Crushed Bricks: Demolition Waste as a Sustainable Raw Material for Geopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Clay Brick Waste-Based Geopolymer
2.2. Mineralogical and Chemical Characterization
2.3. Physical Characterization of the Geopolymers
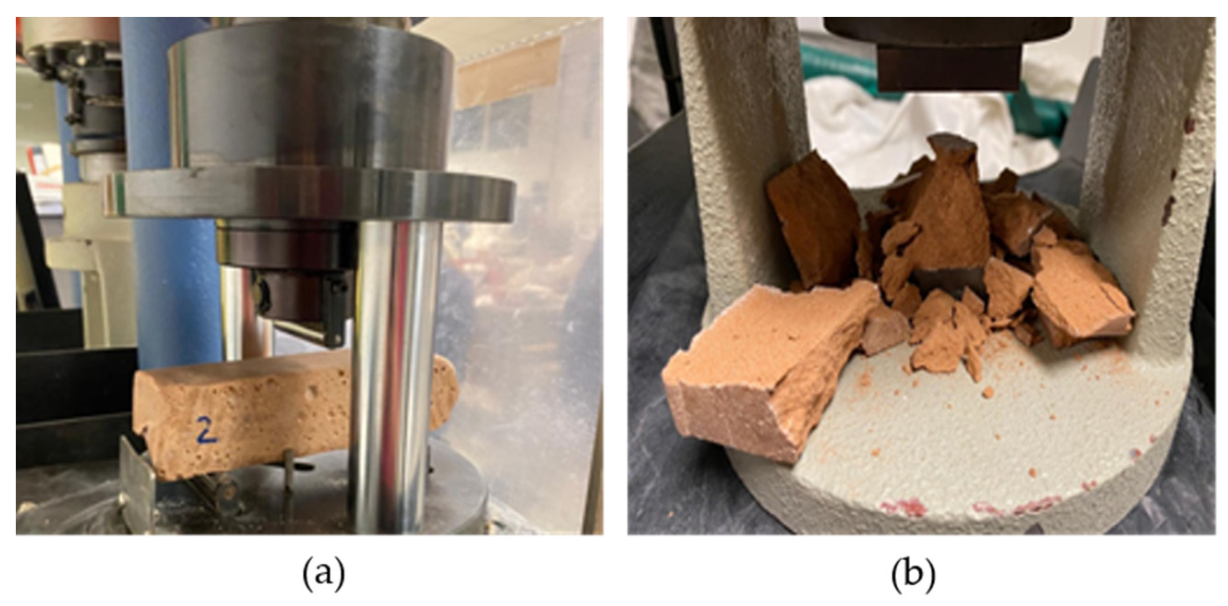
2.4. Mechanical Characterization
3. Results and Discussion
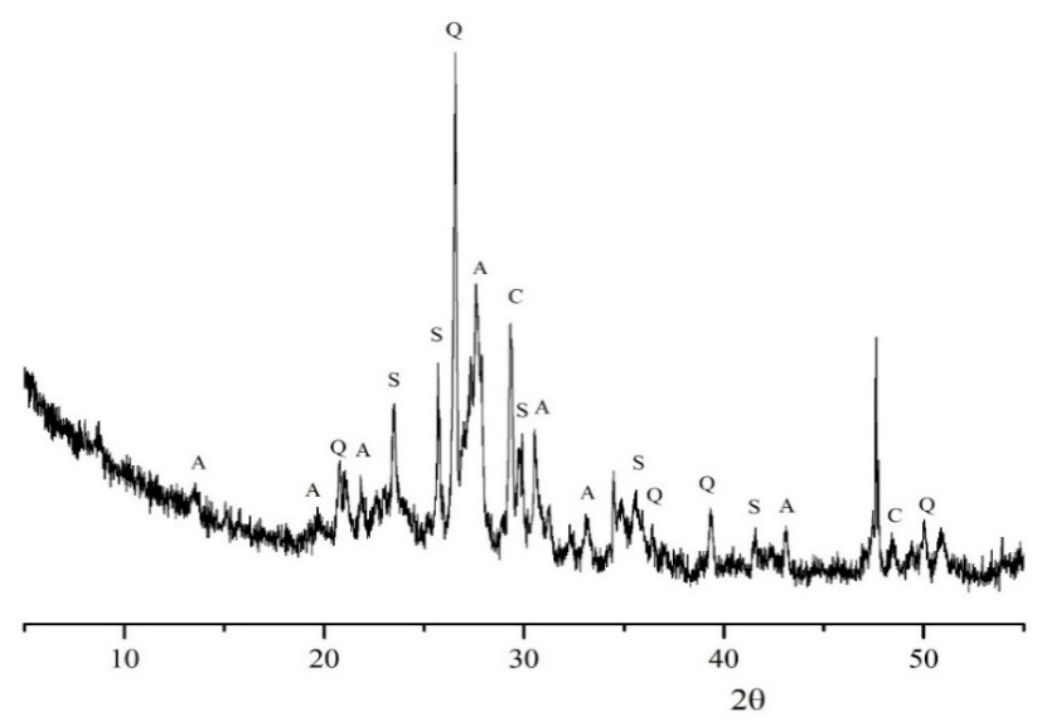
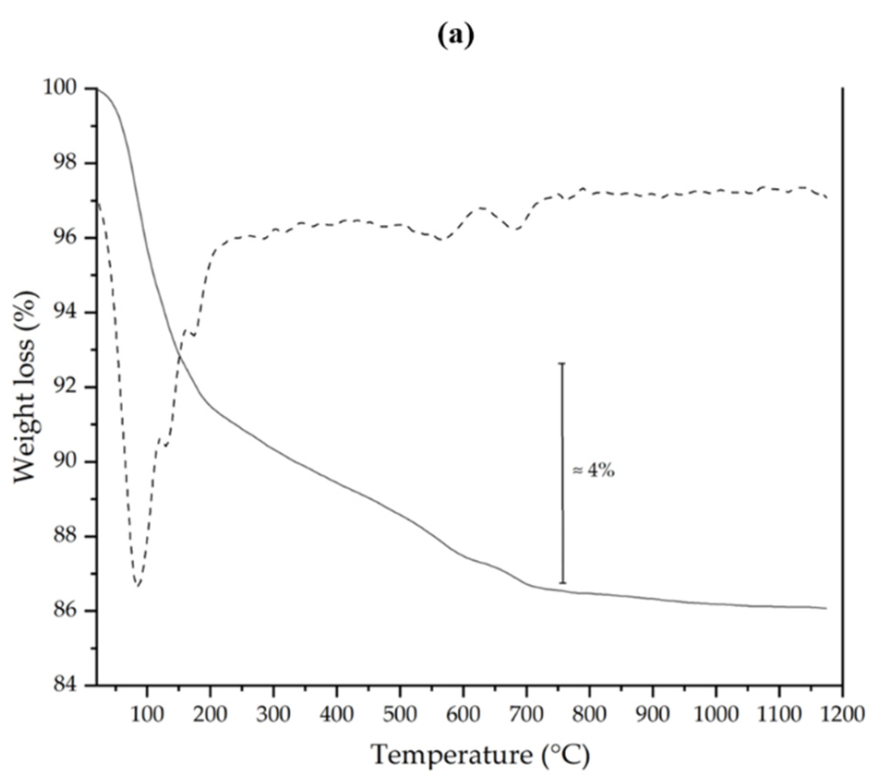
3.1. Preliminary Characterization of Clay Brick Waste
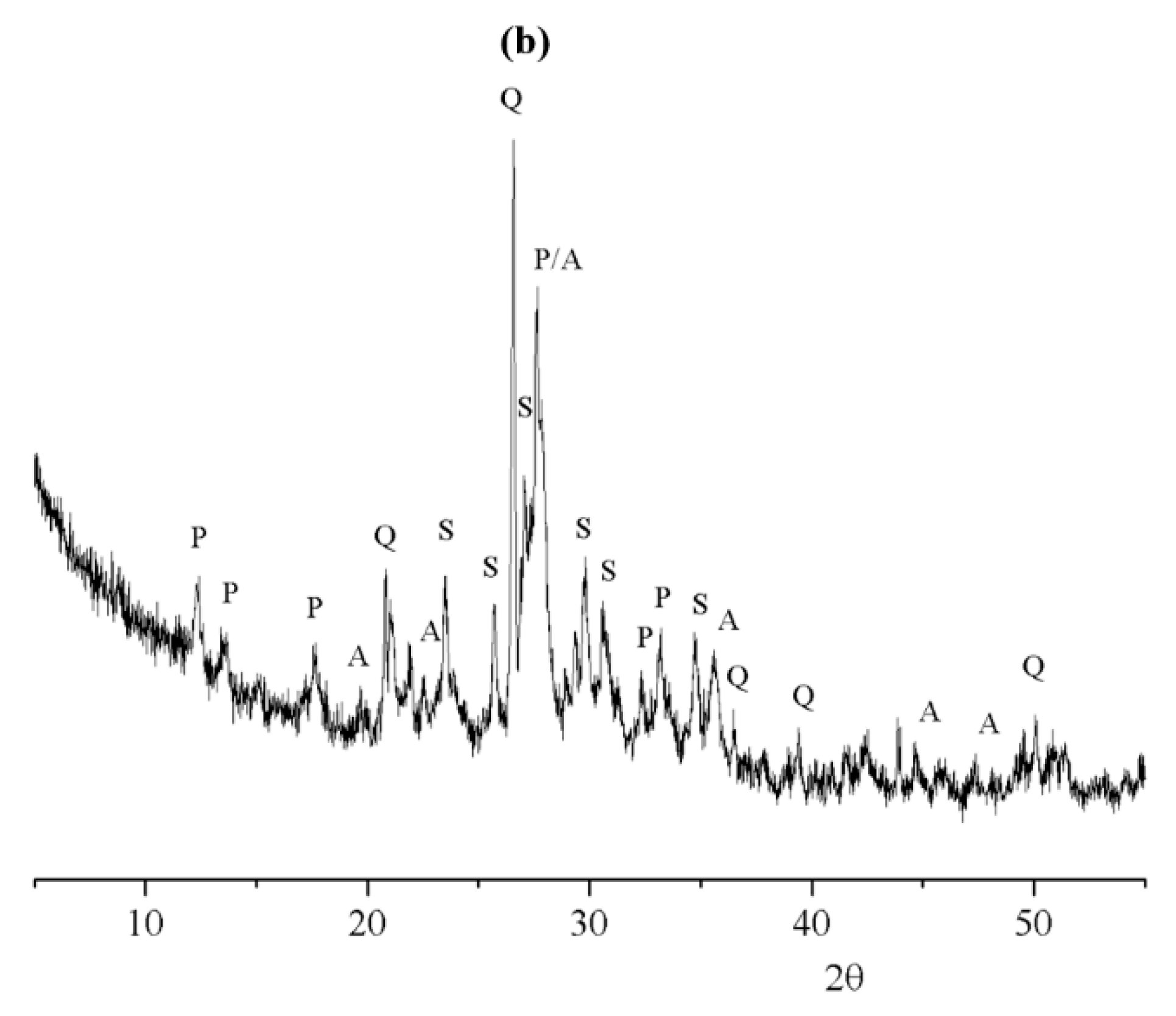
3.2. Characterization of Geopolymeric Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, H.; Shen, L. Trend of the research on construction and demolition waste management. Waste Manag. 2011, 31, 670–679. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Burman, M.; Braimah, N. Pathways to circular construction: An integrated management of construction and demolition waste for resource recovery. J. Clean. Prod. 2020, 244. [Google Scholar] [CrossRef]
- Fischer, C.; Werge, M. EU as a Recycling Society Present Recycling Levels of Municipal Waste and Construction & Demolition Waste in the EU; European Topic Centre on Sustainable Consumption and Production: Copenhagen, Denmark, 2009. [Google Scholar]
- Gálvez-Martos, J.L.; Styles, D.; Schoenberger, H.; Zeschmar-Lahl, B. Construction and demolition waste best management practice in Europe. Resour. Conserv. Recycl. 2018, 136, 166–178. [Google Scholar] [CrossRef] [Green Version]
