Medfly Phenotypic Plasticity as A Prerequisite for Invasiveness and Adaptation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Ccollection
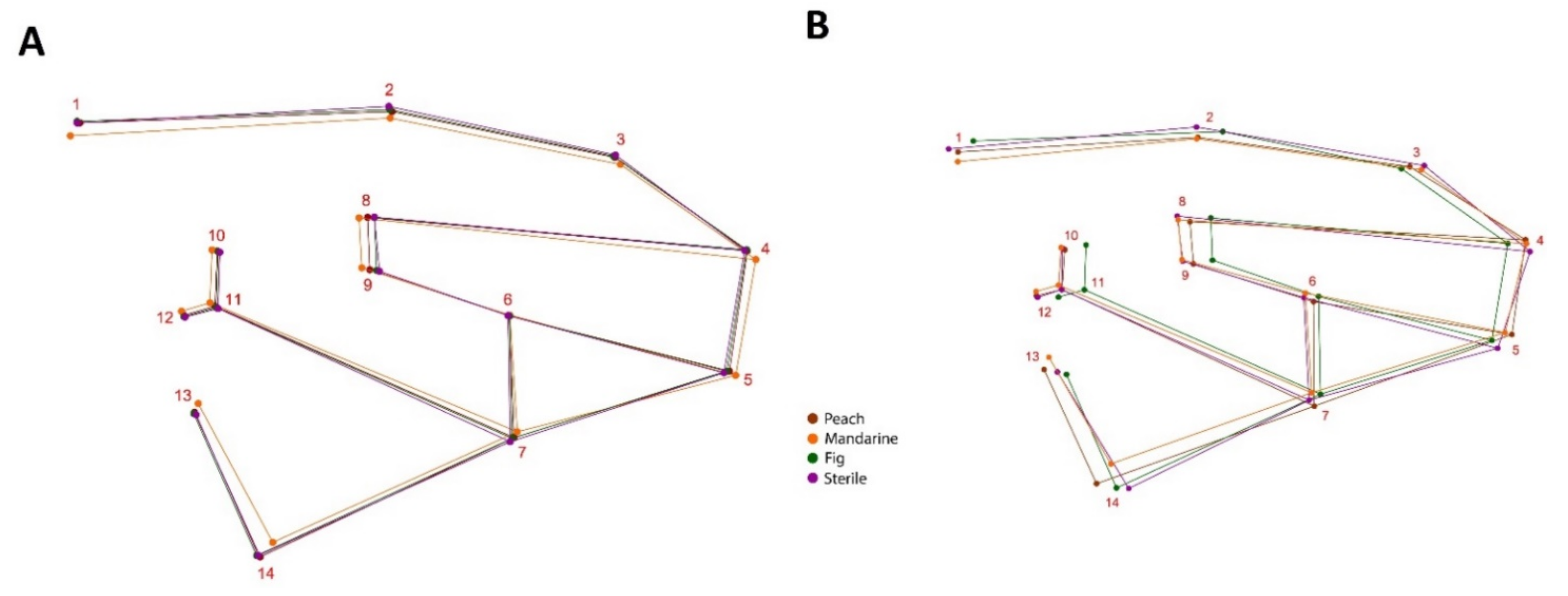
2.2. Data Curation and Geometric Morphometric Analyses
3. Results
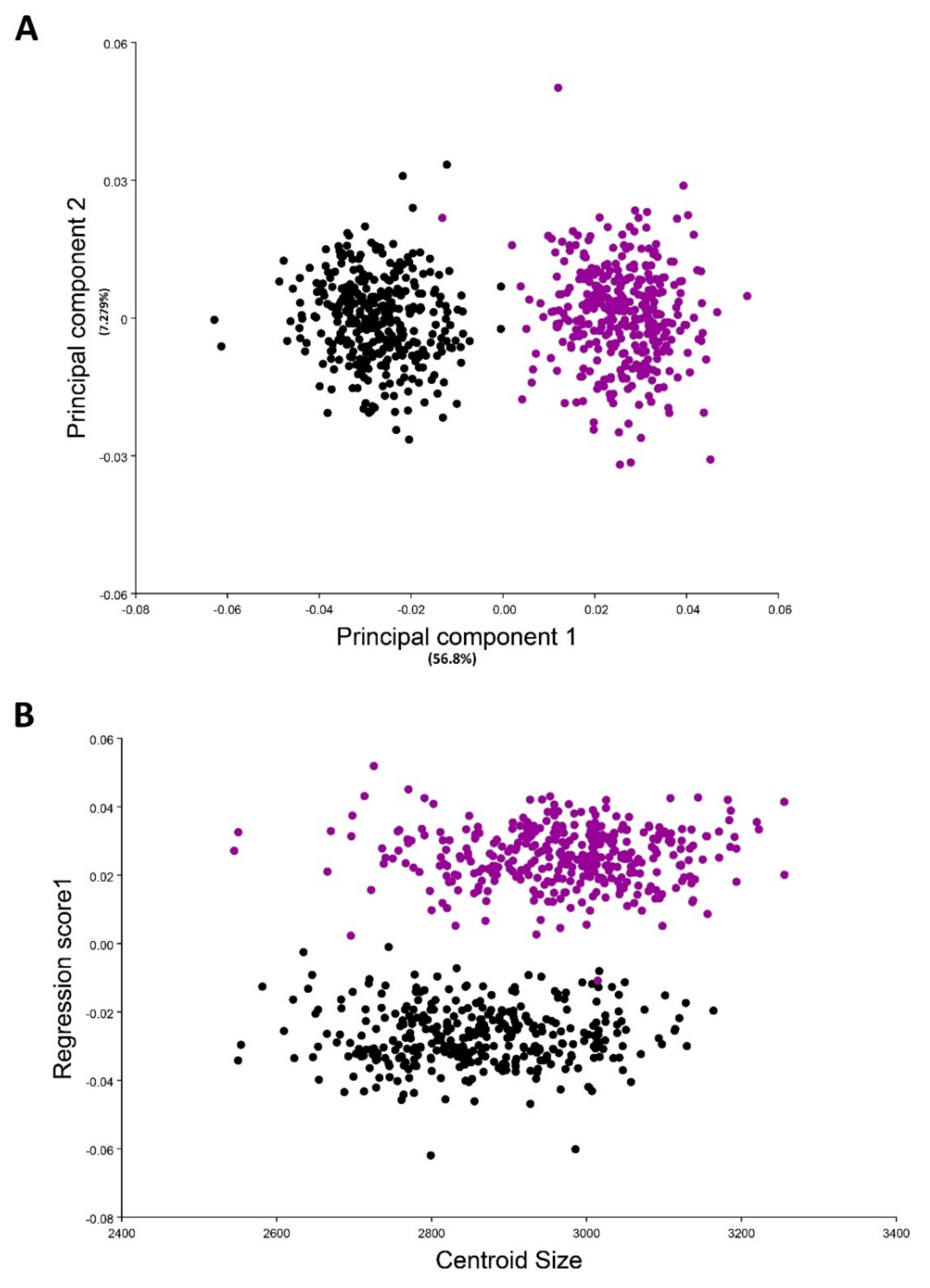
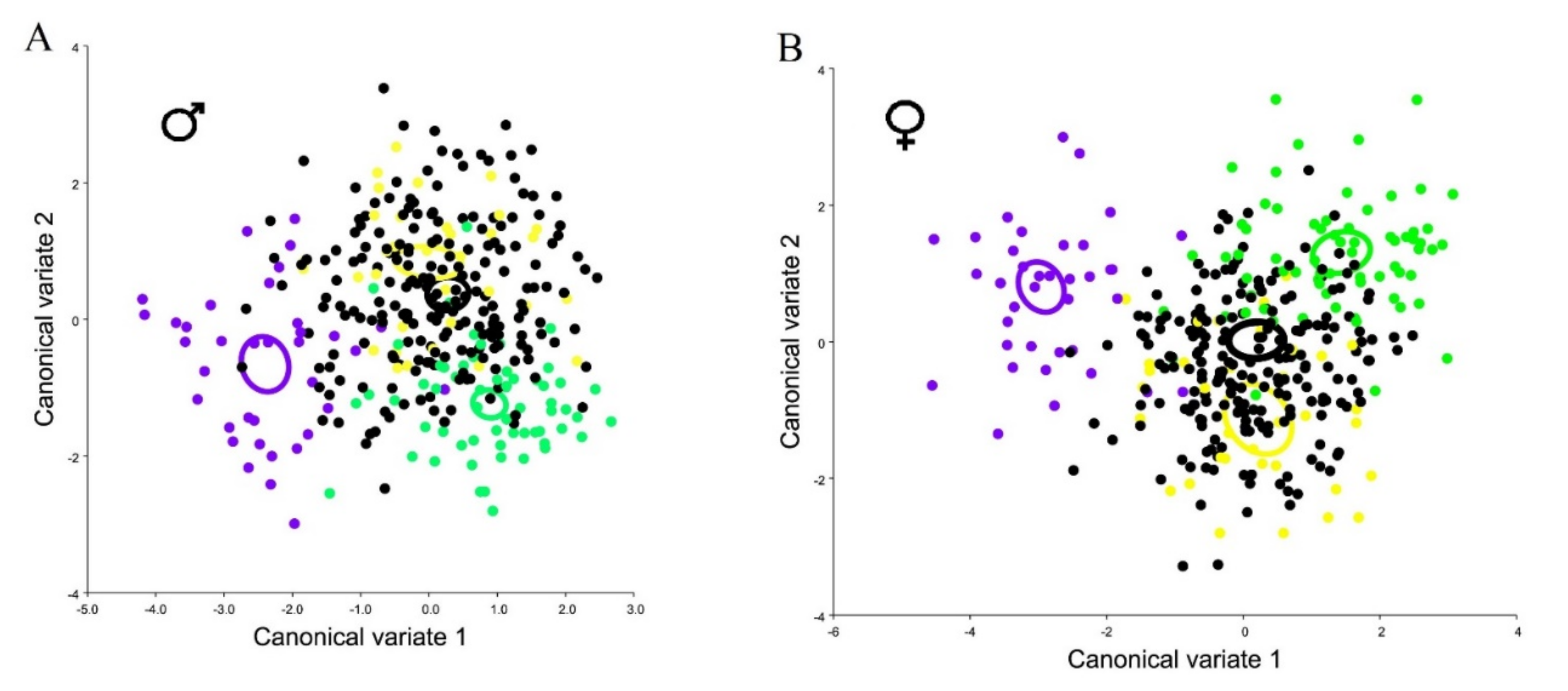
3.1. Variability in the Shape of the Wings of the Medfly from Different Fruit Hosts
3.2. Variability in the Shape of the Wings of a Medfly from Different Locations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilstrap, F.E.; Hart, W.G. Biological Control of the Mediterranean Fruit Fly in the United States and Central America; Hart, W.G., Ed.; U.S. Department of Agriculture, Agricultural Research Services Publication, Texas University: Washington, DC, USA, 1987; ARS-56. [Google Scholar]
- De Meyer, M.; Copeland, R.S.; Wharton, R.A.; McPheron, B.A.; Barnes, B.N. On the geographic origin of the Medfly Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). In Proceedings of the 6th International Symposium on Fruit Flies of Economic Importance, Stellenbosch, South Africa, 6–10 May 2002. [Google Scholar]
- Malacrida, A.R.; Gomulski, L.M.; Bonizzoni, M.; Bertin, S.; Gasperi, G.; Guglielmino, C.R. Globalization and Fruitfly Invasion and Expansion: The Medfly Paradigm. Genetica 2007, 131, 1. [Google Scholar] [CrossRef]
- Fimiani, P. Mediterranean region. In Fruit Flies: Their Biology, Natural Enemies and Control. World Crop Pests; Robinson, A.S., Hooper, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 37–50. [Google Scholar]
- Deschepper, P.; Terrance, T.N.; Virgilio, M.; De Meyer, M.; Barr, N.; Ruiz-Arce, R. Looking at the big picture: Worldwide population structure and range expansion of the cosmopolitan pest Ceratitis capitata (Diptera, Tephritidae). Biol. Invasions 2021, 23, 3529–3543. [Google Scholar] [CrossRef]
- Tominić, A. Muha voćnih plodova (Ceratitis capitata Wied.) na primorju. Biljn. Proizv. 1951, 3, 132–136. [Google Scholar]
- Tominić, A.; Brnetić, D. Biološka ispitivanja voćne muhe (Ceratitis capitata) u 1959. godini. Biljn. Zaštita 1960, 3, 59–65. [Google Scholar]
- Gasperi, G.; Bonizzoni, M.; Gomulski, L.M.; Murelli, V.; Torti, C.; Malacrida, A.R.; Guglielmino, C.R. Genetic Differentiation, Gene Flow and the Origin of Infestations of the Medfly, Ceratitis capitata. Genetica 2002, 116, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Radonjić, S. The Mediterranean fruit fly Ceratitis capitata Wiedemann (Diptera, Tephritidae), a new pest in Montenegro. Bull. OILB/SROP 2006, 29, 217–224. [Google Scholar]
