Transformation of Glass Fiber Waste into Mesoporous Zeolite-Like Nanomaterials with Efficient Adsorption of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Fabrication of MZN by Taguchi Experimental Design
2.3. Characterization
2.4. Adsorption of MB onto Mesoporous Zeolite-Like Nanomaterials
2.5. Thermal Reusability Study
3. Result and Discussion
3.1. Optimization Using Taguchi Approach
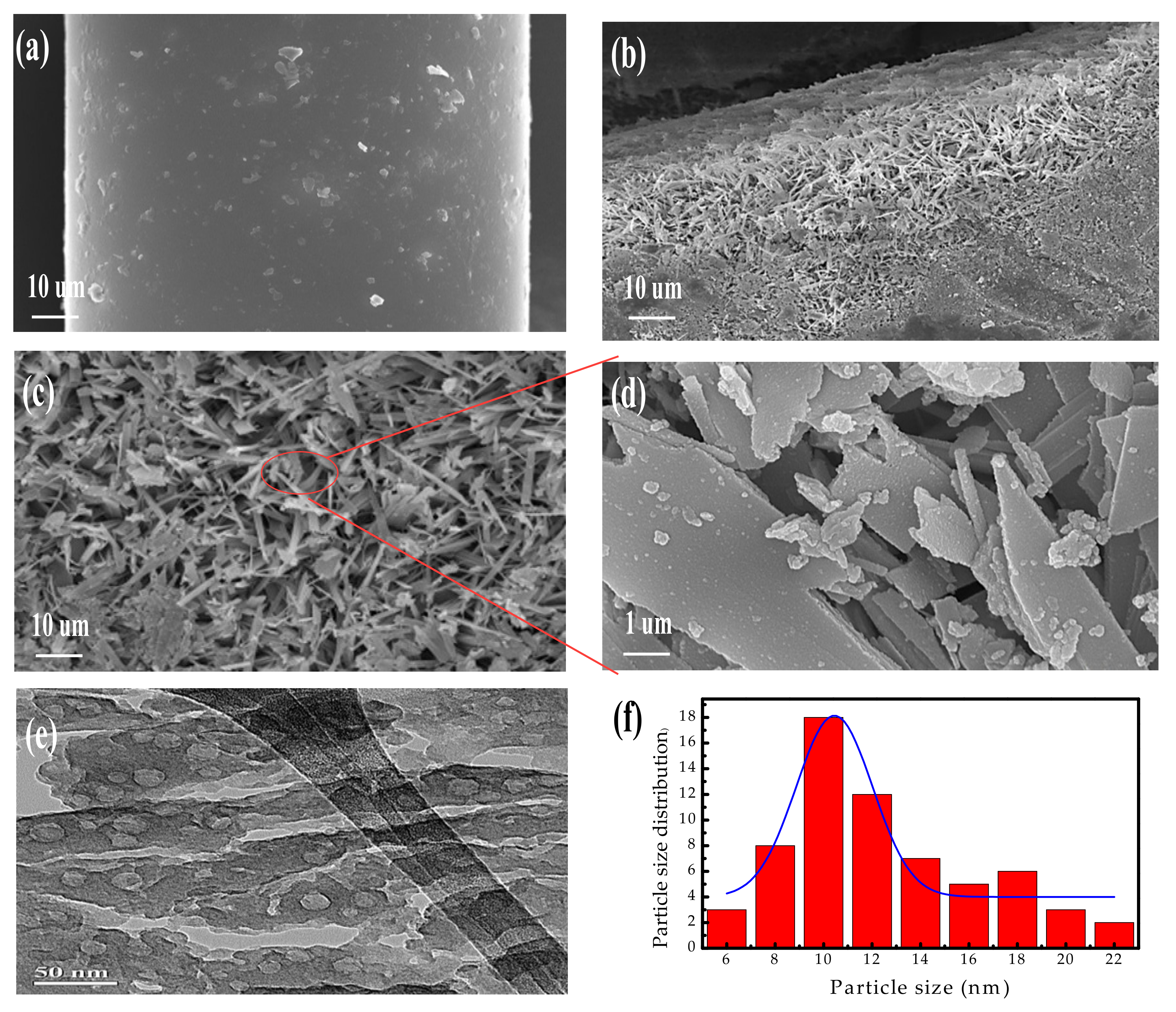
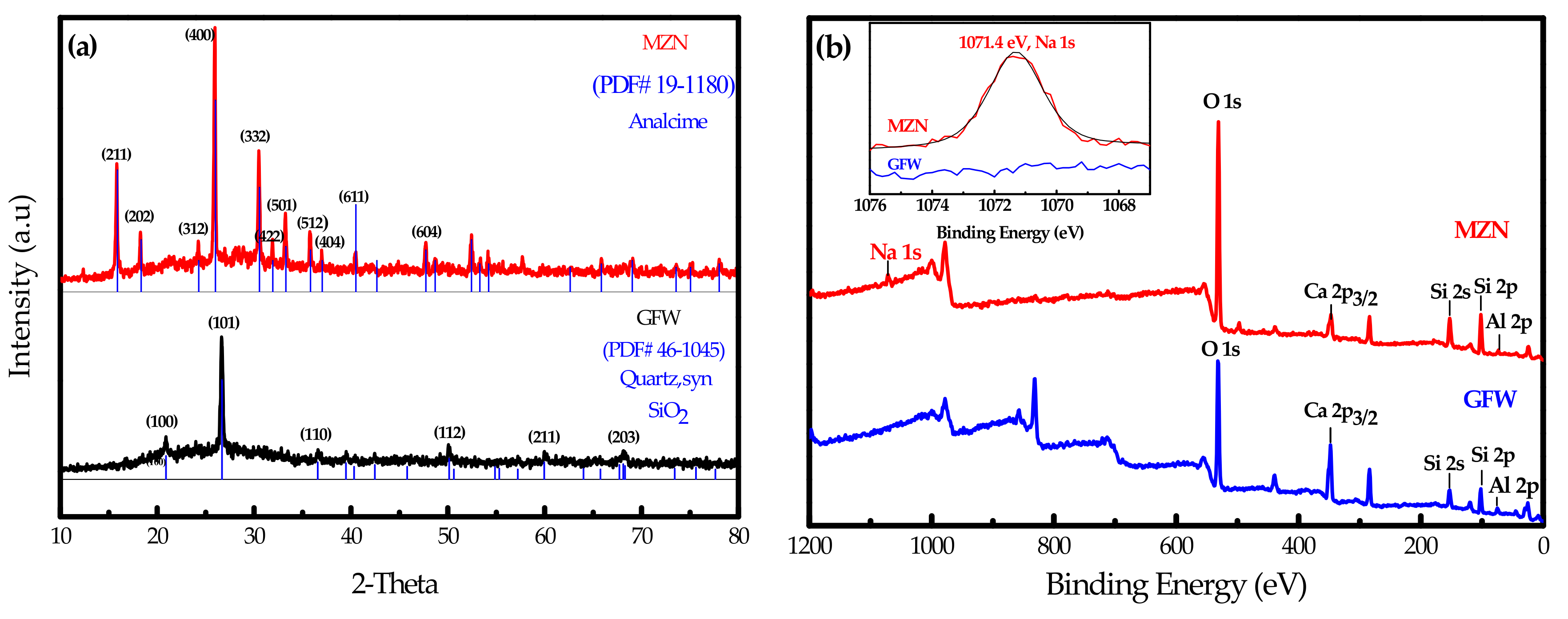
3.2. Surface Characterization of Mesoporous Zeolite-Like Nanomaterial
3.3. The Pore Texture of Mesoporous Zeolite-Like Nanomaterials
3.4. Adsorption of Methylene Blue by MZN
3.4.1. Effect of pH
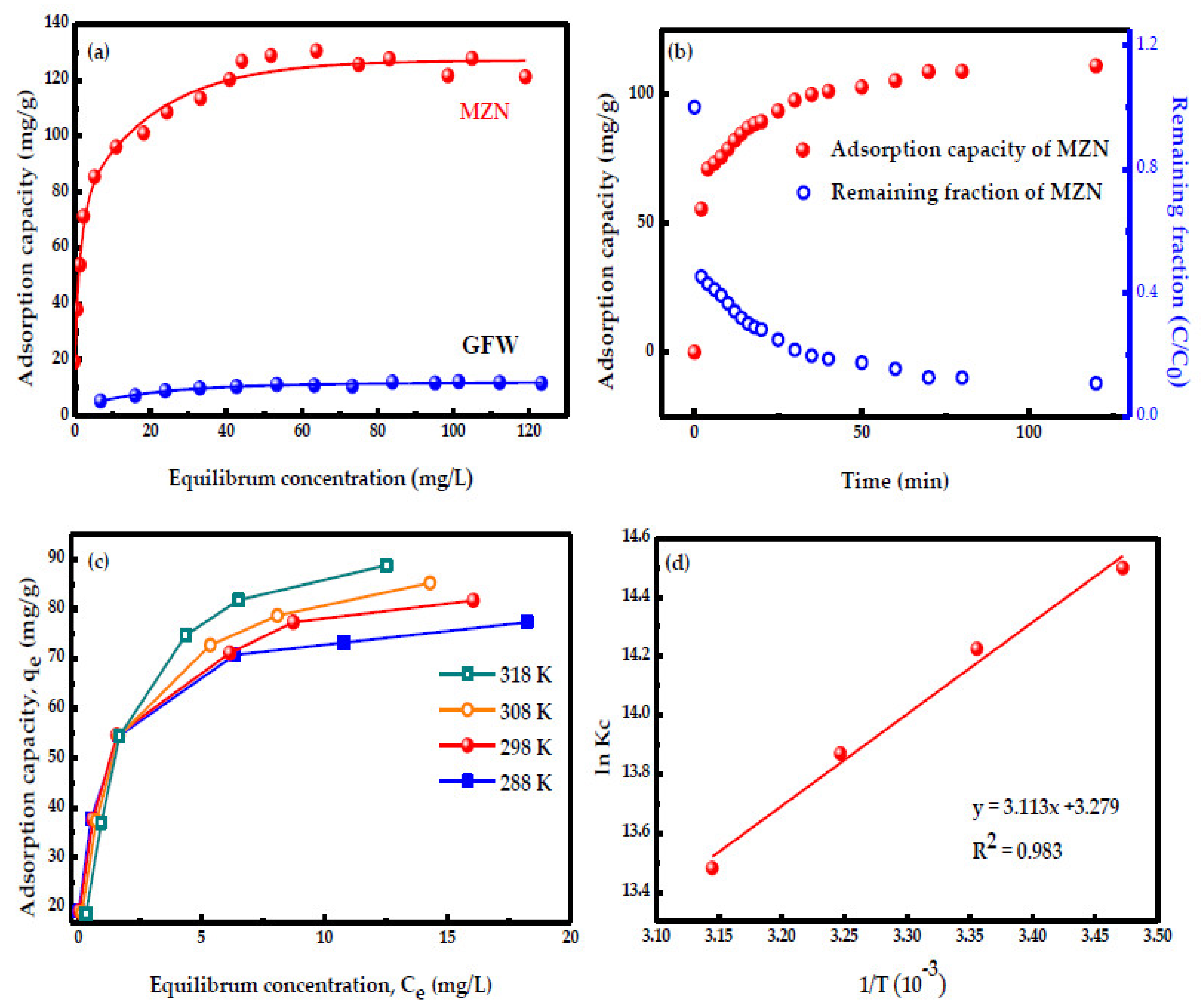
3.4.2. Adsorption Isotherm of MB by MZN
3.4.3. Adsorption Kinetics
3.4.4. Adsorption Thermodynamics
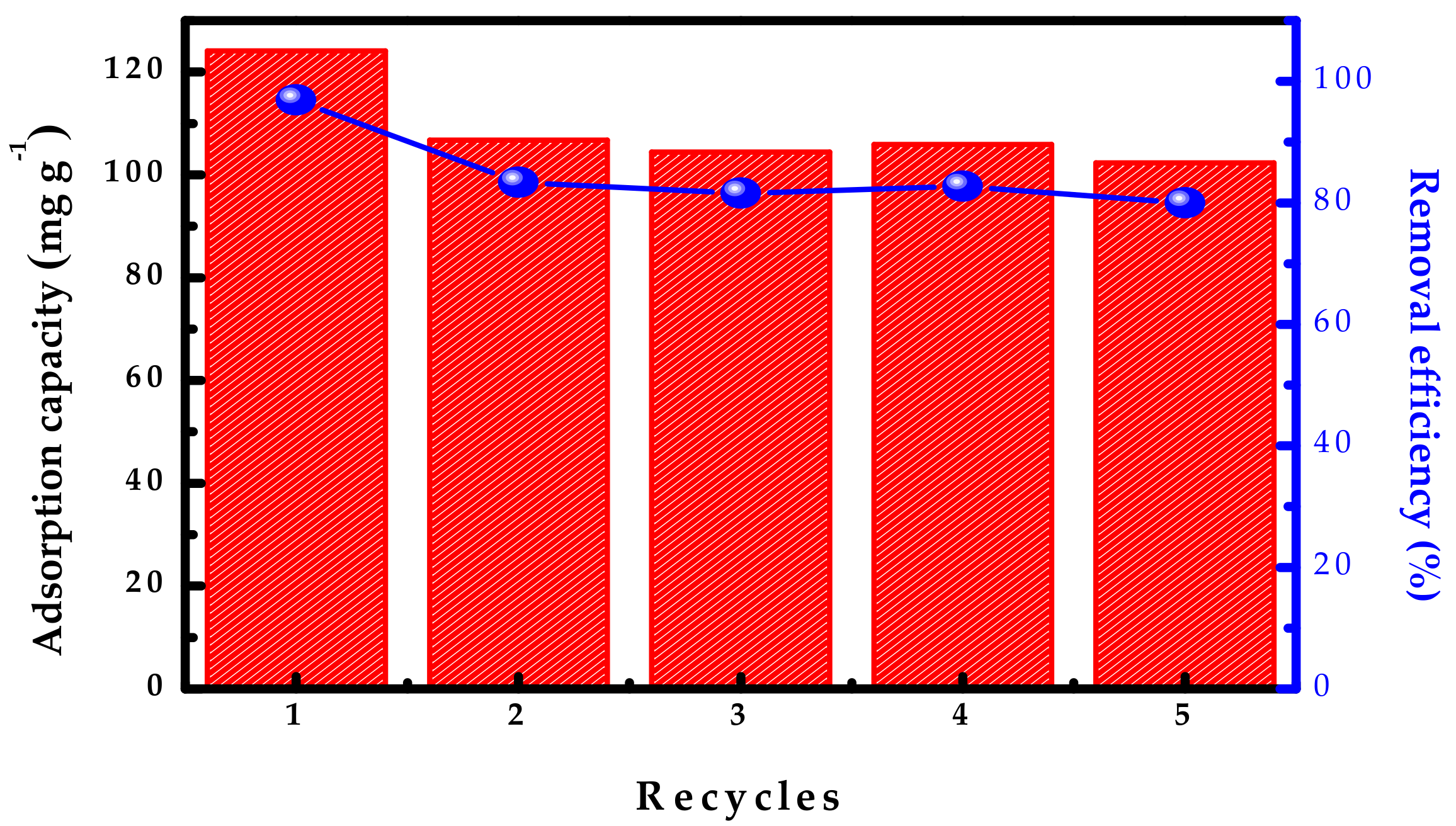
3.5. Reusability Evaluation of MZN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, P.; Meng, F.; Barlow, C.Y. Wind turbine blade end-of-life options: An eco-audit comparison. J. Clean. Prod. 2019, 212, 1268–1281. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zou, B.; Yin, G. Wear mechanisms of Ti (C7N3)-based cermet micro-drill and machining quality during ultra-high speed micro-drilling multi-layered PCB consisting of copper foil and glass fiber reinforced plastics. Ceram. Int. 2019, 45, 24578–24593. [Google Scholar] [CrossRef]
- Joo, S.-J.; Yu, M.-H.; Kim, W.S.; Lee, J.-W.; Kim, H.-S. Design and manufacture of automotive composite front bumper assemble component considering interfacial bond characteristics between over-molded chopped glass fiber polypropylene and continuous glass fiber polypropylene composite. Compos. Struct. 2020, 236, 111849. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Nikafshar, S.; Preyeswary, K.C.; Naebe, M. A technical review on epoxy-clay nanocomposites: Structure, properties, and their applications in fiber reinforced composites. Compos. Part B Eng. 2018, 135, 1–24. [Google Scholar] [CrossRef]
- Nechifor, G.; Totu, E.E.; Nechifor, A.C.; Constantin, L.; Constantin, A.M.; Cărăuşu, M.E.; Isildak, I. Added value recyclability of glass fiber waste as photo-oxidation catalyst for toxic cytostatic micropollutants. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-K.; Lee, N.-T.; Huang, G.-H.; Suzuki, Y.; Doong, R.