Long-Term Changes in Floristic Diversity as an Effect of Transforming the Lake into a Retention Reservoir
Abstract
:1. Introduction
2. Methods and Study Area
2.1. Study Area
2.2. Cartographic Analyses
2.3. Floristic Analyses
2.4. Statistical Analyses
3. Results
3.1. Changes in the Development of the Lake Surroundings
3.2. Vegetation Structure
- BCd (t1, t2) = 0.4539
- BCd (t1, t3) = 0.5164
- BCd (t2, t3) = 0.4073
- t1: 2018–2020
- t2: 1977–1980
- t3: 1950–1960
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dirzo, R.; Raven, P.H. Global state of biodiversity and loss. Annu. Rev. Environ. Res. 2003, 28, 137–167. [Google Scholar] [CrossRef] [Green Version]
- Gopal, B. Biodiversity in wetlands. Wetl. Handb. 2009, 2, 65–95. [Google Scholar]
- Ramsar Convention Secretariat. Global Wetland Outlook: State of the World’s Wetlands and Their Services to People; Ramsar Convention on Wetlands: Gland, Switzerland, 2018; p. 24. [Google Scholar]
- Hassan, R.; Scholes, R.; Ash, N. Ecosystems and Human Well-Being. Current State and Trends; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Maltby, E. Functional Assessment of Wetlands: Towards Evaluation of Ecosystem Services; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- De Groot, R.S.; Wilson, M.A.; Boumans, R.M. A typology for the classification, description and valuation of ecosystem functions, goods and services. Ecol. Econom. 2002, 41, 393–408. [Google Scholar] [CrossRef] [Green Version]
- Ola, O.; Benjamin, E. Preserving biodiversity and ecosystem services in west african forest, watersheds, and wetlands: A review of incentives. Forests 2019, 10, 479. [Google Scholar] [CrossRef] [Green Version]
- Zedler, J.B.; Kercher, S. Wetland resources: Status, trends, ecosystem services and restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wen, L.; Wang, Y.; Liu, C.; Zhou, Y.; Lei, G. Can constructed wetlands be wildlife refuges? A review of their potential biodiversity conservation value. Sustainability 2020, 12, 1442. