaption and inorganic carbon level. Bioresour. Technol. 2017, 233, 256–263. [Google Scholar] [CrossRef]
- Boontawee, S.; Koonaphapdeelert, S. In-situ biomethane enrichment by recirculation of biogas channel digester effluent using gas stripping column. Energy Procedia 2016, 89, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Marin, D.; Vega, M.; Lebrero, R.; Munoz, R. Influence of the diffuser type and liquid-to-biogas ratio on biogas upgrading performance in an outdoor pilot scale high rate algal pond. J. Water Process Eng. 2020, 37. [Google Scholar] [CrossRef]
- Armah, E.K.; Chetty, M.; Deenadayalu, N. Biogas production from sugarcane bagasse with South African industrial wastewater and novel kinetic study using response surface methodology. Sci. Afr. 2020, 10. [Google Scholar] [CrossRef]
- Marin, D.; Carmona-Martinez, A.A.; Lebrero, R.; Munoz, R. Optimization of a chemical scrubbing process based on a Fe-EDTA-carbonate based solvent for the simultaneous removal of CO2 and H2S from biogas. Fuel 2020, 275. [Google Scholar] [CrossRef]
- Jamalluddin, M.F.; Zainol, N.; Sharif, N.S.A.M. Troubleshooting on biogas production by using factorial analysis in sewage treatment plant (STP). Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
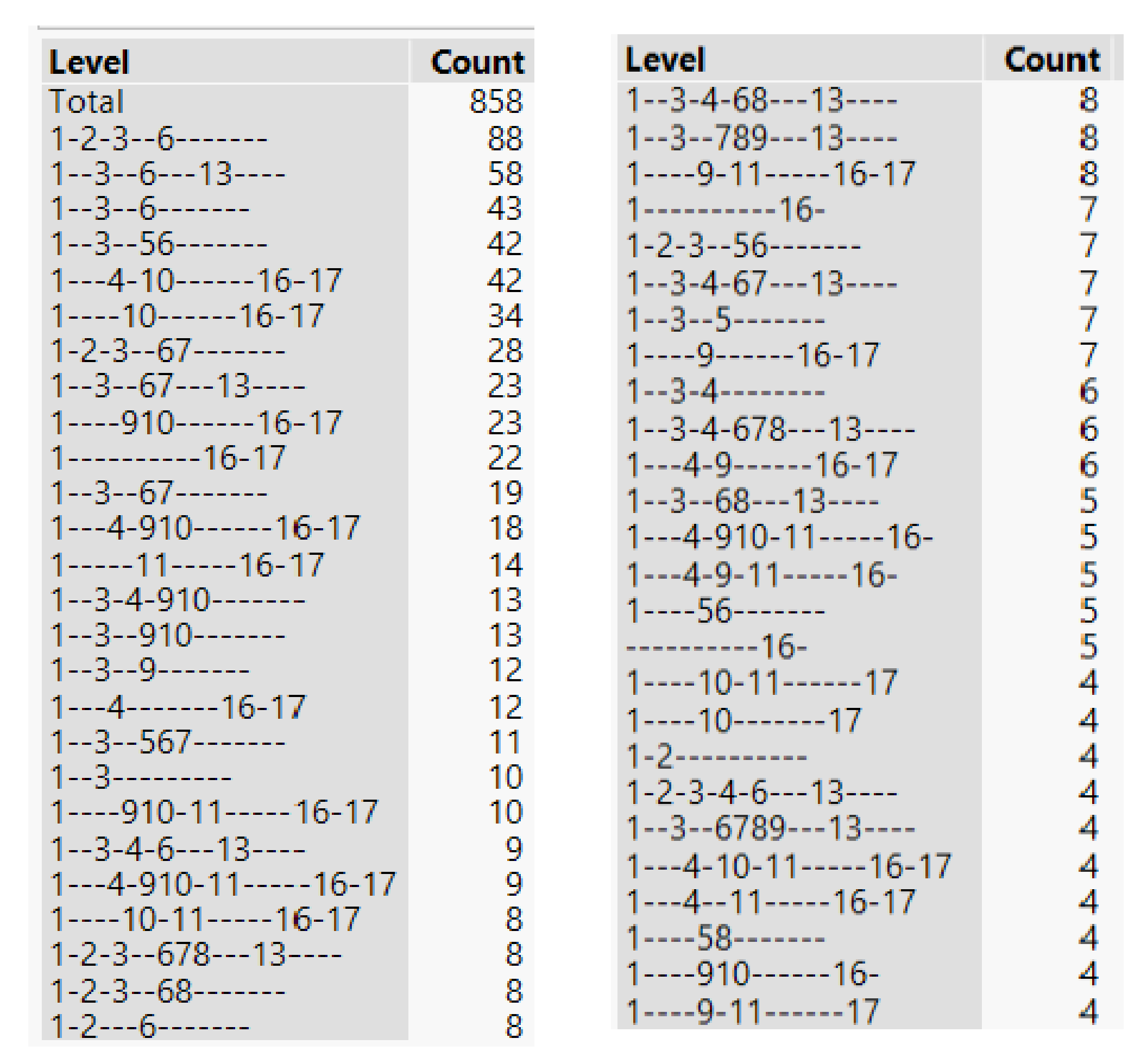
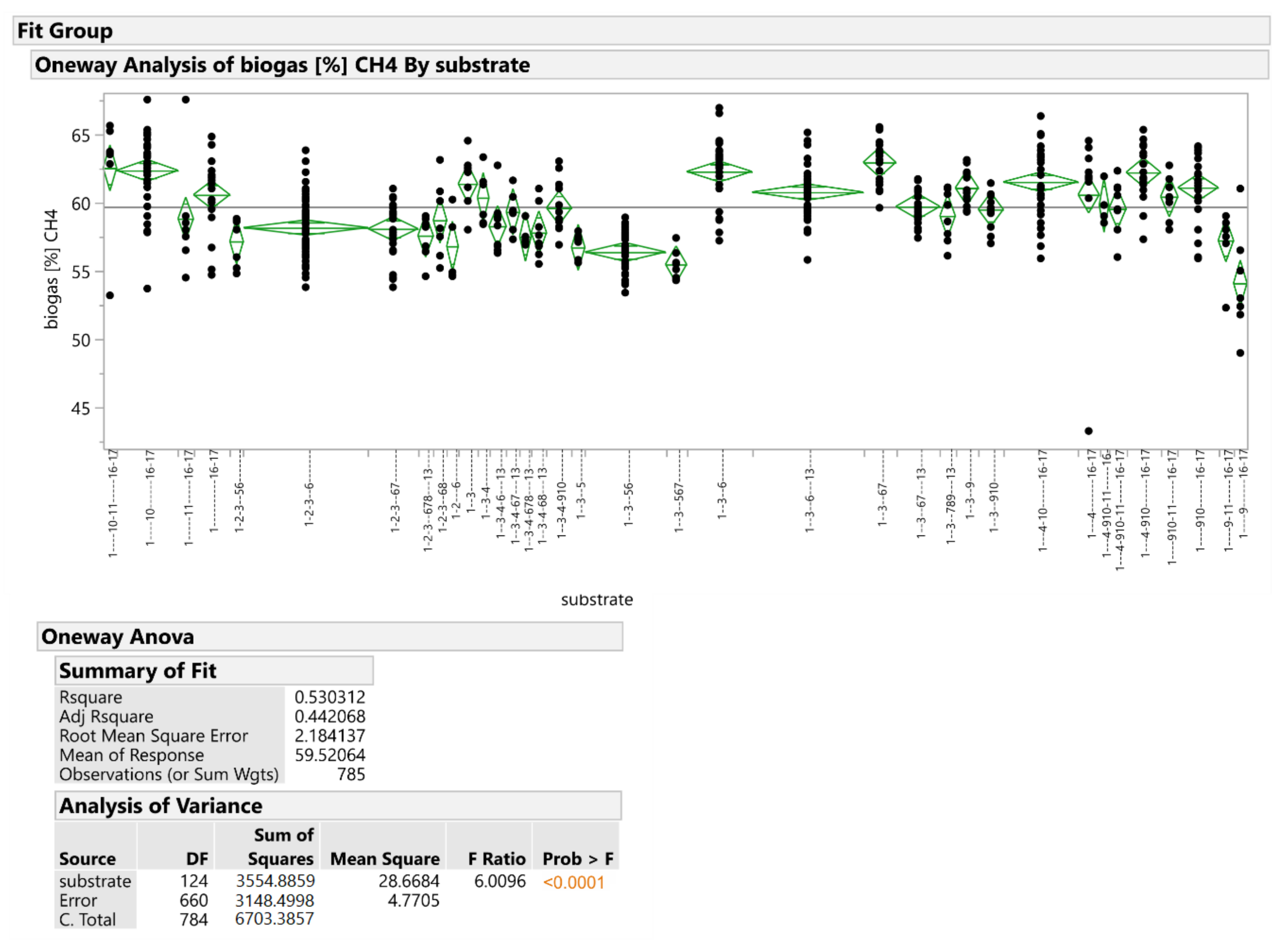
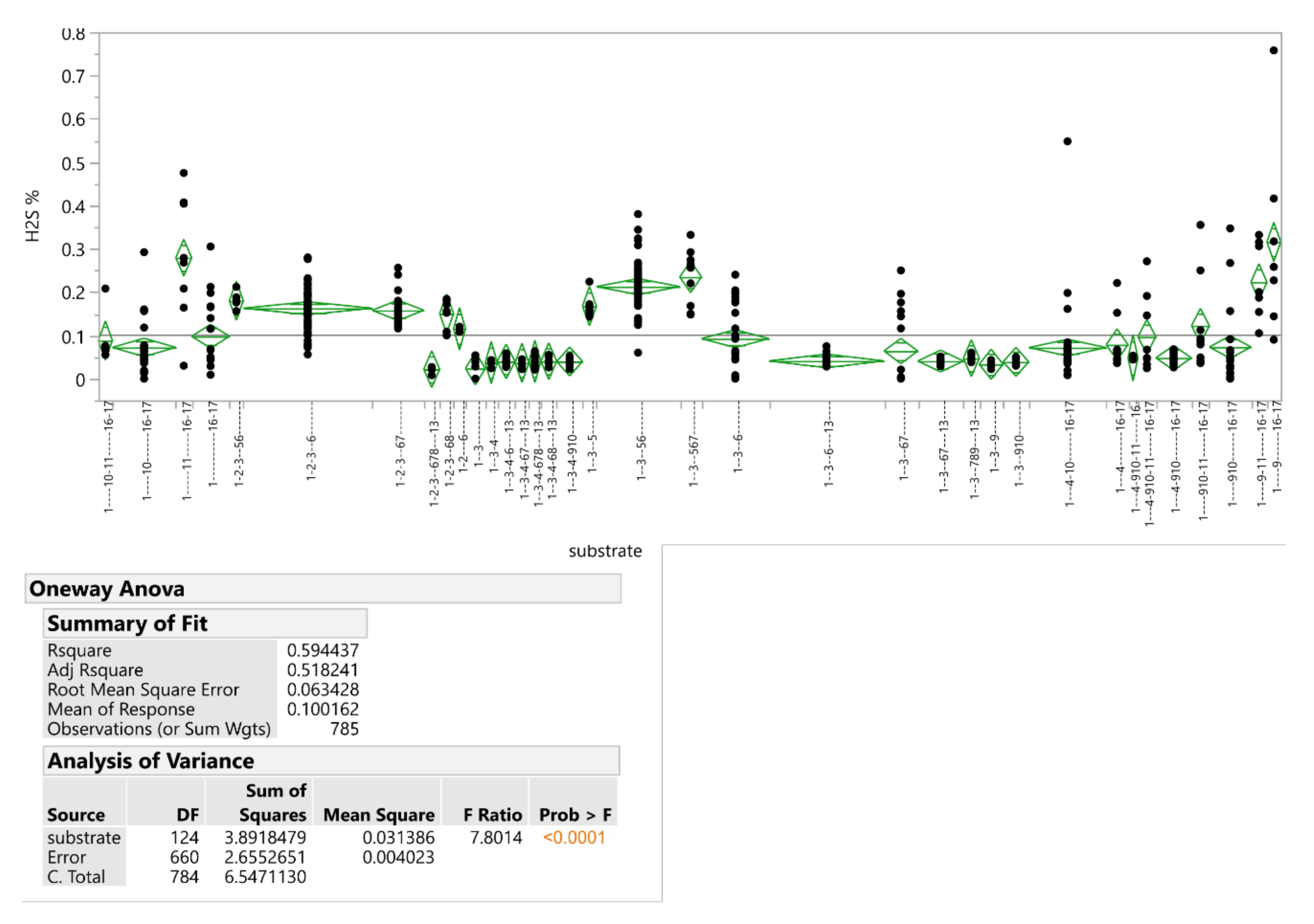


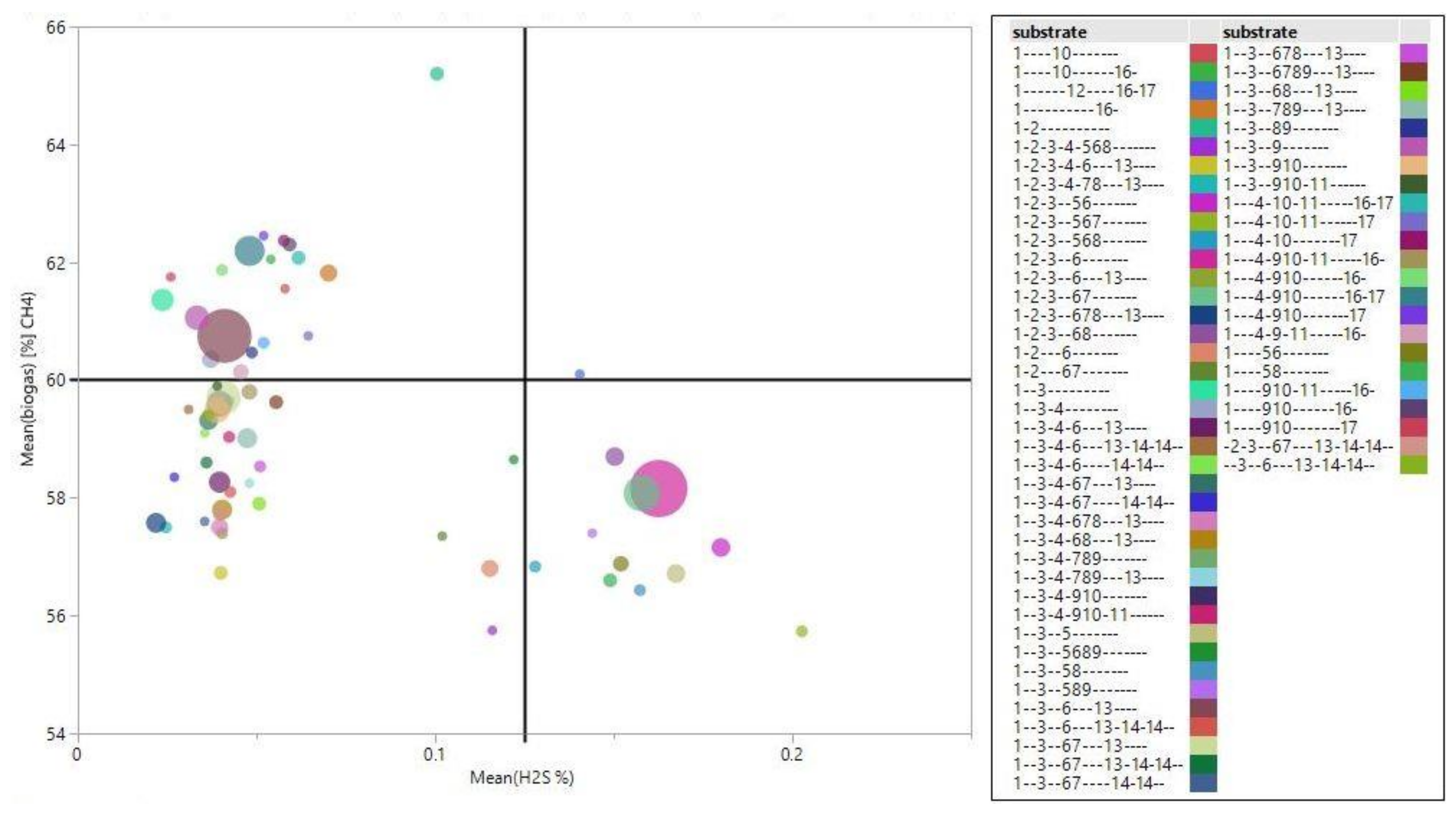

| Num. | Folder | N | Mean | Std. Dev. | Minimum | Maximum |
|---|---|---|---|---|---|---|
| 1 | Corn silage/Maize silage [kg] | 775 | 28,358.10 | 7392.65 | 1500 | 39,860 |
| 2 | Sorghum silage [kg] | 155 | 8258.97 | 4496.66 | 1000 | 17,800 |
| 3 | Receiving tank [kg] | 492 | 15,634.3 | 6102.37 | 1000 | 34,000 |
| 4 | Pastry [kg] | 191 | 1155.76 | 770.8 | 100 | 4000 |
| 5 | Rye [kg] | 94 | 10,245.4 | 2933.97 | 1540 | 17,580 |