-A. Simultaneous recovery of display panel waste glass and wastewater boron by chemical oxo-precipitation with fluidized-bed heterogeneous crystallization. ACS Omega 2019, 4, 14057–14066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.-K.; Doong, R.-A.; Hung, H.-Y. Sustainable valorization of mesoporous aluminosilicate composite from display panel glasses waste for adsorption of heavy metal ions. Sci. Total Environ. 2019, 673, 337–346. [Google Scholar] [CrossRef]
- Yasui, K.; Sasaki, K.; Ikeda, N.; Kinoshita, H. Dye adsorbent materials based on porous ceramics from glass fiber-reinforced plastic and clay. Appl. Sci. 2019, 9, 1574. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Guo, J.; Kim, J. Structural characteristics of hazardous organic dyes and relationship between membrane fouling and organic removal efficiency in fluidized ceramic membrane reactor. J. Clean. Prod. 2019, 232, 608–616. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, M.; Liu, H.; Ouyang, S.; Ding, N.; Zhang, P. Adsorption behavior and mechanism of acid orange 7 and methylene blue on self-assembled three-dimensional MgAl layered double hydroxide: Experimental and DFT investigation. Appl. Surf. Sci. 2020, 522, 146370. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Wang, B.; Wu, Y.; Ma, M.; Zhang, X. Synthesis of magnetic lignin-based hollow microspheres: A highly adsorptive and reusable adsorbent derived from renewable resources. ACS Sustain. Chem. Eng. 2016, 4, 5523–5532. [Google Scholar] [CrossRef]
- Domingues, J.T.; Orlando, R.M.; Millán, R.D.S.; García, A.D.P.; Rodrigues, G.D. Polymer-bixin nanofibers: A promising environmentally friendly material for the removal of dyes from water. Sep. Purif. Technol. 2020, 248, 117118. [Google Scholar] [CrossRef]
- Aslam, S.; Subhan, F.; Yan, Z.; Yaseen, M.; Shujahat, M.H. Fabrication of gold nanoparticles within hierarchically ZSM-5-based micro-/mesostructures (MMZ) with enhanced stability for catalytic reduction of p-nitrophenol and methylene blue. Sep. Purif. Technol. 2021, 254, 117645. [Google Scholar] [CrossRef]
- Grover, A.; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kim, K.-H. Zn-Al layered double hydroxides intercalated with surfactant: Synthesis and applications for efficient removal of organic dyes. J. Clean. Prod. 2019, 240, 118090. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, J.; Wang, D.; Liu, J.; Wang, L.; Xiao, H. Construction of three-dimensional g-C3N4/attapulgite hybrids for Cd (II) adsorption and the reutilization of waste adsorbent. Appl. Surf. Sci. 2020, 504, 144456. [Google Scholar] [CrossRef]