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, J.T.; Arheimer, B.; Yin, C.; Hefting, M.M. Regional and global concerns over wetlands and water quality. Trends Ecol. Evol. 2006, 21, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.P. Wetland loss and biodiversity conservation. Conserv. Biol. 2000, 14, 314–317. [Google Scholar] [CrossRef] [Green Version]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Wetlands and Water Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Verhoeven, J.T.A.; Setter, T.L. Agricultural use of wetlands: Opportunities and limitations. Ann. Bot. 2010, 105, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Weng, B.; Yan, D.; Wang, K.; Li, X.; Bi, W.; Liu, Y. Wetlands of international importance: Status, threats, and future protection. Int. J. Environ. Res. Public Health 2019, 16, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalczyk, Z. Położenie polesia lubelskiego. In Polesie—Środowisko, Melioracje, Polesie Polskie; Urban, D., Dobrowolski, R., Jeznach, J., Eds.; SGGW: Warsaw, Poland, 2020; Volume 3, pp. 79–84. [Google Scholar]
- Wilgat, T.; Michalczyk, Z.; Turczynski, M.; Wojciechowski, K.H. Jeziora łęczyńsko-włodawskie. Studia Ośrodka Dok. Fizjogr. 1991, 19, 100. [Google Scholar]
- Kondracki, J. Geografia Regionalna Polski; Wydawnictwo PWN: Warsaw, Poland, 2002; p. 440. [Google Scholar]
- Borowiec, J. Torfowiska Regionu lubelskiego; PWN: Warsaw, Poland, 1990; p. 348. [Google Scholar]
- Michalczyk, Z.; Chmiel, S.; Głowacki, S.; Mięsiak-Wójcik, K.; Raczyński, K.; Sposób, J.; Turczyński, M. Charakterystyka wód. In Polesie—Środowisko, Melioracje, Polesie Polskie; Urban, D., Dobrowolski, R., Jeznach, J., Eds.; SGGW: Warsaw, Poland, 2020; Volume 3, pp. 162–237. [Google Scholar]
- Łoś, M.J. Systemy melioracyjne. In Polesie—Środowisko, Melioracje, Polesie Polskie; Urban, D., Dobrowolski, R., Jeznach, J., Eds.; SGGW: Warsaw, Poland, 2020; Volume 3, pp. 892–908. [Google Scholar]
- Fijałkowski, D. The vegetation cover of lakes in the Łęczna and Włodawa area and of peat bogs adjacent to these lakes. Ann. UMCS 1959, 14, 131–206. [Google Scholar]
- Fijałkowski, D. Charakterystyka geobotaniczna kompleksu wodno-torfowiskowego koło Wytyczna w województwie lubelskim. Folia Soc. Sci. Lub. 1971, 11, 3–10. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Vienna, Austria, 2013. [Google Scholar]
- Matuszkiewicz, W. Guide to the Identification of Plant Communities in Poland; PWN: Warsaw, Poland, 2008. (In Polish) [Google Scholar]