- Dos Reis, G.S.; Quattrone, M.; Ambrós, W.M.; Cazacliu, B.G.; Sampaio, C.H. Current applications of recycled aggregates from construction and demolition: A review. Materials 2021, 14, 1700. [Google Scholar] [CrossRef]
- Capasso, I.; Liguori, B.; Ferone, C.; Caputo, D.; Cioffi, R. Strategies for the valorization of soil waste by geopolymer production: An overview. J. Clean. Prod. 2021, 288, 125646. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Valencia-Saavedra, W.; de Gutiérrez, R.M. Construction and demolition waste (Cdw) recycling—As both binder and aggregates—In alkali-activated materials: A novel re-use concept. Sustainability 2020, 12, 5775. [Google Scholar] [CrossRef]
- Kvočka, D.; Lešek, A.; Knez, F.; Ducman, V.; Panizza, M.; Tsoutis, C.; Bernardi, A. Life cycle assessment of prefabricated geopolymeric façade cladding panels made from large fractions of recycled construction and demolition waste. Materials 2020, 13, 3931. [Google Scholar] [CrossRef]
- De Brito, J.; Agrela, F.; Silva, R.V. Construction and demolition waste. In New Trends in Eco-Efficient and Recycled Concrete; Woodhead Publishing: Cambridge, UK, 2018; ISBN 9780081024805. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, Z. Reuse of Clay Brick Waste in Mortar and Concrete. Adv. Mater. Sci. Eng. 2020, 31, 218–226. [Google Scholar] [CrossRef]