- Tran, V.A.; Kadam, A.N.; Lee, S.-W. Adsorption-assisted photocatalytic degradation of methyl orange dye by zeolite-imidazole-framework-derived nanoparticles. J. Alloys Compd. 2020, 835, 155414. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Yu, S.; Zou, Y.; Wang, J.; Chen, Z.; Alharbi, N.S.; Alsaedi, A.; Hayat, T.; Chen, Y. Silica coated Fe3O4 magnetic nanospheres for high removal of organic pollutants from wastewater. Chem. Eng. J. 2016, 306, 280–288. [Google Scholar] [CrossRef]
- Qin, P.; Yang, Y.; Zhang, X.; Niu, J.; Yang, H.; Tian, S.; Zhu, J.; Lu, M. Highly efficient, rapid, and simultaneous removal of cationic dyes from aqueous solution using monodispersed mesoporous silica nanoparticles as the adsorbent. Nanomaterials 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Meng, S.; Yu, S.; Tang, F.; Hu, X.; Lu, J.; Fei, X.; Zhu, M. Fiber engineering of silica-based aerogels with surface specificity and regenerability for continuous removal of dye pollutants from wastewaters. Microporous Mesoporous Mater. 2021, 314, 110874. [Google Scholar] [CrossRef]
- Dingal, S.; Pradhan, T.; Sundar, J.S.; Choudhury, A.R.; Roy, S. The application of Taguchi’s method in the experimental investigation of the laser sintering process. Int. J. Adv. Manuf. Technol. 2008, 38, 904–914. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Chen, J.C.; Kirby, E.D. Surface roughness optimization in an end-milling operation using the Taguchi design method. J. Mater. Process. Technol. 2007, 184, 233–239. [Google Scholar] [CrossRef]
- Sushil, K.; Satsangi, P.; Prajapati, D. Optimization of green sand casting process parameters of a foundry by using Taguchi method. Int. J. Adv. Manuf. Technol. 2010, 55, 23–34. [Google Scholar]
- Wang, J.-M.; Yan, H.-J.; Zhou, J.-M.; Li, S.-X.; Gui, G.-C. Optimization of parameters for an aluminum melting furnace using the Taguchi approach. Appl. Therm. Eng. 2012, 33, 33–43. [Google Scholar] [CrossRef]
- Thakker, M.R.; Parikh, J.K.; Desai, M.A. Synergism between ionic liquid and ultrasound for greener extraction of geraniol: Optimization using different statistical tools, comparison and prediction. Chem. Eng. Res. Des. 2018, 134, 162–171. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Yilmaz, E.; Deveci, H. A novel silica xerogel synthesized from volcanic tuff as an adsorbent for high-efficient removal of methylene blue: Parameter optimization using Taguchi experimental design. J. Chem. Technol. Biotechnol. 2019, 94, 2729–2737. [Google Scholar] [CrossRef]