- Mirek, Z.; Piękosz-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland. A Checklist; Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002. [Google Scholar]
- Ochyra, R.; Zarnowiec, J.; Bednarek-Ochyra, H. Census Catalogue of Polish Mosses; Polish Academy of Sciences, Institute of Botany: Kraków, Poland, 2003. [Google Scholar]
- Sender, J. Aquatic and rush macroflora. In Lake Skomielno (Łęczna-Włodawa Lake-Land, Eastern Poland), Environmental Monograph; Kornijów, R., Buczyński, P., Eds.; Mantis: Olsztyn, Poland, 2012; pp. 83–120. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulissen, D. Zeigerwerte von pflanzen in mitteleuropa. Scripta Geobot. 1991, 28, 248. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Simpson, E. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Appeltans, W.; Ahyong, S.T.; Anderson, G.; Angel, M.V.; Artois, T.; Bailly, N.; Costello, M.J. The magnitude of global marine species diversity. Curr. Biol. 2012, 22, 2189–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladle, R.J. Forecasting extinctions: Uncertainties and limitations. Diversity 2009, 1, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Blindow, I.; Andersson, G.; Hargeby, A.; Johansson, S. Long-term pattern of alternative stable states in two shallow eutrophic lakes. Freshw. Biol. 1993, 30, 159–167. [Google Scholar] [CrossRef]
- López Moreira, M.G.A.; Hinegk, L.; Salvadore, A.; Zolezzi, G.; Hölker, F.; Monte Domecq, S.R.A.; Toffolon, M. Eutrophication, research and management history of the shallow Ypacaraí Lake (Paraguay). Sustainability 2018, 10, 2426. [Google Scholar] [CrossRef] [Green Version]
- Hellsten, S.; Marttunen, M.; Palomäki, R.; Riihimäki, J.; Alasaarela, E. Towards an ecologically based regulation practice in Finnish hydroelectric lakes. Regul. Rivers Res. Manag. 1996, 12, 535–545. [Google Scholar] [CrossRef]
- Soszka, H.; Pasztaleniec, A.; Koprowska, K.; Kolada, A.; Ochocka, A. Influence of hydromorphological transformations of lakes on pools of aquatic organisms—A review of the literature. Ochr. Sr. Zasobów Nat. 2012, 50, 24–52. (In Polish) [Google Scholar]
- Kutyła, S. Characteristics of water level fluctuations in Polish lakes–A review of the literature. Ochr. Sr. Zasobów Nat. 2014, 25, 27–34. [Google Scholar]