- Papadopoulos, N.T. Mediterranean fruit fly, Ceratitis capitata (wiedemann) (Diptera: Tephritidae). In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 2318–2322. [Google Scholar]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International, Wallingford and ACIAR: Canberra, Australia, 1992. [Google Scholar]
- De Meyer, M. Systematic revision of the subgenus Ceratitis Mac Leays.s. (Diptera, Tephritidae). Zool. J. Linn. Soc. 2000, 128, 439–467. [Google Scholar] [CrossRef]
- Ordax, M.; Piquer-Salcedo, J.E.; Santander, R.D.; Sabater-Munon, B.; Biosca Lopez, E.G.; Marco-Noales, E. Medfly Ceratitis capitata as potential vector for fire blight pathogen Erwinia amylovora: Survival and transmission. PLoS ONE 2015, 10, e127560. [Google Scholar]
- Bjeliš, M.; Popović, L.; Kiridžija, M.; Ortiz, G.; Pereira, R. Suppression of Mediterranean Fruit Fly Using Sterile Insect Technique in Neretva River Valley of Croatia. In Proceedings of the 9th International Symposium on Fruit Flies of Economic Importance, Bangkok, Thailand, 12–16 May 2014; pp. 29–45. [Google Scholar]
- Papadopoulos, N.T.; Katsoyannos, B.I.; Carey, J.R.; Kouloussis, N.A. Seasonal and Annual Occurance of the Mediterranean Fruit Fly (Diptera: Tephritidae) in Northen Greece. Ecol. Popul. Biol. 2001, 94, 41–50. [Google Scholar]
- Navarro-Campos, C.; Campos, J.M.; Martinez-Ferrer, M.T.; Fibla, J.M. The Influence of Host Fruit and Temperature on the Body Size of Adult Ceratitis capitata (Diptera: Tephritidae) Under Laboratory and Field Conditions. Environ. Entomol. 2011, 40, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, D.; Lance, D.; Stefan, M. Mediterranean fruit flies and their management in the U.S.A. R. Soc. Chem. 1999, 10, 207–212. [Google Scholar]
- Yuval, B.; Hendrichs, J. Behavior of Flies in the Genus Ceratitis. Phylogeny and Evolution of Fruit Flies (Tephritidae) Behavior; CRC Press: Boca Raton, FL, USA, 2000; pp. 429–457. [Google Scholar]
- Prakash, A.; Rao, J.; Mukherjee, A.K.; Berliner, J.; Pokhare, S.S.; Adak, T.; Munda, S.; Shashank, P.R. Climate Change: Impact on Crop Pests; Applied Zoologists Research Association (AZRA), Central Rice Research Institute: Odisha, India, 2014; ISBN 81-900947-2-7. [Google Scholar]
- De Meyer, M.; Robertson, M.P.; Peterson, A.T.; Mansell, M.W. Ecological niches and potential geographical distributions of Mediterranean fruit fly (Ceratitis capitata) and Natal fruit fly (Ceratitis rosa). J. Biogeogr. 2008, 35, 270–281. [Google Scholar] [CrossRef]
- Liquido, N.J.; Shinoda, L.A.; Cunningham, R.T. Host plants of the Mediterranean fruit fly (Diptera: Tephritidae). An annotated world list. Ann. Entomol. Soc. Am. 1991, 77, 1–52. [Google Scholar]