- Googerdchian, F.; Moheb, A.; Emadi, R.; Asgari, M. Optimization of Pb (II) ions adsorption on nanohydroxyapatite adsorbents by applying Taguchi method. J. Hazard. Mater. 2018, 349, 186–194. [Google Scholar] [CrossRef]
- Barly, S.H.; Okhrimenko, D.V.; Solvang, M.; Yue, Y.; Stipp, S.L. Dissolution of Stone Wool Fibers with Phenol-urea-formaldehyde Binder in a Synthetic Lung Fluid. Chem. Res. Toxicol. 2019, 32, 2398–2410. [Google Scholar] [CrossRef]
- Zolfaghari, G.; Esmaili-Sari, A.; Anbia, M.; Younesi, H.; Amirmahmoodi, S.; Ghafari-Nazari, A. Taguchi optimization approach for Pb (II) and Hg (II) removal from aqueous solutions using modified mesoporous carbon. J. Hazard. Mater. 2011, 192, 1046–1055. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Tashakori, C.; Kamboh, M.A.; Korrani, Z.S.; Saidur, R.; Nodeh, H.R.; Bidhendi, M.E. Kinetic and equilibrium adsorption of lead from water using magnetic metformin-substituted SBA-15. Environ. Sci. Water Res. Technol. 2018, 4, 549–558. [Google Scholar] [CrossRef]
- Guo, D.; Li, Y.; Cui, B.; Hu, M.; Luo, S.; Ji, B.; Liu, Y. Natural adsorption of methylene blue by waste fallen leaves of Magnoliaceae and its repeated thermal regeneration for reuse. J. Clean. Prod. 2020, 267, 121903. [Google Scholar] [CrossRef]
- Nia, P.M.; Jenatabadi, H.S.; Woi, P.M.; Abouzari-Lotf, E.; Alias, Y.J.M. The optimization of effective parameters for electrodeposition of reduced graphene oxide through Taguchi method to evaluate the charge transfer. Measurement 2019, 137, 683–690. [Google Scholar]
- Ning, C.; Hadi, P.; Xu, M.; Lin, C.S.K.; McKay, G. Valorization of an electronic waste-derived aluminosilicate: Surface functionalization and porous structure tuning. ACS Sustain. Chem. Eng. 2016, 4, 2980–2989. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Shao, N.; Hu, T.; Han, L.; Wang, D. Geopolymerization enhanced hydrothermal synthesis of analcime from steel slag and CFBC fly ash and heavy metal adsorption on analcime. Environ. Technol. 2020, 41, 1753–1765. [Google Scholar] [CrossRef]
- Bortolini, H.R.; Lima, D.S.; Perez-Lopez, O.W. Hydrothermal synthesis of analcime without template. J. Cryst. Growth 2020, 532, 125424. [Google Scholar] [CrossRef]