| 6 | Manure [kg] | 408 | 1562.55 | 571.93 | 0 | 2660 |
| 7 | Oil [kg] | 147 | 276.74 | 158.13 | 0 | 820 |
| 8 | Juniper [kg] | 88 | 3649.77 | 2192.42 | 300 | 6880 |
| 9 | Other 1 [kg] | 189 | 3198.52 | 2868.25 | 60 | 15,000 |
| 10 | Other 2 [kg] | 210 | 1981.86 | 1890.45 | 60 | 20,000 |
| 11 | Others 3 [kg] | 82 | 1830.00 | 2535.58 | 50 | 10,700 |
| 12 | Others 4 [kg] | 6 | 2573.33 | 2009.25 | 600 | 6300 |
| 13 | Haylage [kg] | 180 | 5321.89 | 2436.56 | 200 | 12,000 |
| 14 | Corn chips silage [kg] | 26 | 27,173.1 | 8368.92 | 6000 | 40,000 |
| 15 | CCM (Corn Cob Mix) [kg] | 23 | 2135.65 | 1075.19 | 1020 | 4740 |
| 16 | Pasteurized biodegradable waste [kg] | 240 | 13,727.00 | 6519.78 | 0 | 36,600 |
| 17 | Fruits/vegetables [kg] | 236 | 2971.50 | 1341.05 | 230 | 6320 |
| # | CH4 [%] | 785 | 59.53 | 2.92 | 43.3 | 67.5 |
| # | O2 [%] | 781 | 0.16 | 0.22 | 0 | 5.7 |
| # | H2S [%] | 785 | 0.10 | 0.09 | 0 | 0.76 |
| N | Mean | Std. Dev. | Minimum | Maximum | |