- Liquido, N.J.; Barr, P.G.; Cunningham, R.T. MEDHOST: An Encyclopedic bibliography of the Host Plants of the Mediterranean Fruit Fly, Ceratitis capitata (Wiedemann). In Fruit Fly Expert Identification System and Biosystematic Information Database; Diptera Data Dissemination Disk (CD, Rom); Thompson, F.C., Ed.; USDA, Agriculture Research Service: Beltsiville, MD, USA, 1998; p. 144. [Google Scholar]
- Vera, M.T.; Rodriguez, R.; Segura, D.F.; Cladera, J.L.; Sutherst, R.W. Potential geographical distribution of the mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), with emphasis on Argentina and Australia. Environ. Entomol. 2002, 31, 1009–1022. [Google Scholar] [CrossRef] [Green Version]
- De Meyer, M.; Copeland, R.S.; Lux, S.A.; Mansell, M.; Quilici, S.; Wharton, R.; White, I.M.; Zenz, N.J. Annotated Check List of Host Plants for Afrotropical Fruit Flies (Diptera: Tephritidae) of the Genus Ceratitis; MRAC: Tervuren, Belgium, 2002; p. 91. ISBN 90-75894-48-12002. [Google Scholar]
- Umeh, V.C.; Olaniyan, A.A.; Ker, J.; Andir, J. Development of citrus fruit fly control strategies for small-holders in Nigeria. Fruits 2004, 59, 265–274. [Google Scholar] [CrossRef]
- Governatori, G. Le barriere fitosanitarie nella libera circolazione dei vegetali e dei prodotti vegetali: L’esempio dell’esportazione dei frutti di kiwi. Notiziario ERSA 2015, 3, 7–9. [Google Scholar]
- Egartner, A.; Lethmayer, C. Invasive Fruit Flies of economic importance in Austria—monitoring activities 2017. In Proceedings of the 9th International Conference on Integrated Fruit Production, Thessaloniki, Greece, 4–8 September 2016; Ioriatti, C., Damos, P., Escudero-Colomar, L.A., Linder, C., Stensvand, A., Eds.; pp. 45–49. [Google Scholar]
- Bjeliš, M.; Popović, L.; Moraiti, C.A.; Papadopoulos, N.T. Overwintering dynamics of the Mediterranean fruit fly in Central Dalmatia of Croatia. In Proceedings of the 4th International TEAM meeting, Book of Abstracts, La Grande-Motte, France, 5–9 October 2020; p. 56. [Google Scholar]
- Chireceanu, C.; Teodoru, A.; Chiriloaie Palade, A. Current status on spreading of Ceratitis capitata in Romania. In Proceedings of the 4th International TEAM meeting, Book of Abstracts, La Grande-Motte, France, 5–9 October 2020; p. 89. [Google Scholar]
- Egartner, A.; Lethmayer, C.; Gottsberger, R.A.; Blümel, S. Recent records of Ceratitis sp. and Bactrocera spp. (Tephritidae, Diptera) in Austria. In Proceedings of the 4th International TEAM meeting, Book of Abstracts, La Grande-Motte, France, 5–9 October 2020; p. 90. [Google Scholar]
- Bradshaw, A.D. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar]
- Schlichting, C.D. The evolution of phenotypic plasticity. Ann. Rev. Ecol. Syst. 1986, 17, 667–693. [Google Scholar] [CrossRef]