- Ya, M.; Deubener, J.; Yue, Y. Enthalpy and anisotropy relaxation of glass fibers. J. Am. Ceram. Soc. 2008, 91, 745–752. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, Z.; Kameda, T.; Yoshioka, T. Hydrothermal synthesis of hardened diatomite-based adsorbents with analcime formation for methylene blue adsorption. RSC Adv. 2016, 6, 26765–26774. [Google Scholar] [CrossRef]
- Hameed, A.M.; Alharbi, A.; Abdelrahman, E.A.; Mabrouk, E.; Hegazey, R.; Algethami, F.K.; Al-Ghamdi, Y.O.; Youssef, H.M. Facile hydrothermal fabrication of analcime and zeolite x for efficient removal of Cd (II) ions from aqueous media and polluted water. J. Inorg. Organmetallic Polym. Mater. 2020, 30, 4117–4128. [Google Scholar] [CrossRef]
- Kazemian, H.; Naghdali, Z.; Kashani, T.G.; Farhadi, F. Conversion of high silicon fly ash to Na-P1 zeolite: Alkaline fusion followed by hydrothermal crystallization. Adv. Powder Technol. 2010, 21, 279–283. [Google Scholar] [CrossRef]
- Wang, F.; Yu, J.; Liang, X.; Lu, S.; Liu, Z.; Liu, L. Effect of heat treatment on the morphology, diameter and mesoporous of superfine glass fiber. J. Text. Inst. 2020, 112, 123–131. [Google Scholar] [CrossRef]
- You, S.-M.; Tasi, C.-K.; Millet, P.; Doong, R.-A. Electrochemically capacitive deionization of copper (II) using 3D hierarchically reduced graphene oxide architectures. Sep. Purif. Technol. 2020, 251, 117368. [Google Scholar] [CrossRef]
- Li, K.; Yu, L.; Cai, J.; Zhang, L.J.M. Removal of dyes from aqueous solution using novel C@ C composite adsorbents. Microporous Mesoporous Mater. 2021, 313, 110840. [Google Scholar] [CrossRef]
- Youssef, H.M.; Shah, R.K.; Algethami, F.K.; Hegazey, R.; Naglah, A.M.; Al-Omar, M.A.; Alluhaybi, A.A.; Alherbish, H.A.; Mabrouk, E.; Abdelrahman, E.A. Facile hydrothermal procedure for the synthesis of sodium aluminum silicate hydrate/analcime and analcime for effective removal of manganese (II) ions from aqueous solutions. J. Inorg. Organometallic Polym. Mater. 2020, 31, 1035–1046. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdelrahman, E.A. Hydrothermal tuning of the morphology and crystallite size of zeolite nanostructures for simultaneous adsorption and photocatalytic degradation of methylene blue dye. J. Mol. Liq. 2017, 242, 364–374. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Alharbi, A.; Subaihi, A.; Hameed, A.M.; Almutairi, M.A.; Algethami, F.K.; Youssef, H.M. Facile fabrication of novel analcime/sodium aluminum silicate hydrate and zeolite Y/faujasite mesoporous nanocomposites for efficient removal of Cu (II) and Pb (II) ions from aqueous media. J. Mater. Res. Technol. 2020, 9, 7900–7914. [Google Scholar] [CrossRef]