- José, J.; Eras, C.; Sagastume, A.; Hernández, D.; Hens, L.; Vandecasteele, C. Improving the environmental performance of an earthwork project using cleaner production strategies. J. Clean. Prod. 2013, 47, 368–376. [Google Scholar] [CrossRef]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction—Part 2. J. Appl. Biomater. Funct. Mater. 2018, 16, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Youssef, N.; Rabenantoandro, A.Z.; Dakhli, Z.; Chapiseau, C.; Waendendries, F.; Hage Chehade, F.; Lafhaj, Z. Reuse of waste bricks: A new generation of geopolymer bricks. SN Appl. Sci. 2019, 1, 1252. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Global Warming Impact on the Cement and Aggregates Industries. World Res. Rev. 1994, 6, 263–278. [Google Scholar]
- Liguori, B.; Capasso, I.; De Pertis, M.; Ferone, C.; Cioffi, R. Geopolymerization Ability of Natural and Secondary Raw Materials by Solubility Test in Alkaline Media. Environments 2017, 4, 56. [Google Scholar] [CrossRef] [Green Version]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Roviello, G.; Capasso, I.; Caputo, D.; Aprea, P.; Liguori, B.; Ferone, C. Thermal cycling stability of fly ash based geopolymer mortars. Compos. Part B Eng. 2017, 129, 11–17. [Google Scholar] [CrossRef]