- Maihemuti, B.; Aishan, T.; Simayi, Z.; Alifujiang, Y.; Yang, S. Temporal scaling of water level fluctuations in shallow lakes and its impacts on the lake eco-environments. Sustainability 2020, 12, 3541. [Google Scholar] [CrossRef]
- Van Geest, G.J.; Coops, H.; Scheffer, M.; Van Nes, E.H. Long transients near the ghost of a stable state in eutrophic shallow lakes with fluctuating water levels. Ecosystems 2007, 10, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Hellsten, S. Environmental Factors and Aquatic Macrophytes in the Littoral of Regulated Lakes: Causes, Consequences and Possibilities to Alleviate Harmful Effects; Oulun Yliopisto: Oulun, Finland, 2000. [Google Scholar]
- Rodrigo, M.A. Wetland restoration with hydrophytes: A review. Plants 2021, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Rørslett, B. Principal determinants of aquatic macrophyte richness in northern European lakes. Aquat. Bot. 1991, 39, 173–193. [Google Scholar] [CrossRef]
- Hellsten, S.; Mjelde, M. Macrophyte responses to water level fluctuation in Fennoscandinavian lakes—Applying a common index. Int. Ver. Theor. Angew. Limnol. Verh. 2009, 30, 765–769. [Google Scholar] [CrossRef]
- Yang, Z.D.; Yuan, S.B.; Liu, X.Q.; Wang, H.Z. Water level fluctuation requirements of emergent macrophyte Typha angustifolia L. Water 2020, 12, 127. [Google Scholar] [CrossRef] [Green Version]
- Jennings, M.J.; Emmons, E.E.; Hatzenbeler, G.R.; Edwards, C.; Bozek, M.A. Is littoral habitat affected by residential development and land use in watersheds of Wisconsin lakes? Lake Reserv. Manag. 2003, 19, 272–279. [Google Scholar] [CrossRef]
- Soja, R. The influence of reservoirs on the inanimate nature of the Pieniny Mountains. Pieniny–Dam–Changes. Monogr. Pienińskie 2010, 2, 37–41. (In Polish) [Google Scholar]
- Wojciechowski, K.H. Connection of lake waters with surface and ground waters. Studia Ośrodka Dok. Fizjogr. 1991, 19, 95–101. (In Polish) [Google Scholar]
- Radwan, S.; Kornijów, R. Hydrobiological and Hydrochemical Characteristics of Surface Waters. Natural Environment in the Impact Zone of the Wieprz-Krzna Canal; AR Lublin, TWWP: Lublin, Poland, 1994; pp. 47–58. (In Polish) [Google Scholar]