- Schlichting, C.D. The role of phenotypic plasticity in diversification. In Phenotypic Plasticity: Functional and Conceptual Approaches; deWitt, T.J., Scheiner, S.M., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 191–200. [Google Scholar]
- Murren, C.J.; Denning, W.; Pigliucci, M. Relationships between vegetative and life history traits and fitness in a novel field environment: Impacts of herbivores. Evol. Ecol. 2005, 19, 58. [Google Scholar] [CrossRef]
- Berrigan, D.M.; Scheiner, S.M. Modelling the evolution of phenotypic plasticity. In Phenotypic Plasticity: Functional and Conceptual Approaches; DeWitt, T.J., Scheiner, S.M., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 82–97. [Google Scholar]
- Helmuth, B.; Kingsolver, J.G.; Carrington, E. Biophysics, physiological ecology, and climate change: Does mechanism matter? Annu. Rev. Physiol. 2005, 67, 177–201. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Fisher, M. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 2005, 166, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.P.; Blackburn, T.M.; Sol, D. The ecology of bird introductions. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 71–98. [Google Scholar] [CrossRef]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix Ital. J. Mammal. 2013, 24, 7–14. [Google Scholar]
- Klingenberg, C.P. Visualizations in geometric morphometrics: How to read and how to make graphs showing shape changes. Hystrix Ital. J. Mammal. 2013, 24, 15–24. [Google Scholar]
- Benitez, H.A.; Püschel, T.A. Modelando la Varianza de la Forma: Morfometría Geome´trica Aplicaciones en Biología Evolutiva. Int. J. Morphol. 2014, 32, 998–1008. [Google Scholar] [CrossRef] [Green Version]
- Camara, M.; Caro-Rian, H.; Ravel, S.; Dujardin, J.P.; Hervouet, J.P.; De Meeus, T.; Kagbadouno, M.S.; Bouyer, J.; Solano, P. Genetic and Morphometric Evidence for Population Isolation of Glossina palpalis gambiensis (Diptera:Glossinidae) on the Loos Islands, Guinea. J. Med. Entomol. 2006, 43, 853–860. [Google Scholar] [CrossRef]
- Bouyer, J.; Ravel, S.; Dujardin, J.P.; De Meeus, T.; Via, L.; Thévenon, S.; Guerrini, L.; Sidibé, I.; Solano, P. Population structuring of Glossina palpalis gambiensis (Diptera: Glossinidae) according to landscape fragmentation in the Mouhoun river, Burkina Faso. J. Med. Entomol. 2007, 44, 788–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingenberg, C.P. Evolution and development of shape: Integrating quantitative approaches. Nat. Rev. Genet. 2010, 11, 623–635. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.M.; de Lima-Filho, P.A.; Molina, W.F.; de Almeida, L.M.; de Gouveia, M.B.; de Macedo, F.P.; Laumann, R.A.; Paranhos, B.A. Wing morphometry and acoustic signals in sterile and wild males: Implications for mating success in Ceratitis capitata. Sci. World J. 2015, 2015, 526969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikac, K.M.; Douglas, J.; Spencer, J.L. Wing Shape and Size of the Western Corn Rootworm (Coleoptera: Chrysomelidae) is Related to Sex and Resistance to Soybean-Maize Crop Rotation. J. Econ. Entomol. 2013, 106, 1517–1524. [Google Scholar] [CrossRef]
- Mikac, K.; Lemić, D.; Bažok, R.; Benitez, H.A. Wing shape changes: A morphological view of the Diabrotica virgifera virgifera European invasion. Biol. Invasions 2016, 18, 3401–3407. [Google Scholar] [CrossRef]
- Benitez, H.A.; Lemic, D.; Bažok, R.; Bravi, R.; Buketa, M.; Püschel, T. Morphological integration and modularity in Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) hind wings. Zool. Anz. 2014, 253, 461–468. [Google Scholar] [CrossRef]
- Lemić, D.; Benitez, H.A.; Bažok, R. Intercontinental effect on sexual shape dimorphism and allometric relationships in the beetle pest Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae). Zool. Anz. 2014, 3, 203–206. [Google Scholar] [CrossRef]
- Pieterse, W.; Benítez, H.A.; Addisona, P. The use of geometric morphometric analysis to illustrate the shape change induced by different fruit hosts on the wing shape of Bactrocera dorsalis and Ceratitis capitata (Diptera: Tephritidae). Zool. Anz. 2017, 269, 110–116. [Google Scholar] [CrossRef]
- Pajač Živković, I.; Lemić, D.; Mešić, A.; Barić, B.; Ordenes, R.; Benítez, A.H. Effect of fruit host on wing morphology in Drosophila suzukii (Diptera: Drosophilidae): A first view using geometric morphometrics. Entomol. Res. 2018, 4, 262–268. [Google Scholar] [CrossRef]
- Lemić, D.; Benitez, H.A.; Bjeliš, M.; Ordenes-Claveria, R.; Ninčević, P.; Mikac, K.M.; Pajač Živković, I. Agroecological effect and sexual shape dimorphism in medfly Ceratitis capitata (Diptera: Tephritidae) an example in Croatian populations. Zool. Anazeiger 2020, 288, 118–124. [Google Scholar] [CrossRef]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International: Oxon, UK, 1994; p. 601. [Google Scholar]
- Upton, M.F.S.; Mantel, B.L. Methods for Collecting, Preserving, and Studying Insects and Other Terrestrial Arthropods. Aust. J. Enomology 2010, 51, 4. [Google Scholar]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Rohlf, F.J. Digitize Landmarks and Outlines, Version 2.12; Department of Ecology and Evolution, State University of New York at Stony Brook, New York, USA: 2008. Available online: http://life.bio.sunysb.edu/morph (accessed on 20 January 2020).