- Luhar, S.; Cheng, T.W.; Nicolaides, D.; Luhar, I.; Panias, D.; Sakkas, K. Valorisation of glass waste for development of Geopolymer composites—Mechanical properties and rheological characteristics: A review. Constr. Build. Mater. 2019, 220, 547–564. [Google Scholar] [CrossRef]
- Kinnunen, P.; Ismailov, A.; Solismaa, S.; Sreenivasan, H.; Räisänen, M.L.; Levänen, E.; Illikainen, M. Recycling mine tailings in chemically bonded ceramics—A review. J. Clean. Prod. 2018, 174, 634–649. [Google Scholar] [CrossRef] [Green Version]
- Lirer, S.; Liguori, B.; Capasso, I.; Flora, A.; Caputo, D. Mechanical and chemical properties of composite materials made of dredged sediments in a fly-ash based geopolymer. J. Environ. Manag. 2017, 191, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Capasso, I.; Lirer, S.; Flora, A.; Ferone, C.; Cioffi, R.; Caputo, D.; Liguori, B. Reuse of mining waste as aggregates in fly ash-based geopolymers. J. Clean. Prod. 2019, 220, 65–73. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Production of eco-friendly bricks from copper mine tailings through geopolymerization. Constr. Build. Mater. 2012, 29, 323–331. [Google Scholar] [CrossRef]
- UNI EN 15801:2010—Conservation of Cultural Property—Test Methods—Determination of Water Absorption by Capillarity 2010. Available online: http://store.uni.com/catalogo/uni-en-15801-2010 (accessed on 10 May 2021).