- Borysiak, J. The Structure of Alluvial Land Vegetation of the Middle and Lower Warta River; UAM Poznań, sect Biologia; Wydawnictwo Naukowe Uniwersytetu im. Adama Mickiewicza: Poznan, Poland, 1994; p. 52. [Google Scholar]
- Matuszkiewicz, J.M.; Roo-Zielińska, E. Inter-Embankment of the Vistula as a Specific Natural System: (Section Pilica-Narew); Dokumentacja Geograficzna: Warsaw, Poland, 2000. [Google Scholar]
- Rodzik, J.; Dobrowolski, R.; Melke, J. Estimation of kind, amount and mechanism of sedimentation in the Zemborzyce reservoir near Lublin. Teka. Kom. Ochr. Środ. Przyr. 2009, 6, 261–276. [Google Scholar]
- Sender, J.; Jaruga, C. Water eutrophication of dam reservoirs and the role of macrophytes in this process. Inżynieria Ekol. 2017, 18, 3. [Google Scholar]
- Serafin, A.; Sender, J.; Bronowicka-Mielniczuk, U. Potential of shrubs, shore vegetation and macrophytes of a lake to function as a phytogeochemical barrier against biogenic substances of various origin. Water 2019, 11, 290. [Google Scholar] [CrossRef] [Green Version]
- Sender, J.; Kułak, A. Phytocenotic structure and physico-chemical properties of a small water body in agricultural landscape. Acta Agrobot. 2014, 67, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Dembek, W. Problems of protection and restitution of wetlands in Poland. Inżynieria Ekol. 2002, 6, 68–85. [Google Scholar]
- Barabach, J.; Milecka, K. Anthropogenic Transformations of Rzęcin Mire Observed in Aerial Photos; Archiwum Fotogrametrii, Kartografii i Teledetekcji: Warsaw, Poland, 2013. [Google Scholar]
- Semeraro, T.; Giannuzzi, C.; Beccarisi, L.; Aretano, R.; De Marco, A.; Pasimeni, M.R.; Zurlini, G.; Petrosillo, I. A constructed treatment wetland as an opportunity to enhance biodiversity and ecosystem services. Ecol. Eng. 2015, 82, 517–526. [Google Scholar] [CrossRef]
- Solon, J. Anthropogenic disturbance and vegetation diversity in agricultural landscapes. Landsc. Urban Plan. 1995, 31, 171–180. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, D.; Dong, J.; Tan, S.K. Application of constructed wetlands for treating agricultural runoff and agro-industrial wastewater: A review. Hydrobiologia 2018, 805, 1–31. [Google Scholar] [CrossRef]
- Gacka-Grzesikiewicz, E. Ecological Corridor of the Vistula Valley; Fundacja IUCN Poland: Warsaw, Poland, 1995. [Google Scholar]