|---|---|---|---|---|---|
| CH4 [%] | 446 | 59.30 | 2.39 | 53.50 | 65.9 |
| H2S [%] | 446 | 0.08 | 0.06 | 0.00 | 0.28 |
| Folder | Mean Suitable Substrates | Mean Unsuitable Substrates | ||
|---|---|---|---|---|
| [kg] | [%] | [kg] | [%] | |
| 1-Corn silage/Maize silage | 29,154 | 61.47% | 29,913 | 56.07% |
| 2-Sorghum silage | 404.228 | 1.29% | 6464.32 | 12.07% |
| 3-Receiving tank | 8799.06 | 18.82% | 12,651.5 | 22.45% |
| 4-Pastry | 436.04 | 0.87% | 0 | 0.00% |
| 5-Rye | 0 | 0.00% | 2686.06 | 5.74% |
| 6-Manure | 414.228 | 0.90% | 1634.7 | 3.01% |
| 7-Oil | 0 | 0.00% | 34.5455 | 0.06% |
| 8-Juniper | 133.02 | 0.26% | 229,848 | 0.49% |
| 9-Other 1 | 894.698 | 1.67% | 47.2727 | 0.10% |
| 10-Other 2 | 585.369 | 1.21% | 0 | 0.00% |
| 11-Other 3 | 158.121 | 0.28% | 0 | 0.00% |
| 12-Other 4 | 0 | 0.00% | 0 | 0.00% |
| 13-Haylage | 1885.1 | 3.95% | 0 | 0.00% |
| 14-Corn chips silage | 0 | 0.00% | 0 | 0.00% |
| 15-CCM (Corn Cob Mix) | 32.7517 | 0.06% | 0 | 0.00% |
| 16-Pasteurized biodegradable waste | 4503.22 | 8.12% | 0 | 0.00% |
| 17-Fruits/vegetables | 556.705 | 1.10% | 0 | 0.00% |
| biogas [%] CH4 | 61.3047 | 57.8091 | ||
| H2S [%] | 0.0453 | 0.1611 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauš, P.; Kudelas, D.; Taušová, M.; Gabániová, Ľ. Statistical Approach for Assessing the Suitability of Substrates for a Biogas Plant. Sustainability 2020, 12, 9044. https://doi.org/10.3390/su12219044
Tauš P, Kudelas D, Taušová M, Gabániová Ľ. Statistical Approach for Assessing the Suitability of Substrates for a Biogas Plant. Sustainability. 2020; 12(21):9044. https://doi.org/10.3390/su12219044
Chicago/Turabian StyleTauš, Peter, Dušan Kudelas, Marcela Taušová, and Ľubomíra Gabániová. 2020. "Statistical Approach for Assessing the Suitability of Substrates for a Biogas Plant" Sustainability 12, no. 21: 9044. https://doi.org/10.3390/su12219044