- Wang, K.; Peng, N.; Sun, J.; Lu, G.; Chen, M.; Deng, F.; Dou, R.; Nie, L.; Zhong, Y. Synthesis of silica-composited biochars from alkali-fused fly ash and agricultural wastes for enhanced adsorption of methylene blue. Sci. Total Environ. 2020, 729, 139055. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.; Ok, Y.S.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Du, X.; Shao, W. Preparation of reusable glass hollow fiber membranes and methylene blue adsorption. J. Eur. Ceram. Soc. 2019, 39, 4891–4900. [Google Scholar] [CrossRef]
- Alizadeh Arasi, M.; Salem, A.; Salem, S. Extraction of nano-porous silica from hydrosodalite produced via modification of low-grade kaolin for removal of methylene blue from wastewater. J. Chem. Technol. Biotechnol. 2020, 95, 1989–2000. [Google Scholar] [CrossRef]
- Verma, M.; Dwivedi, P.K.; Saxena, N.J.C. Hollow silica nanoparticles synthesized from core-shell nanoparticles as highly efficient adsorbent for methylene blue and its invitro release: Mechanism and Kinetics study. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124333. [Google Scholar] [CrossRef]
- Hu, M.; Yan, X.; Hu, X.; Feng, R.; Zhou, M. Synthesis of silver decorated silica nanoparticles with rough surfaces as adsorbent and catalyst for methylene blue removal. J. Sol-Gel Sci. Technol. 2019, 89, 754–763. [Google Scholar] [CrossRef]
- Han, H.; Wei, W.; Jiang, Z.; Lu, J.; Zhu, J.; Xie, J. Removal of cationic dyes from aqueous solution by adsorption onto hydrophobic/hydrophilic silica aerogel. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 539–549. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, W.; He, S.; Yu, S.; Chen, Y.; Lu, L.; Shu, Z.; Cui, H.; Zhang, Y.; Jin, H. Rapid adsorption of cationic dye-methylene blue on the modified montmorillonite/graphene oxide composites. Appl. Clay Sci. 2019, 168, 304–311. [Google Scholar] [CrossRef]
- Brião, G.V.; Jahn, S.L.; Foletto, E.L.; Dotto, G.L. Highly efficient and reusable mesoporous zeolite synthetized from a biopolymer for cationic dyes adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 43–50. [Google Scholar] [CrossRef]
