Effect of Cover Crop Type and Application Rate on Soil Nitrogen Mineralization and Availability in Organic Rice Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Cover Crops Collection and Properties
2.2. Laboratory Incubation and Determinations
2.3. Soil Carbon and Nitrogen Mineralization Kinetics
2.4. Statistical Analysis
3. Results
3.1. Soil CO2 Evolution and the Estimation of Carbon (C) Mineralization
3.2. Estimation of N Mineralization Potential (N0) and Half-Life (t1/2)
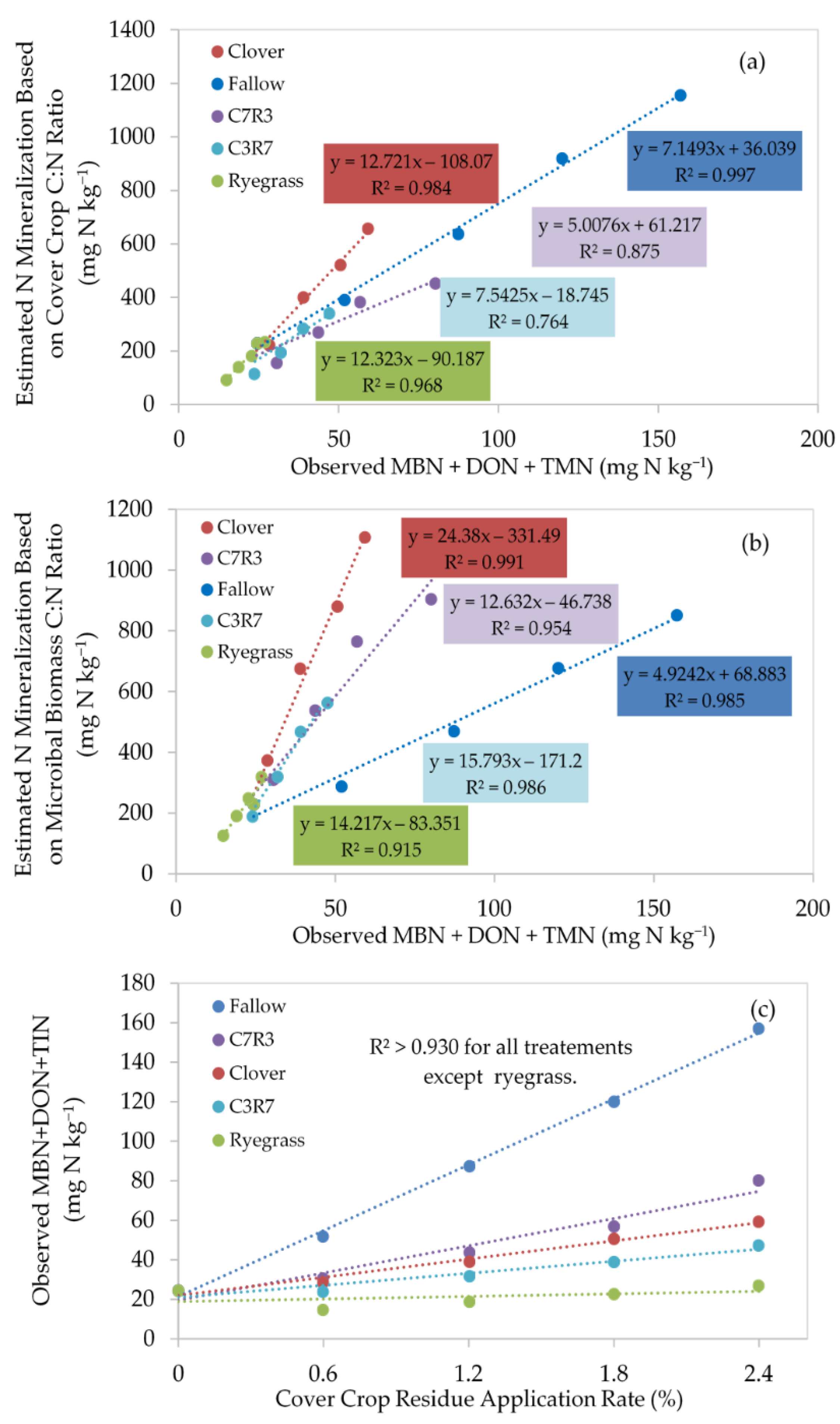
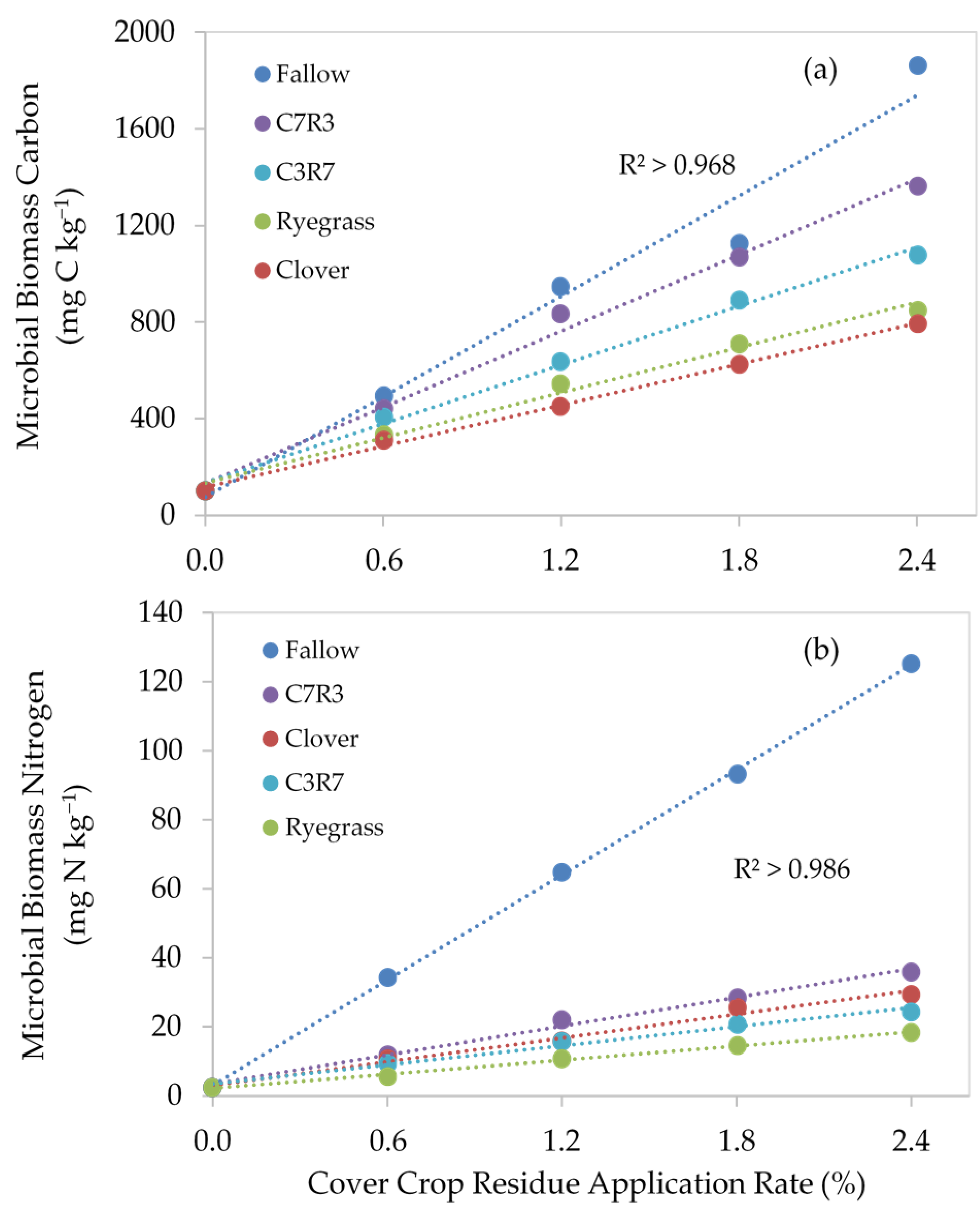