- UNI 11060:2003—Beni Culturali—Materiali Lapidei Naturali ed Artificiali—Determinazione della Massa Volumica e Della Percentuale di Vuoti 2003. Available online: http://store.uni.com/catalogo/uni-11060-2003?josso_back_to=http://store.uni.com/josso-security-check.php&josso_cmd=login_optional&josso_partnerapp_host=store.uni.com (accessed on 10 May 2021).
- EN 13279-2: 2014—Gypsum Binders and Gypsum Plasters—Part 2: Test Methods. 2014. Available online: https://standards.iteh.ai/catalog/standards/cen/7404ca86-7b9d-4584-945c-18b20c974ba2/en-13279-2-2014 (accessed on 10 May 2021).
- Ferone, C.; Liguori, B.; Capasso, I.; Colangelo, F.; Cioffi, R.; Cappelletto, E.; Di Maggio, R. Thermally treated clay sediments as geopolymer source material. Appl. Clay Sci. 2015, 107, 195–204. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. Attenuated Total Reflectance Fourier Transform Infrared Analysis of Fly Ash Geopolymer Gel Aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef]
- Swanepoel, J.C.; Strydom, C.A. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 1143–1148. [Google Scholar] [CrossRef]
- Verdolotti, L.; Iannace, S.; Lavorgna, M.; Lamanna, R. Geopolymerization reaction to consolidate incoherent pozzolanic soil. J. Mater. Sci. 2008, 43, 865–873. [Google Scholar] [CrossRef]
- Alexandre Bogas, J.; Gomes, M.G.; Real, S. Capillary absorption of structural lightweight aggregate concrete. Mater. Struct. Constr. 2015, 48, 2869–2883. [Google Scholar] [CrossRef]
- Karagiannis, N.; Karoglou, M.; Bakolas, A.; Moropoulou, A. Building Materials Capillary Rise Coefficient: Concepts, Determination and Parameters Involved. In Building Pathology and Rehabilitation; Springer: Singapore, 2016; Volume 6, pp. 27–44. [Google Scholar] [CrossRef]
- Villoria Sáez, P.; del Río Merino, M.; Atanes Sánchez, E.; Santa Cruz Astorqui, J.; Porras-Amores, C. Viability of Gypsum Composites with Addition of Glass Waste for Applications in Construction. J. Mater. Civ. Eng. 2019, 31, 04018403. [Google Scholar] [CrossRef]
- San-Antonio-González, A.; Del Río Merino, M.; Viñas Arrebola, C.; Villoria-Sáez, P. Lightweight material made with gypsum and extruded polystyrene waste with enhanced thermal behaviour. Constr. Build. Mater. 2015, 93, 57–63. [Google Scholar] [CrossRef]