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Slice, D. Extensions of the Procustes methods for the optimal superimposition of landmarks. Syst. Biol. 1990, 39, 40–59. [Google Scholar]
- Fierst, J.L. A history of phenotypic plasticity accelerates adaptation to a new enwironment. J. Evol. Biol. 2011, 24, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Bonduriansky, R. Convergent evolution of sexual shape dimorphism in Diptera. J. Morphol. 2006, 267, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Gidaszewski, N.A.; Baylac, M.; Klingenberg, C.P. Evolution of sexual dimorphism of wing shape in the Drosophila melanogaster subgroup. BMC Evol. Biol. 2009, 9, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsteller, S.; Adams, D.C.; Collyer, M.L.; Condon, M. Six cryptic species on a single species of host plant: Morphometric evidence for possible reproductive character displacement. Ecol. Entomol. 2009, 34, 66–73. [Google Scholar] [CrossRef]
- Benitez, H.; Parra, L.; Sepulveda, E.; Sanzana, M. Geometric Perspectives of Sexual Dimorphism in the Wing Shape of Lepidoptera: The Case of Synneuria sp. (Lepidoptera: Geometridae). J. Entomol. Res. Soc. 2011, 13, 53–60. [Google Scholar]
- Wootton, R.J. Functional morphology of insect wings. Annu. Rev. Entomol. 1992, 37, 113–140. [Google Scholar] [CrossRef]
- Nylin, S.; Gotthard, K. Plasticity in life history-traits. Annu. Rev. Entomol. 1998, 43, 63–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isard, S.A.; Spencer, J.L.; Mabry, T.R.; Levine, E. Influence of atmospheric conditions on high-elevation flight of western corn rootworm (Coleoptera: Chrysomelidae). Environ. Entomol. 2004, 33, 650–656. [Google Scholar] [CrossRef]
- Hernández, N.; Barragán, Á.R.; Dupas, S.; Silvain, J.F.; Dangles, O. Wing shape variations in an invasive moth are related to sexual dimorphism and altitude. Bull. Entomol. Res. 2010, 100, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Hendrichs, J.; Robinson, A.S.; Cayol, J.P.; Enkerlin, W.R. Medfly Areawide Sterile Insect Technique Programmes for Prevention, Suppression or Eradication: The Importance of Mating Behavior Studies. Fla. Entomoogist 2002, 85, 1–13. [Google Scholar] [CrossRef]
- Atkinson, D.; Sibly, R.M. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 1997, 12, 235–239. [Google Scholar] [CrossRef]
- Angilletta, M.J.; Dunham, A.E. The temperaturesize rule in ectotherms: Simple evolutionary explanations may not be general. Am. Nat. 2003, 162, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; Ratna, E.; Sgro, C.M.; Barton, M.; Blacket, M.; Hallas, R.; De Garis, S.; Weeks, A.R. Antagonistic selection between adult thorax and wing size in Þeld released Drosophila melanogaster independent of thermal conditions. J. Evol. Biol. 2007, 20, 2219–2227. [Google Scholar] [CrossRef]
- Stamp, N.E. Growth versus molting time of caterpillars as a function of temperature, nutrient concentration and the phenolic rutin. Oecologia 1990, 82, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Inglesfield, C. Larval hosts, adult body size and population quality in Ceratitis capitata Wied.: A laboratory study. Ann. Della Fac. Di Agrar. Dellõuniversita Di Sassari 1982, 28, 25–39. [Google Scholar]
- Vinson, J. The functional food properties of figs. Cereal Foods World 1999, 44, 82–87. [Google Scholar]
- Asharf, C.M.; Iqbal, S.; Dildar, A. Nutritional and physicochemical studies on fruit pulp, seed and shell of indigenous Prunus persica. J. Med. Plants Res. 2011, 5, 3917–3921. [Google Scholar]
- Ferenčić, D.; Gluhić, D.; Dudaš, S. Hranjiva vrijednost mandarina (Citrus reticulata Blanco, Citrus nobilis Lour). Glas. Zaštite Bilja 2016, 3, 39. [Google Scholar]