3.3. Soil C and N in Microbial and Organic Pools
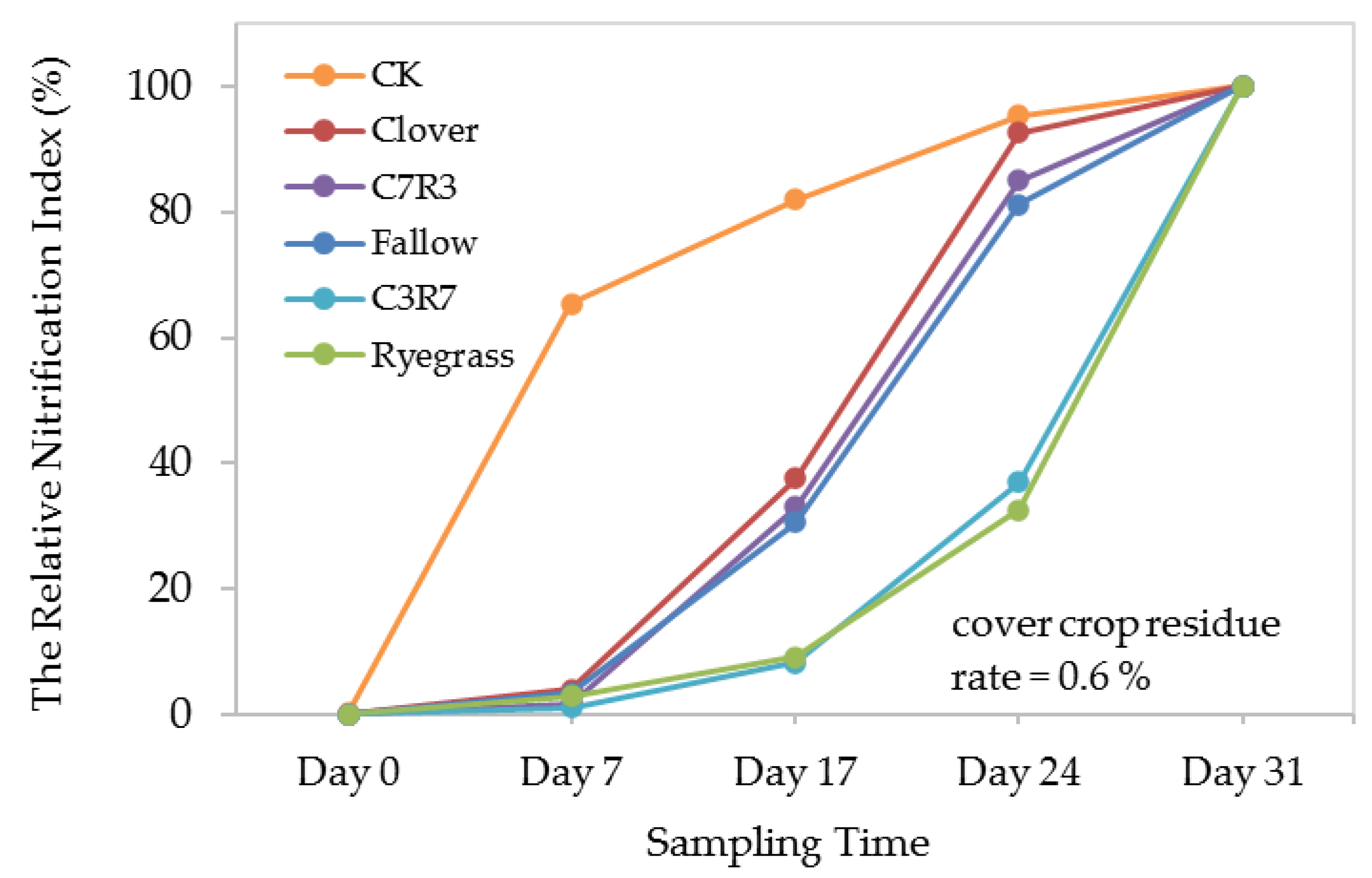
3.4. Soil Mineral Nitrogen Availability
4. Discussion
4.1. Carbon and Nitrogen Mineralization under Different Cover Crops and Application Rates
4.2. Effect of Cover Crop Types and Application Rates on Microbial Biomass Carbon and Nitrogen
4.3. Soil Mineral N Availability under Different Cover Crop Types and Application Rates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- USDA-ERS. Organic Production. Table 3. Certified Organic and Total U.S. Acreage, Selected Crops and Livestock, 1995–2011 (Last Updated on 24 October 2013). Economic Research Service, United States Department of Agriculture. Available online: https://www.ers.usda.gov/data-products/organic-production/ (accessed on 16 July 2020).
- USDA-NASS. Certified Organic Survey 2016 Summary Report (Released on 20 September 2017). National Agriculture Statistics Service, United States Department of Agriculture. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/zg64tk92g/70795b52w/4m90dz33q/OrganicProduction-09-20-2017_correction.pdf (accessed on 16 July 2020).