- Kowalska, A. Influence of Embankments on the Diversity of Vegetation of the Floodplain in the Middle Vistula Valley, Mazowiecka Lowland; Prace Komisji Krajobrazu Kulturowego: Sosnowiec, Poland, 2010. [Google Scholar]
- Solis, M. The influence of the Wieprz-Krzna Canal on the physical, chemical and biological properties of water in selected retention reservoirs. Inżynieria Ekol. 2012, 29, 182–191. [Google Scholar]
- Sender, J. Impact of the drainage system on water vegetation of the lowland lakes (Eastern Poland). Turk. J. Fish. Aquat. Sci. 2018, 18, 611–622. [Google Scholar] [CrossRef]
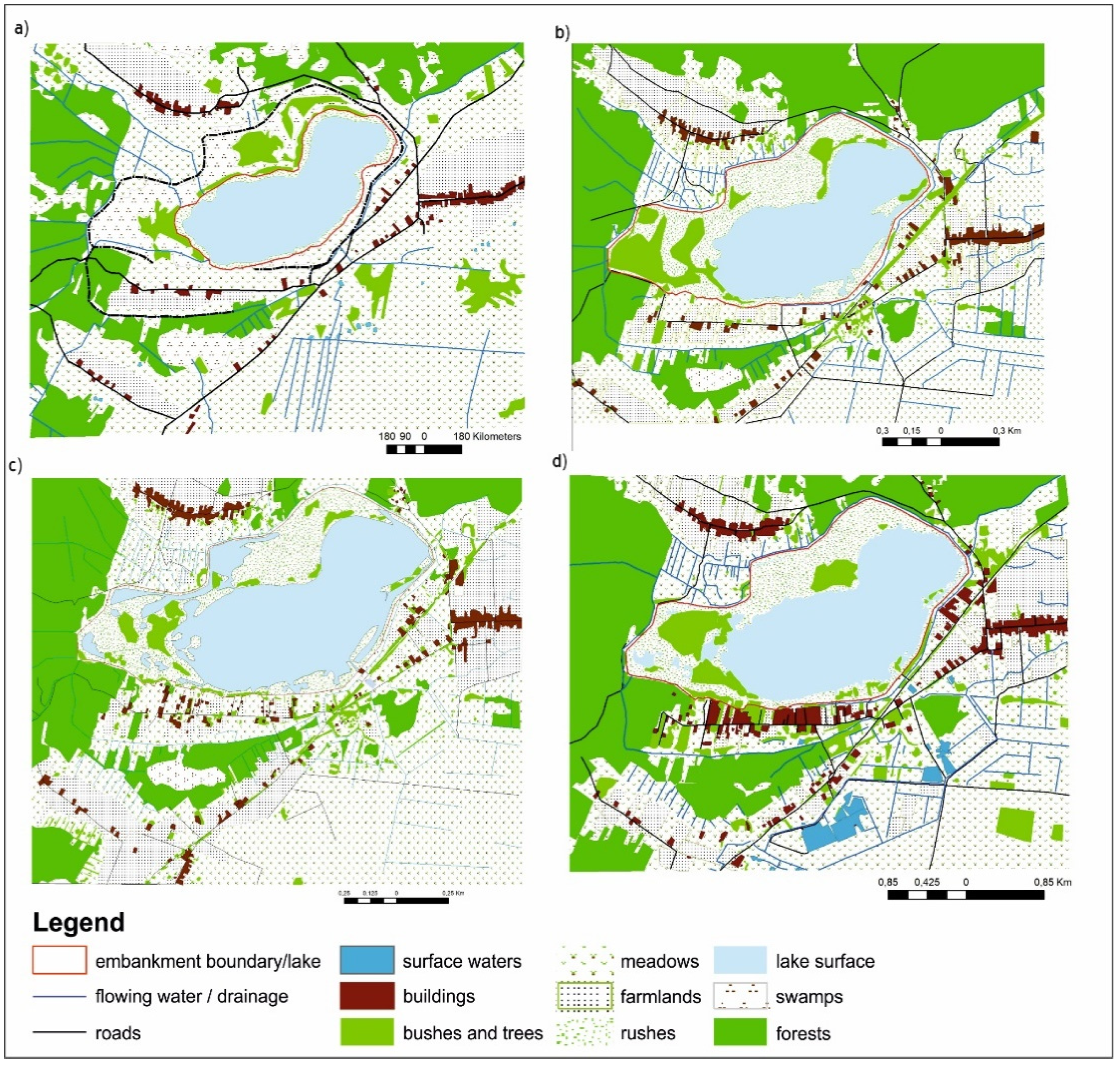
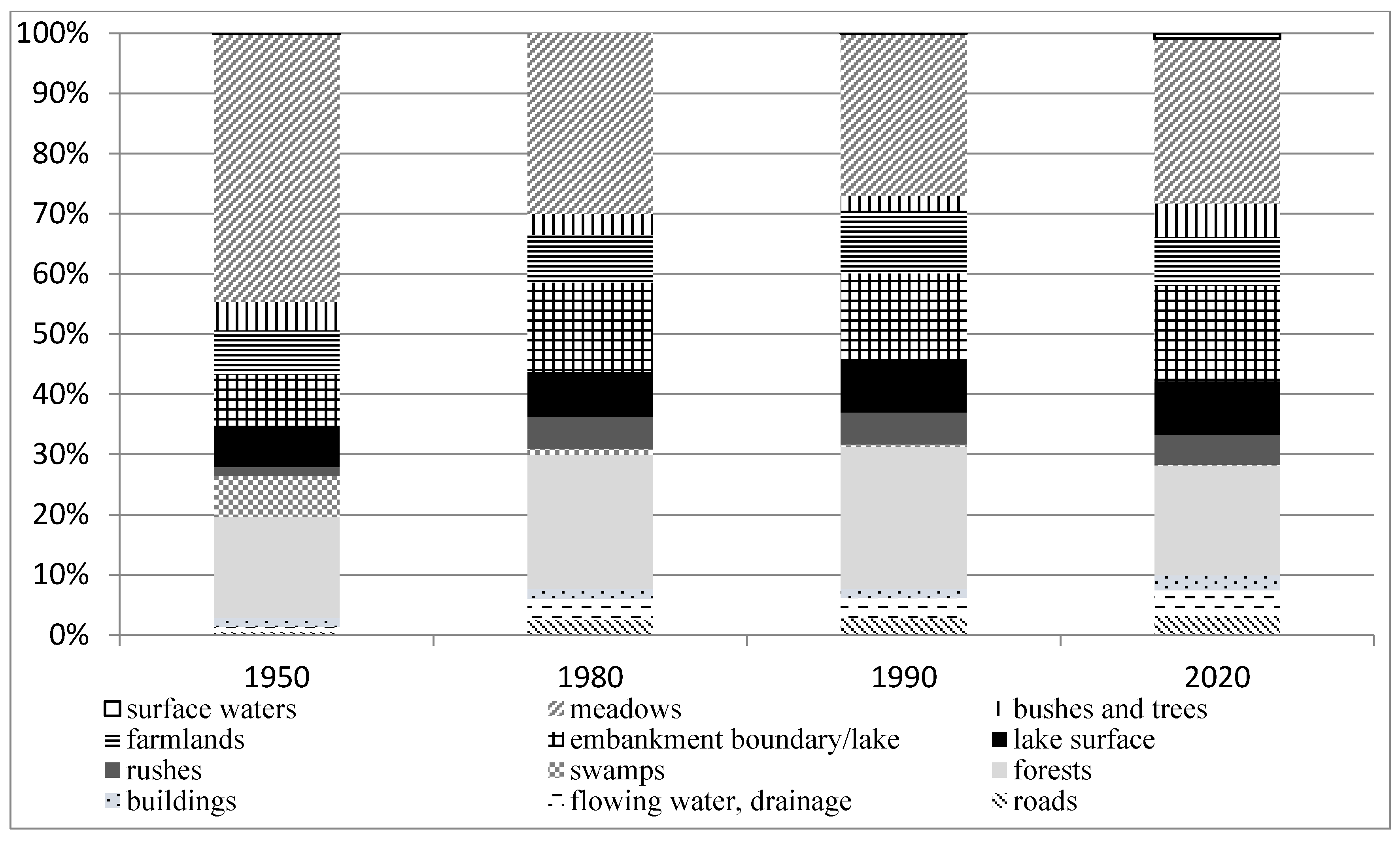

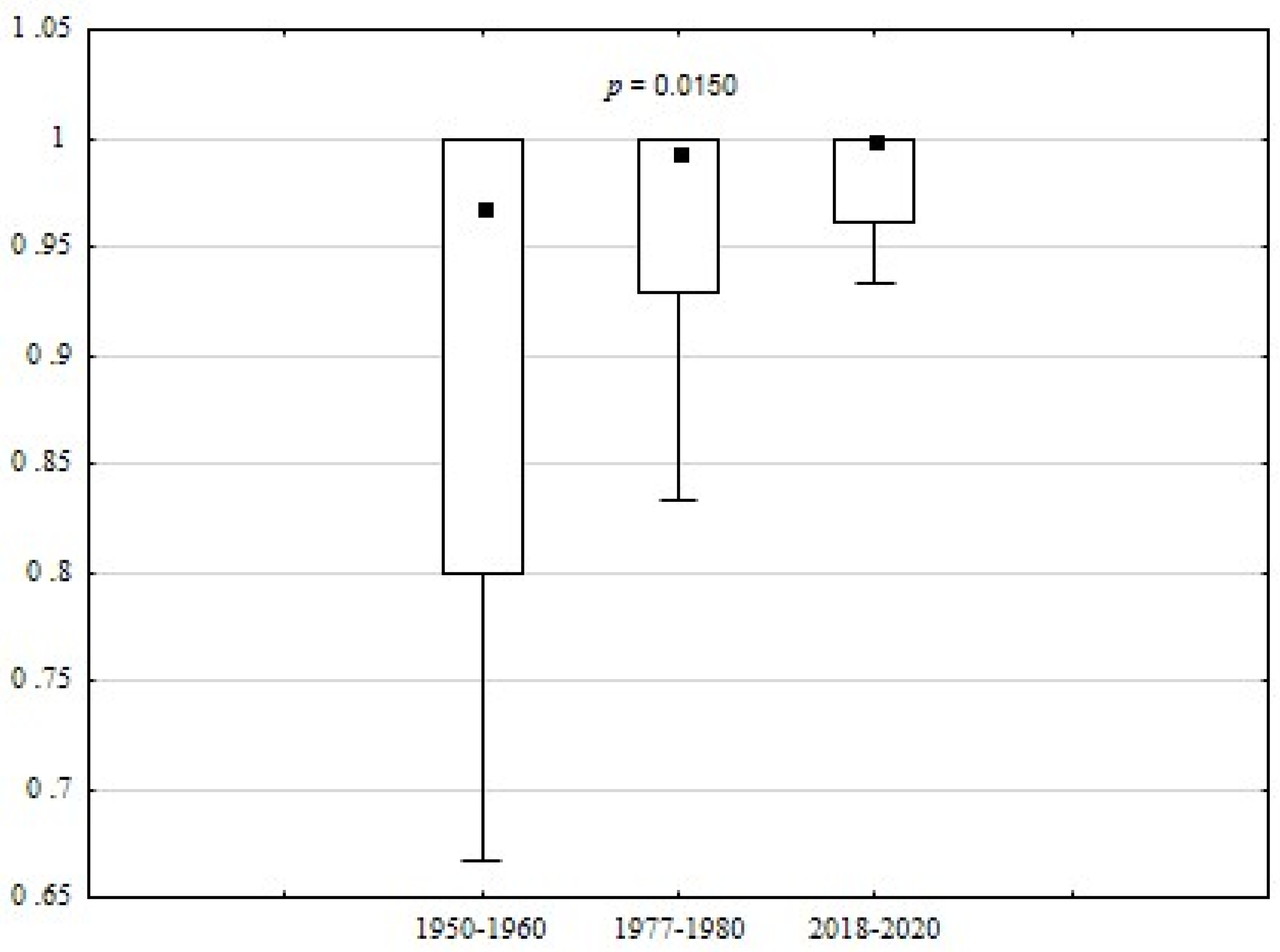

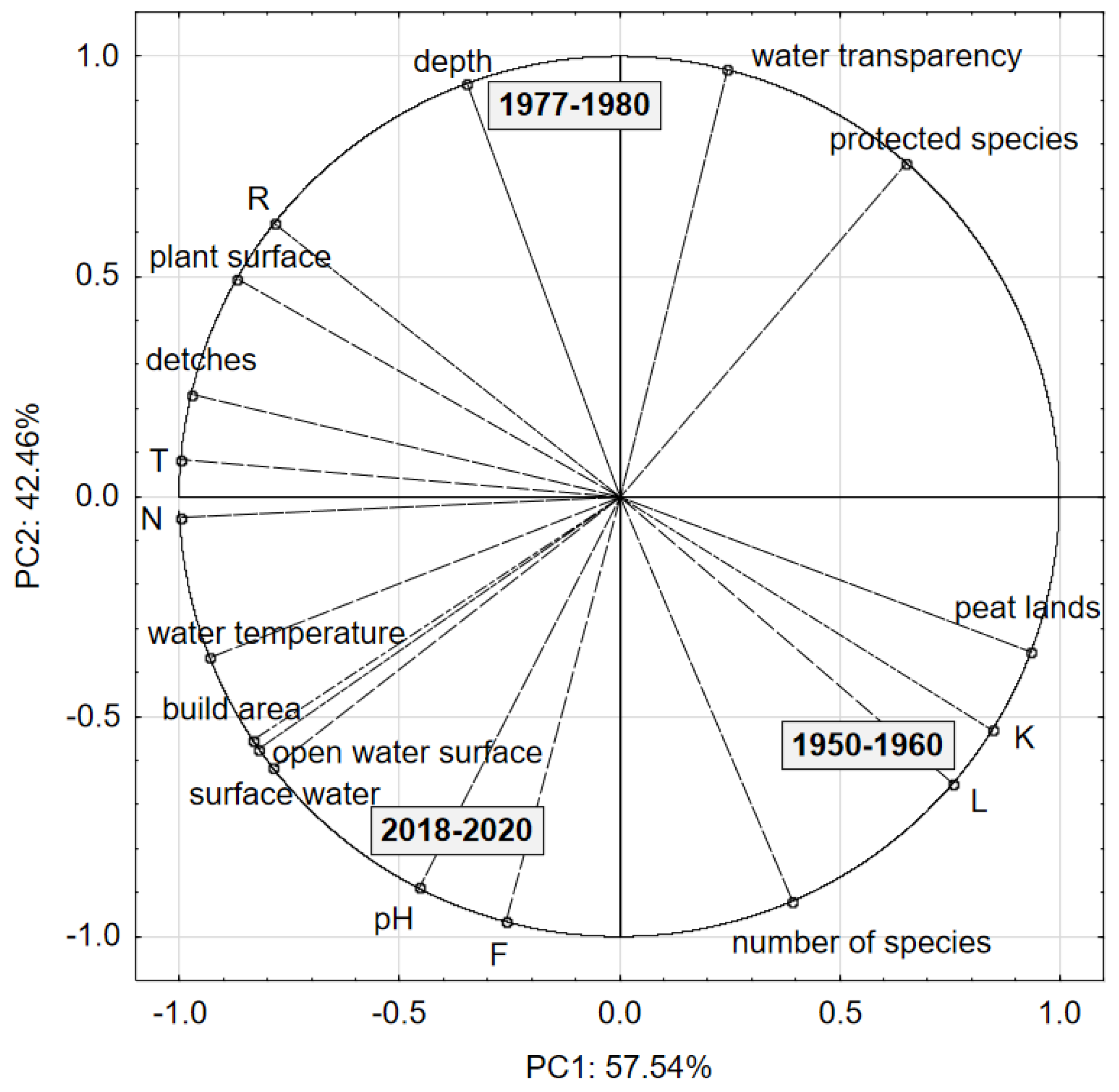
| Feature | p-Value |
|---|---|
| roads | 0.1617 |
| flowing waters/ drainage | 0.0461 |
| buildings | 0.0229 |
| forests | 0.4048 |
| swamps | 0.0511 |
| rushes | 0.0814 |
| water surface | 0.7699 |
| lake surface | 0.1035 |
| farmlands | 0.8491 |
| bushes and trees | 0.2360 |
| meadows | 0.0215 |
| surface waters | 0.0313 |
| Class | Number of Communities | 1950–1960 | 1977–1980 | 2018–2020 |
|---|---|---|---|---|
| Lemnetea minoris | 3 | 0 | 3 | 3 |
| Bidentetea tripartiti | 1 | 0 | 1 | 1 |
| Charetea | 4 | 4 | 2 | 1 |
| Potametea | 18 | 11 | 16 | 15 |
| Utricularietea intermeio-mnoris | 1 | 0 | 1 | 0 |
| Phragmitetea | 24 | 12 | 22 | 19 |
| Molinio-Arrhenatheretea | 8 | 5 | 8 | 0 |
| Scheuchzerio-Caricetea | 6 | 5 | 4 | 0 |
| Alnetea glutinosae | 3 | 3 | 3 | 2 |
| Querco-Fagetea | 1 | 1 | 1 | 0 |
| In total | 69 | 41 | 61 | 41 |
| Habitat | Conservation Status | 1950 | 1980 | 2020 |
|---|---|---|---|---|
| Nardus grasslands and moors | + | |||
| Xerothermic grasslands | 6210 | + | + | |
| Variable wet Molinia meadows | 64,140 | + | + | |
| Transitional bogs and quaking bogs | 7140 | + | + | |
| Deciduous forest | + | + | ||
| Swamp forest | + | + | + | |
| Alder thickets | + | + | + | |
| Willow riparian forest | + | + | ||
| Farmland | + | + | ||
| Forest clearance | + | |||
| Ruderal | + | + | + | |
| Dry on a substrate rich in minerals | + | |||
| Ecotone | + | |||
| Water | + | + | + | |
| In total | 10 | 14 | 4 |