- Khanday, W.; Asif, M.; Hameed, B. Cross-linked beads of activated oil palm ash zeolite/chitosan composite as a bio-adsorbent for the removal of methylene blue and acid blue 29 dyes. Int. J. Biol. Macromol. 2017, 95, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Sumalinog, D.A.G.; Capareda, S.C.; de Luna, M.D.G. Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ. Manag. 2018, 210, 255–262. [Google Scholar] [CrossRef]
- Dong, G.; Tian, G.; Gong, L.; Tang, Q.; Li, M.; Meng, J.; Liang, J. Mesoporous zinc silicate composites derived from iron ore tailings for highly efficient dye removal: Structure and morphology evolution. Microporous Mesoporous Mater. 2020, 305, 110352. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Lightweight geopolymer-expanded glass composites for removal of methylene blue from aqueous solutions. Ceram. Int. 2020, 46, 19785–19791. [Google Scholar] [CrossRef]
- Supelano, G.; Cuaspud, J.G.; Moreno-Aldana, L.C.; Ortiz, C.; Trujillo, C.; Palacio, C.; Vargas, C.P.; Gómez, J.M. Synthesis of magnetic zeolites from recycled fly ash for adsorption of methylene blue. Fuel 2020, 263, 116800. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Milonjić, S.K. A consideration of the correct calculation of thermodynamic parameters of adsorption. J. Serb. Chem. Soc. 2007, 72, 1363–1367. [Google Scholar] [CrossRef]
- Huang, T.; Yan, M.; He, K.; Huang, Z.; Zeng, G.; Chen, A.; Peng, M.; Li, H.; Yuan, L.; Chen, G.J. Efficient removal of methylene blue from aqueous solutions using magnetic graphene oxide modified zeolite. J. Colloid Interface Sci. 2019, 543, 43–51. [Google Scholar] [CrossRef]
- Gal, Y.-S.; Jin, S.-H.; Park, J.W.; Lim, K.T. Comparative study between liquid. liquid extraction and bulk liquid membrane for the removal and recovery of methylene blue from wastewater. J. Ind. Eng. Chem. 2015, 30, 261–265. [Google Scholar] [CrossRef]
- Maneechakr, P.; Karnjanakom, S. Adsorption behaviour of Fe (II) and Cr (VI) on activated carbon: Surface chemistry, isotherm, kinetic and thermodynamic studies. J. Ind. Eng. Chem. 2017, 106, 104–112. [Google Scholar] [CrossRef]



| Factors | qe (mg g−1) | S/N Ratio | |||
|---|---|---|---|---|---|
| Agent Ratio (w/w) | Agent Conc. (M) | Temp. (K) | Time (HR) | ||
| 1:2.5 | 1 | 393 | 8 | 36.6 | 31.28 |
| 1:2.5 | 2.5 | 423 | 16 | 114 | 41.18 |
| 1:2.5 | 5 | 453 | 24 | 88.1 | 38.90 |
| 1:5 | 1 | 423 | 24 | 107 | 40.58 |
| 1:5 | 2.5 | 453 | 8 | 84.6 | 38.54 |