- Roshanzamir, F.; Safavi, S.M. The putative effects of D-Aspartic acid on blood testosterone levels: A systematic review. Int. J. Reprod. Biomed. 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjeliš, M.; Popović, L.; Marušić, I.; Gakić, S.; Buljubašić, I.; Ivanović, A.; Arnaut, P.; Cardoso-Pereira, R. Medfly suppression by sterile insect technique in Neretva valley of Croatia. In Abstracts of the 2nd TEAM International Meeting Biological Invasions of Tephritidae, Scological and Economic Impacts, Crete, Greece, 3–6 July 2012; ZITI Publications: Thessaloniki, Greece, 2012; p. 88. [Google Scholar]
- Anjos-Duarte, C.S.; Costa, A.M.; Joachim-Bravo, I.S. Sexual behaviour of the Mediterranean fruit fly (Diptera: Tephritidae): The influence of female size on mate choice. J. Appl. Entomol. 2010, 1, 367–373. [Google Scholar] [CrossRef]
- De Aquino, J.C.; Joachim-Bravo, I.S. Relevance of Male Size to Female Mate Choice in Ceratitis capitata (Diptera: Tephritidae): Investigations with Wild and Laboratory-Reared Flies. J. Insect Behav. 2014, 27, 162–176. [Google Scholar] [CrossRef]
- Van Cann, J.; Virgilio, M.; Jordaens, K.; De Meyer, M. Wing morphometrics as a possible tool for the diagnosis of the Ceratitis fasciventris, C. anonae, C. rosa complex (Diptera, Tephritidae). ZooKeys 2015, 540, 489–506. [Google Scholar]
- Calkins, C.O.; Parker, A.G. Sterile insect quality. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 269–296. [Google Scholar]
- Hendrichs, J.; Vreysen, M.J.B.; Enkerlin, W.R.; Cayol, J.P. Strategic options in using sterile insects for area-wide integrated pest management. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2021; pp. 563–600. [Google Scholar]
- Gasparich, G.E.; Silva, J.G.; Han, H.Y.; Mcpheron, B.A.; Steck, G.J.; Sheppard, W.S. Population Genetic Structure of Mediterranean Fruit Fly (Diptera: Tephritidae) and Implications for Worldwide Colonization Patterns. Ann. Entomol. Soc. Am. 1997, 90, 790–797. [Google Scholar] [CrossRef]
- Hulme, P.E. Phenotypic plasticity and plant invasions: Is it all Jack? Funct. Ecol. 2008, 22, 3–7. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A metaanalysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z. Voćna mušica Ceratitis capitata W. (Diptera, Tephritidae) kao novi problem. Agron. Glas. 1960, 10, 161–170. [Google Scholar]
- Bjeliš, M. Pilot project of medfly—Ceratitis capitata Wied. (Diptera, Tephritidae) suppression by sterile insect technique in the Neretva river valley of Croatia. In Proceedings of the 10th Slovenian Conference on Plant Protection with International Participation, Podčetrtek, Slovenija, 1–2 March 2011; Društvo za Varstvo Rastlin Slovenije: Ljubljana, Slovenija, 2011; p. 23. [Google Scholar]




| Location | Plant Host | Control Measures * | Total Number of Individuals | Adult Emergence from Field Collected Fruits |
|---|---|---|---|---|
| Sućuraj | Fig | CON | 40 | 10.10.–26.10.2020. |
| Hvar | Fig | CON | 40 | 10.10–26.10.2020. |
| Hvar | Mandarin | CON | 40 | 20.10.–30.10.2019. |
| Podstrana | Fig | CON | 36 | 10.10–28.10.2020. |
| Podstrana | Peach | CON | 39 | 1.09.–7.09.2020. |
| Metković | Mandarin | IPM | 40 | 1.11.–14.11.2019. |
| Opuzen | Peach | SIT | 40 | 13.07.–27.07.2020. |
| Opuzen | Fig | SIT | 31 | 27.08.–8.09.2020. |
| Ston | Mandarin | CON | 40 | 10.–28.11.2019. |
| Laboratory | Sterile | - | 40 | 2019. |
| Peach | Mandarin | Fig | Peach | Mandarin | Fig | |
|---|---|---|---|---|---|---|
| Mahalanobis Distance p Value | Procrustes Distance p Value | |||||
| MALES | ||||||
| Mandarin | 1.8115 p < 0.0001 | 0.0099 p < 0.0001 | ||||
| Fig | 1.1727 p < 0.0001 | 1.2301 p < 0.0001 | 0.0084 p < 0.0001 | 0.0054 p = 0.0023 | ||
| Sterile | 2.9715 p < 0.0001 | 2.8492 p < 0.0001 | 2.841 p < 0.0001 | 0.0178 p < 0.0001 | 0.0186 p < 0.0001 | 0.0175 p < 0.0001 |
| FEMALES | ||||||
| Mandarin | 1.6085 p < 0.0001 | 0.0093 p < 0.0001 | ||||
| Fig | 1.1564 p < 0.0001 | 1.3039 p < 0.0001 | 0.0061 p = 0.0014 | 0.0065 p < 0.0001 | ||