- Capasso, I.; Iucolano, F. Production of lightweight gypsum using a vegetal protein as foaming agent. Mater. Struct. Constr. 2020, 53, 35. [Google Scholar] [CrossRef]
- Verdolotti, L.; Liguori, B.; Capasso, I.; Errico, A.; Caputo, D.; Lavorgna, M.; Iannace, S. Synergistic effect of vegetable protein and silicon addition on geopolymeric foams properties. J. Mater. Sci. 2014, 50, 2459–2466. [Google Scholar] [CrossRef]
- Liguori, B.; Capasso, I.; Romeo, V.; D’Auria, M.; Lavorgna, M.; Caputo, D.; Iannace, S.; Verdolotti, L. Hybrid geopolymeric foams with diatomite addition: Effect on chemico-physical properties. J. Cell. Plast. 2017, 53, 525–536. [Google Scholar] [CrossRef]
- Iucolano, F.; Liguori, B.; Colella, C. Fibre-reinforced lime-based mortars: A possible resource for ancient masonry restoration. Constr. Build. Mater. 2013, 38, 785–789. [Google Scholar] [CrossRef]
- Verdolotti, L.; Iucolano, F.; Capasso, I.; Lavorgna, M.; Iannace, S.; Liguori, B. Recycling and recovery of PE-PP-PET-based fiber polymeric wastes as aggregate replacement in lightweight mortar: Evaluation of environmental friendly application. Environ. Prog. Sustain. Energy 2014, 33, 1445–1451. [Google Scholar] [CrossRef]
- de Oliveira Andrade, J.J.; Possan, E.; Squiavon, J.Z.; Ortolan, T.L.P. Evaluation of mechanical properties and carbonation of mortars produced with construction and demolition waste. Constr. Build. Mater. 2018, 161, 70–83. [Google Scholar] [CrossRef]
- San-Antonio-Gonzalez, A.; Santos Jimenez, R.; del Rio Merino, M.; Gonzalez Cortina, M.; Vinas Arrebola, C. Feasibility of Recycling CDW as Raw Material in Gypsum Composites. Athens J. Τechnol. Eng. 2015, 2, 149–160. [Google Scholar] [CrossRef]
- Iucolano, F.; Boccarusso, L.; Langella, A. Hemp as eco-friendly substitute of glass fibres for gypsum reinforcement: Impact and flexural behaviour. Compos. Part B Eng. 2019, 175. [Google Scholar] [CrossRef]





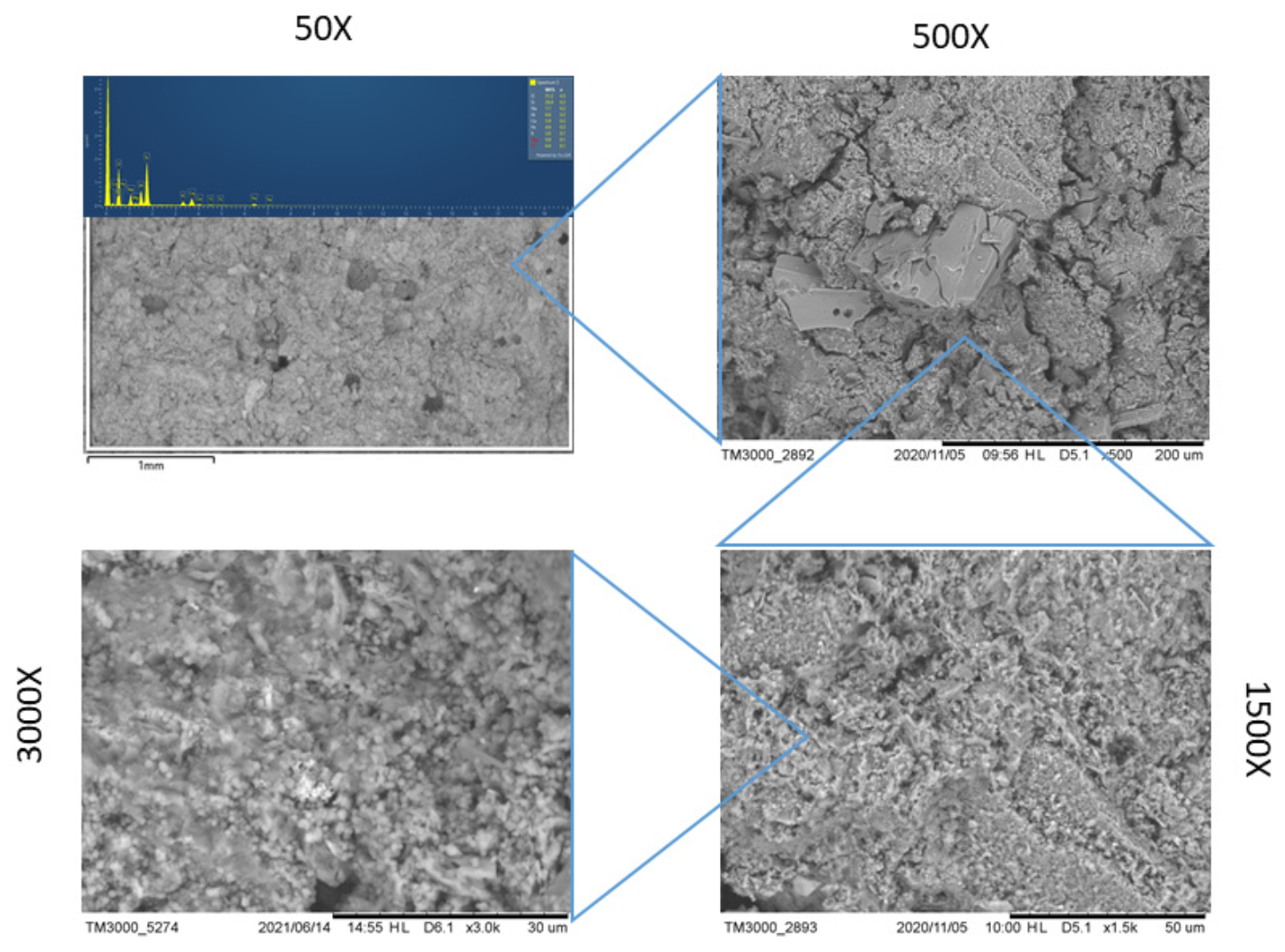

| Major Elements (wt%) | ||||||
|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | CaO |
| 47.90 | 31.82 | 2.99 | 4.14 | 3.75 | 3.59 | 4.52 |
| XRD mineralogical phases | ||||||
| Quartz | Calcium Carbonate | Sanidine | Albite | |||