| 1:5 | 5 | 393 | 16 | 114 | 41.15 |
| 1:10 | 1 | 453 | 16 | 106 | 40.53 |
| 1:10 | 2.5 | 393 | 24 | 111 | 40.93 |
| 1:10 | 5 | 423 | 8 | 103 | 40.27 |
| Level | Agent Ratio (w/w) | Agent Conc. (M) | Temp. (K) | Time (HR) |
|---|---|---|---|---|
| L1 | 37.12 | 37.46 | 37.79 | 36.7 |
| L2 | 40.09 | 40.22 | 40.68 | 40.95 |
| L3 | 40.58 | 40.11 | 39.32 | 40.14 |
| Delta | 3.46 | 2.75 | 2.89 | 4.25 |
| Rank | 2 | 4 | 3 | 1 |
| Adsorbent | pH | Surface Area (m2 g−1) | Temp. (K) | Adsorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|
| PVDF/glass as-prepared membrane | 7 | 278 | 298 | 44.4 | [Zhang et al., 2019] |
| Silica xerogel | 5 | 195 | 313 | 51.9 | [Guzel Kaya et al., 2019] |
| Pure silica | 6.5 | 421 | - | 102 | [Alizadeh Arasi, et al., 2020] |
| Hollow silica | 7 | 67.0 | 298 | 64.0 | [Verma, et al., 2020] |
| Ag/SiO2 | 7 | 208 | - | 55.0 | [Hu, et al., 2019] |
| Hydroxyl group silica aerogel | 7 | 628 | - | 47.2 | [Han, et al., 2016] |
| Montomorillonite/graphene oxide | 7 | 74.6 | 303 | 471 | [Yang et al., 2019] |
| Mesoporous zeolite (ZSM-5) | 7 | 164 | 298 | 115 | [Brião, et al., 2018] |
| Zeolite/chitosan composite | - | 83.0 | 303 | 152 | [Khanday, et al., 2017] |
| Mesoporous zeolite-like material | 6 | 166 | 298 | 132 | This study |
| Kinetic Model | Equation a | Parameters | MB |
|---|---|---|---|
| Adsorbed amount | - | qe, exp (mg g−1) | 111.0 |
| Pseudo-first order | qe, cal (mg g−1) | 23.8 | |
| k1 (min−1) | 0.0046 | ||
| R2 | 0.858 | ||
| Pseudo-second order | qe, cal (mg g−1) | 113.0 | |
| k2 (g mg−1 min−1) | 0.0023 | ||
| R2 | 0.998 | ||
| Intra-particle diffusion | kid (mg g−1 min0.5) | 5.12 | |
| C | 64.6 | ||
| R2 | 0.921 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-K.; Horng, J.-J. Transformation of Glass Fiber Waste into Mesoporous Zeolite-Like Nanomaterials with Efficient Adsorption of Methylene Blue. Sustainability 2021, 13, 6207. https://doi.org/10.3390/su13116207
Tsai C-K, Horng J-J. Transformation of Glass Fiber Waste into Mesoporous Zeolite-Like Nanomaterials with Efficient Adsorption of Methylene Blue. Sustainability. 2021; 13(11):6207. https://doi.org/10.3390/su13116207
Chicago/Turabian StyleTsai, Cheng-Kuo, and Jao-Jia Horng. 2021. "Transformation of Glass Fiber Waste into Mesoporous Zeolite-Like Nanomaterials with Efficient Adsorption of Methylene Blue" Sustainability 13, no. 11: 6207. https://doi.org/10.3390/su13116207