| Sterile | 3.7637 p < 0.0001 | 3.3428 p < 0.0001 | 3.4391 p < 0.0001 | 0.0229 p < 0.0001 | 0.0212 p < 0.0001 | 0.0207 p < 0.0001 |
| Hvar | Podstrana | Opuzen | Hvar | Podstrana | Opuzen | |
|---|---|---|---|---|---|---|
| Mahalanobis Distance p Value | Procrustes Distance p Value | |||||
| MALES | ||||||
| Mandarin vs. Fig | 2.0194 p < 0.0001 | 0.0093 p = 0.001 | ||||
| Fig vs. Peach | 1.8782 p < 0.0001 | 1.5926 p = 0.01 | 0.0095 p = 0.008 | 0.0078 p = 0.08 | ||
| FEMALES | ||||||
| Mandarin vs. Fig | 2.1394 p < 0.0001 | 0.0104 p < 0.0001 | ||||
| Fig vs. Peach | 1.3576 p = 0.06 | 1.8811 p < 0.0001 | 0.009 p = 0.006 | 0.009 p = 0.001 | ||
| FEMALE | Hvar | Metković | Opuzen | Ston | Podstrana | Sterile |
|---|---|---|---|---|---|---|
| Mahalanobis Distance p Value | ||||||
| Metković | 1.4832 p = 0.0014 | |||||
| Opuzen | 2.5498 p < 0.0001 | 2.7942 p < 0.0001 | ||||
| Ston | 1.9459 p < 0.0001 | 2.3817 p < 0.0001 | 2.0943 p < 0.0001 | |||
| Podstrana | 2.2512 p < 0.0001 | 2.4622 p < 0.0001 | 2.5266 p < 0.0001 | 2.0517 p < 0.0001 | ||
| Sterile | 3.5225 p < 0.0001 | 3.8136 p < 0.0001 | 4.2868 p < 0.0001 | 3.4827 p < 0.0001 | 3.4432 p < 0.0001 | |
| Sućuraj | 1.4768 p < 0.0001 | 1.7915 p < 0.0001 | 2.2037 p < 0.0001 | 2.2459 p < 0.0001 | 2.3188 p < 0.0001 | 3.8186 p < 0.0001 |
| Procrustes distance p value | ||||||
| Metković | 0.0072 p = 0.0276 | |||||
| Opuzen | 0.013 p < 0.0001 | 0.0155 p < 0.0001 | ||||
| Ston | 0.0108 p < 0.0001 | 0.0125 p < 0.0001 | 0.0114 p < 0.0001 | |||
| Podstrana | 0.0103 p < 0.0001 | 0.0111 p < 0.0001 | 0.0128 p < 0.0001 | 0.0096 p < 0.0001 | ||
| Sterile | 0.0227 p < 0.0001 | 0.0232 p < 0.0001 | 0.0256 p < 0.0001 | 0.0195 p < 0.0001 | 0.019 p < 0.0001 | |
| Sućuraj | 0.0074 p = 0.0061 | 0.0084 p = 0.0126 | 0.013 p < 0.0001 | 0.0125 p < 0.0001 | 0.011 p < 0.0001 | 0.0223 p < 0.0001 |
| MALE | Hvar | Metković | Opuzen | Ston | Podstrana | Sterile |
|---|---|---|---|---|---|---|
| Mahalanobis Distance p Value | ||||||
| Metković | 1.5215 p = 0.0006 | |||||
| Opuzen | 2.3725 p < 0.0001 | 2.2746 p < 0.0001 | ||||
| Ston | 1.837 p < 0.0001 | 1.4733 p = 0.0589 | 1.8769 p < 0.0001 | |||
| Podstrana | 2.224 p < 0.0001 | 1.8551 p < 0.0001 | 2.1022 p < 0.0001 | 1.7606 p < 0.0001 | ||
| Sterile | 3.1992 p < 0.0001 | 3.0333 p < 0.0001 | 3.3603 p < 0.0001 | 2.7428 p < 0.0001 | 2.7072 p < 0.0001 | |
| Sućuraj | 1.3312 p = 0.0023 | 1.6089 p = 0.0045 | 1.0232 p < 0.0001 | 1.8999 p < 0.0001 | 1.9145 p < 0.0001 | 3.3065 p < 0.0001 |
| Procrustes distance p value | ||||||
| Metković | 0.0072 p = 0.037 | |||||
| Opuzen | 0.0139 p < 0.0001 | 0.0113 p < 0.0001 | ||||
| Ston | 0.0112 p < 0.0001 | 0.0071 p = 2187 | 0.0078 p = 0035 | |||
| Podstrana | 0.0117 p < 0.0001 | 0.008 p = 0414 | 0.0109 p < 0.0001 | 0.0081 p = 0.0097 | ||
| Sterile | 0.0209 p < 0.0001 | 0.0187 p < 0.0001 | 0.0178 p < 0.0001 | 0.0158 p < 0.0001 | 0.0172 p < 0.0001 | |
| Sućuraj | 0.0069 p = 0.0291 | 0.0082 p = 0.0745 | 0.0113 p < 0.0001 | 0.0103 p = 0.0008 | 0.0096 p = 0.0009 | 0.0195 p < 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemic, D.; Bjeliš, M.; Ninčević, P.; Živković, I.P.; Popović, L.; Gašparić, H.V.; Benitez, H.A. Medfly Phenotypic Plasticity as A Prerequisite for Invasiveness and Adaptation. Sustainability 2021, 13, 12510. https://doi.org/10.3390/su132212510
Lemic D, Bjeliš M, Ninčević P, Živković IP, Popović L, Gašparić HV, Benitez HA. Medfly Phenotypic Plasticity as A Prerequisite for Invasiveness and Adaptation. Sustainability. 2021; 13(22):12510. https://doi.org/10.3390/su132212510
Chicago/Turabian StyleLemic, Darija, Mario Bjeliš, Pave Ninčević, Ivana Pajač Živković, Luka Popović, Helena Virić Gašparić, and Hugo A. Benitez. 2021. "Medfly Phenotypic Plasticity as A Prerequisite for Invasiveness and Adaptation" Sustainability 13, no. 22: 12510. https://doi.org/10.3390/su132212510