| 1950–1960 | 1977–1980 | 2018–2020 | |
|---|---|---|---|
| Class in years | |||
| DMg | 3.274 | 4.520 | 3.609 |
| HS | 2.505 | 2.537 | 1.672 |
| S | 0.813 | 0.783 | 0.607 |
| Communities in the years | |||
| DMg | 7.485 | 8.528 | 4.910 |
| HS | 3.304 | 3.469 | 2.679 |
| S | 0.869 | 0.866 | 0.789 |
| L Light | T Temperature | K Continentalism | F Moisture | R Reaction | N Trophy | |||
|---|---|---|---|---|---|---|---|---|
| 1950–1960 | ||||||||
| Number of species | 153 | 110 | 112 | 145 | 116 | 134 | ||
| Mean | 7.05 | 5.34 | 4.38 | 8.62 | 5.87 | 4.33 | ||
| V | 6.18 | 6 | 2.81 | 4.09 | 3.07 | 2.15 | ||
| SD | 1.14 | 0.83 | 1.56 | 2.11 | 1.91 | 2.01 | ||
| Range | 4–9 | 2–8 | 1–8 | 2–12 | 1–9 | 1–8 | ||
| 1977–1980 | ||||||||
| Number of species | 273 | 185 | 206 | 249 | 190 | 238 | ||
| Mean | 6.90 | 5.48 | 4.13 | 7.72 | 6.22 | 4.90 | ||
| V | 5.90 | 7.31 | 2.85 | 3.27 | 3.72 | 2.34 | ||
| SD | 1.17 | 0.75 | 1.45 | 2.36 | 1.67 | 2.09 | ||
| Range | 3–9 | 2–8 | 1–8 | 2–12 | 1–9 | 1–9 | ||
| 2018–2020 | ||||||||
| Number of species | 111 | 85 | 83 | 108 | 82 | 97 | ||
| Mean | 6.94 | 5.56 | 4.16 | 9.07 | 6.14 | 5.42 | ||
| V | 7.08 | 8.42 | 3.22 | 5.24 | 3.77 | 2.91 | ||
| SD | 0.98 | 0.66 | 1.29 | 1.73 | 1.63 | 1.86 | ||
| Range | 4–9 | 4–8 | 1–7 | 4–12 | 1–9 | 1–9 | ||
| Protection Rank | 1950–60 | 1977–80 | 2018–20 |
|---|---|---|---|
| Rare | 7 | 9 | 4 |
| Partial protection | 9 | 17 | 4 |
| Strict protection | 19 | 19 | 0 |
| In total | 35 | 45 | 8 |
| Year | Parameter | min | max | Mean | SD | CV | p |
|---|---|---|---|---|---|---|---|
| 1955 | water temperature (°C) | 16 | 20 | 18.3 | 1.7 | 9.36 | 0.0597 |
| depth (m) | 0.5 | 3.5 | 1.3 | 0.2 | 15.38 | 0.0096 * | |
| open water surface (ha) | 238.6 | 0.00 | |||||
| plant surface (ha) | 52.9 | 0.00 | |||||
| pH | 7.1 | 7.7 | 7.4 | 0.3 | 3.98 | 0.2818 | |
| water transparency (m) | 0.7 | 0.9 | 0.8 | 0.1 | 12.50 | 0.0498 * | |
| ditches | 31.0 | 0.00 | |||||
| built-up area | 49.4 | 0.00 | |||||
| surface water | 3.3 | 0.00 | |||||
| peat lands | 236.0 | 0.00 | |||||
| protected species | 7 | 19 | 11.7 | 6.4 | 55.11 | ||
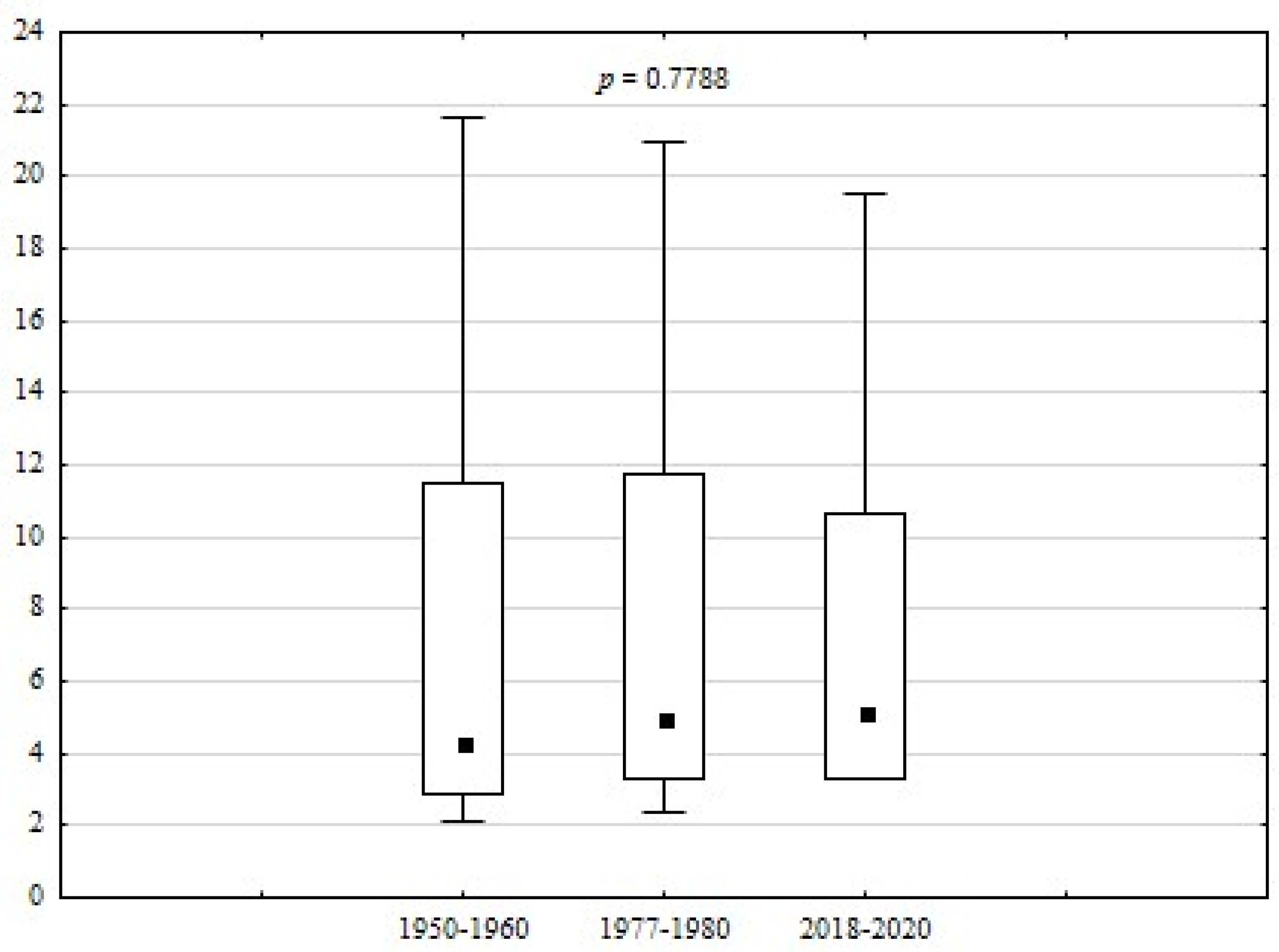
| DMg | 2.1 | 21.7 | 7.4 | 6.2 | 83.26 | 0.7788 | |
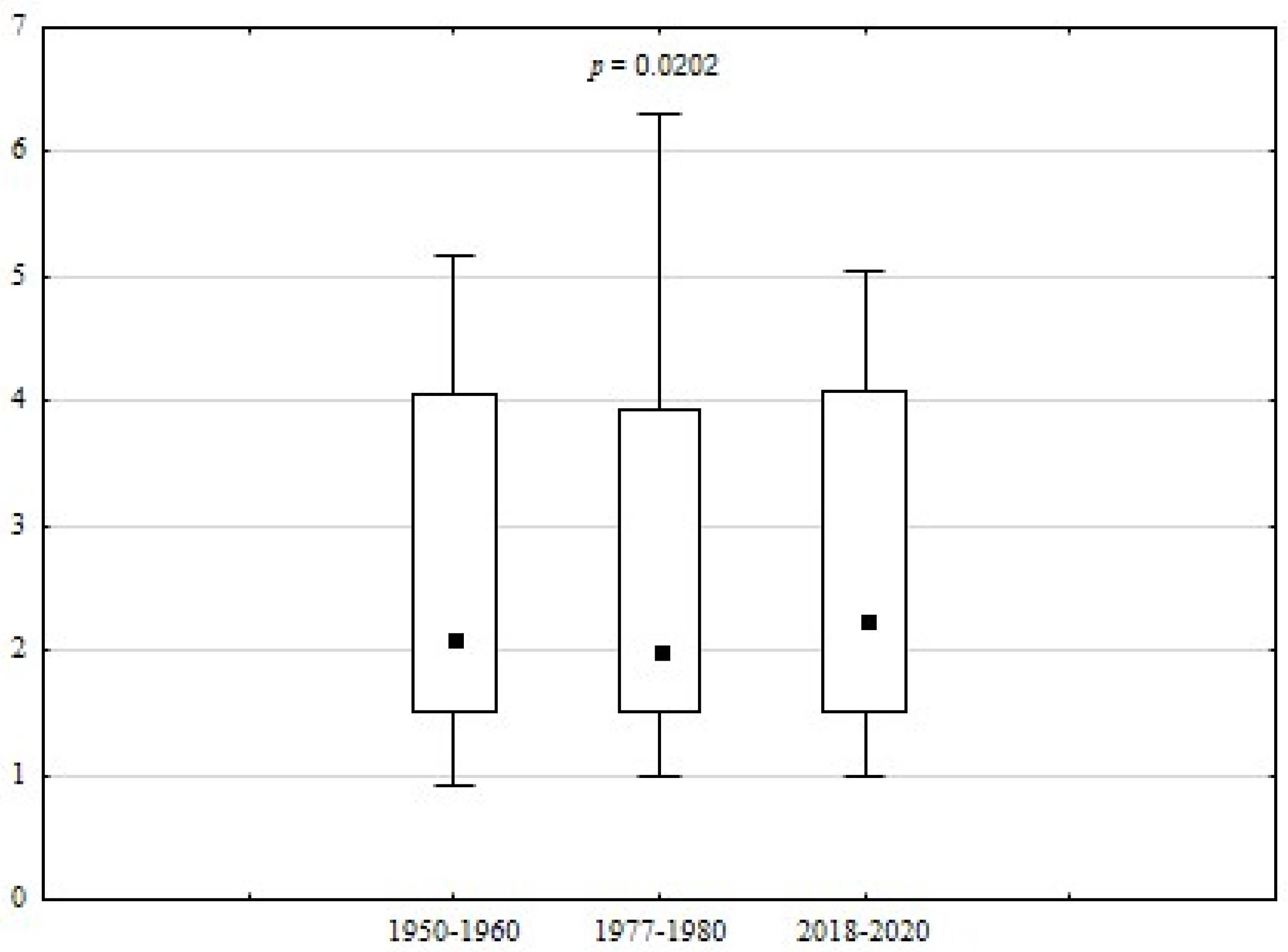