| Apparent Density (g/cm3) | Real Density (g/cm3) | Open Porosity (%) |
|---|---|---|
| 1.39 ± 0.02 | 2.56 ± 0.02 | 45.68 ± 0.37 |
| Water absorption * (%) | Water absorption (%) | CA (mg/cm2 s−1/2) |
| 16.65 ± 0.35 | 32.81 ± 0.67 | 18.24 ± 0.25 |
| Sample | Point 1 | Point 2 | Point 3 | Point 4 | Point 5 |
|---|---|---|---|---|---|
| Geo_CBW 1 | 85 | 76 | 85 | 86 | 90 |
| 86 | 86 | 72 | 80 | 86 | |
| Geo_CBW 2 | 84 | 86 | 86 | 82 | 89 |
| 76 | 85 | 73 | 81 | 80 | |
| Geo_CBW 3 | 85 | 80 | 75 | 86 | 86 |
| 73 | 81 | 74 | 84 | 87 |
| Sample | Reference | Density (kg/m3) | RF (MPa) | RC (MPa) |
|---|---|---|---|---|
| Geo_CBW | 1390 | 2.85 | 5.34 | |
| Lightweight gypsum | [36] | 910 | 1.52 | 2.17 |
| Metakaolin-based foam | [37] | 1000 | 0.14 | 4.62 |
| Diatomite-based foam | [38] | 423 | 0.63 | 1.49 |
| NHL | [39] | 2.15 | 5.55 | |
| NHL + 2% glass fibers | [39] | 1590 | 2.41 | 3.62 |
| NHL with plastic waste aggregate | [40] | 1670 | 0.60 | 1.25 |
| Dredged sediments geocomposite | [20] | / | / | 1.90 |
| Cement mortar with mixed recycled aggregate | [41] | 1660 | 2.38 | 5.20 |
| Gypsum + 1% polystyrene waste | [35] | 970 | 2.89 | 5.64 |
| Gypsum Plaster with ceramic waste from bricks | [42] | 1180 | 2.80 | 5.40 |
| Gypsum composites with glass waste | [34] | 1270 | 2.93 | 6.01 |
| Gypsum plaster with hemp fibers | [43] | / | 2.50 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, G.; Fumo, M.; Merino, M.d.R.; Capasso, I.; Campanile, A.; Iucolano, F.; Caputo, D.; Liguori, B. Crushed Bricks: Demolition Waste as a Sustainable Raw Material for Geopolymers. Sustainability 2021, 13, 7572. https://doi.org/10.3390/su13147572
D’Angelo G, Fumo M, Merino MdR, Capasso I, Campanile A, Iucolano F, Caputo D, Liguori B. Crushed Bricks: Demolition Waste as a Sustainable Raw Material for Geopolymers. Sustainability. 2021; 13(14):7572. https://doi.org/10.3390/su13147572
Chicago/Turabian StyleD’Angelo, Gigliola, Marina Fumo, Mercedes del Rio Merino, Ilaria Capasso, Assunta Campanile, Fabio Iucolano, Domenico Caputo, and Barbara Liguori. 2021. "Crushed Bricks: Demolition Waste as a Sustainable Raw Material for Geopolymers" Sustainability 13, no. 14: 7572. https://doi.org/10.3390/su13147572