| HS | 0.9 | 5.2 | 2.6 | 1.5 | 56.72 | 0.0202 * | |
| S | 0.0 | 1.0 | 0.8 | 0.00 | 0.0150 * | ||
| 1980 | water temperature (°C) | 18.0 | 20.0 | 19.0 | 0.8 | 4.30 | |
| depth (m) | 1.0 | 5.5 | 2.0 | 0.3 | 12.59 | ||
| open water surface (ha) | 237.6 | 0.00 | |||||
| plant surface (ha) | 173.7 | 0.00 | |||||
| pH | 6.7 | 7.4 | 7.1 | 0.3 | 4.67 | ||
| water transparency (m) | 1.2 | 1.7 | 1.4 | 0.3 | 17.56 | ||
| ditches | 114.1 | 0.00 | |||||
| build-up area | 49.8 | 0.00 | |||||
| surface water | 1.1 | 0.00 | |||||
| peat lands | 28.1 | 0.00 | |||||
| protected species | 9.0 | 19.0 | 15.0 | 5.3 | 35.28 | ||
| DMg | 2.4 | 41.2 | 8.7 | 8.9 | 102.22 | ||
| HS | 1.0 | 6.3 | 2.7 | 1.5 | 57.64 | ||
| S | 1.0 | 22.0 | 6.1 | 7.2 | 118.83 | ||
| species number | 0.8 | 1.0 | 0,9 | 0,1 | 8.97 | ||
| 2020 | water temperature (°C) | 20.0 | 23.0 | 21.5 | 1.3 | 6.00 | |
| depth (m) | 0.5 | 4.0 | 1.5 | 0.4 | 26.67 | ||
| open water surface (ha) | 295.6 | 0.00 | |||||
| plant surface (ha) | 164.7 | 0.00 | |||||
| pH | 7.3 | 8.2 | 7.8 | 0.4 | 5.42 | ||
| water transparency (m) | 0.4 | 0.6 | 0.5 | 0.1 | 20.00 | ||
| ditches | 138.4 | 0.00 | |||||
| build-up area | 82.9 | 0.00 | |||||
| surface water | 30.4 | 0.00 | |||||
| peat lands | 5.0 | 0.00 | |||||
| protected species | 0.0 | 4.0 | 2.7 | 2.3 | 86.60 | ||
| DMg | 3.3 | 19.5 | 7.6 | 5.5 | 72.26 | ||
| HS | 1.0 | 5.0 | 2.6 | 1.4 | 52.61 | ||
| S | 0.0 | 1.0 | 0.8 | 0.3 | 41.10 | ||
| species number | 0.0 | 19.0 | 4.1 | 6.9 | 169.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sender, J.; Urban, D.; Różańska-Boczula, M.; Grzywna, A. Long-Term Changes in Floristic Diversity as an Effect of Transforming the Lake into a Retention Reservoir. Sustainability 2021, 13, 7642. https://doi.org/10.3390/su13147642
Sender J, Urban D, Różańska-Boczula M, Grzywna A. Long-Term Changes in Floristic Diversity as an Effect of Transforming the Lake into a Retention Reservoir. Sustainability. 2021; 13(14):7642. https://doi.org/10.3390/su13147642
Chicago/Turabian StyleSender, Joanna, Danuta Urban, Monika Różańska-Boczula, and Antoni Grzywna. 2021. "Long-Term Changes in Floristic Diversity as an Effect of Transforming the Lake into a Retention Reservoir" Sustainability 13, no. 14: 7642. https://doi.org/10.3390/su13147642