- Jerkins, D.; Ory, J. National Organic Research Agenda: Outcomes and Recommendations from the 2015 National Organic Farmer Survey and Listening Sessions. 2016. Available online: https://ofrf.org/wp-content/uploads/2019/09/NORA_2016_final9_28.pdf (accessed on 27 May 2020).
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef] [Green Version]
- Claire, C.; John, D.L.; Bill, D.; Laura, L.V.E. Legume cover crop management on nitrogen dynamics and yield in grain corn systems. Field Crops Res. 2017, 201, 75–85. [Google Scholar]
- Sainju, U.M.; Singh, H.P.; Singh, B.P.; Whitehead, W.F.; Chiluwal, A.; Paudel, R. Cover crop and nitrogen fertilization influence soil carbon and nitrogen under bioenergy sweet sorghum. Agron. J. 2018, 110, 463–477. [Google Scholar] [CrossRef] [Green Version]
- Ritter, W.F.; Scarborough, R.W.; Chirnside, A.E.M. Winter cover crops as a best management practice for reducing nitrogen leaching. J. Contam. Hydrol. 1998, 34, 1–15. [Google Scholar] [CrossRef]
- Shelton, R.E.; Jacobsen, K.L.; McCulley, R.L. Cover crops and fertilization alter nitrogen loss in organic and conventional conservation agriculture systems. Front. Plant Sci. 2018, 8, 2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapa, R.; Mirsky, S.B.; Tully, K.L. Cover crops reduce nitrate leaching in agroecosystems: A global meta-analysis. J. Environ. Qual. 2018, 47, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Reberg-Horton, S.C.; Burton, J.D.; Danehower, D.A.; Ma, G.; Monks, D.W.; Murphy, J.P.; Ranells, N.N.; Williamson, J.D.; Creamer, N.G. Changes over time in the allelochemical content of ten cultivars of rye (Secale cereale L.). J. Chem. Ecol. 2005, 31, 179–193. [Google Scholar] [CrossRef]
- Cordeau, S.; Guillemin, J.P.; Reibel, C.; Chauvel, B. Weed species differ in their ability to emerge in no-till systems that include cover crops. Ann. Appl. Biol. 2015, 166, 444–455. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Barbercheck, M.; Douglas, M.R.; Finney, D.M.; Haider, K.; Kaye, J.P.; Kemanian, A.R.; Mortensen, D.A.; Ryan, M.R.; Tooker, J.; et al. A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agric. Syst. 2014, 125, 12–22. [Google Scholar] [CrossRef]
- Finney, D.M.; White, C.M.; Kaye, J.P. Biomass production and carbon/nitrogen ratio influence ecosystem services from cover crop mixtures. Agron. J. 2016, 108, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.C.; Gallagher, R.S.; Kaye, J.P.; Lynch, J.; Bradley, B. Optimizing vetch nitrogen production and corn nitrogen accumulation under no-till management. Agron. J. 2010, 102, 1491–1499. [Google Scholar] [CrossRef]
- White, K.E.; Brennan, E.B.; Cavigelli, M.A.; Smith, R.F. Winter cover crops increase readily decomposable soil carbon, but compost drives total soil carbon during eight years of intensive, organic vegetable production in California. PLoS ONE 2020, 15, e0228677. [Google Scholar] [CrossRef]
- Dabney, S.M.; Breitenbeck, G.A.; Griffin, J.L.; Hoff, B.J. Subterranean clover cover crop used to increase rice yield. Agron. J. 1989, 81, 483–487. [Google Scholar] [CrossRef]
- Yu, Y.; Xue, L.; Yang, L. Winter legumes in rice crop rotations reduces nitrogen loss, and improves rice yield and soil nitrogen supply. Agron. Sustain. Dev. 2014, 34, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Wang, Z.; Yang, B.; Zheng, L.; Yao, Z.; Seyrek, U.A.; Chung, H.; Wei, H. Effects of winter cover crops on rice pests, natural enemies, and grain yield in a rice rotation system. J. Insect. Sci. 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.L.; Silva, P.R.F.; Boeni, M.; Bredemeier, C.; Anghinoni, I.; Menegati, G.B.; Maass, M.B.; Miozzo, L.C. Performance of flooded rice grown in succession to winter cover crops. Rev. Bras. Cienc. Solo. 2018, 42, e0160461. [Google Scholar] [CrossRef] [Green Version]
- Portugal, J.R.; Arf, O.; Buzetti, S.; Portugal, A.R.P.; Garcia, N.F.S.; Meirelles, F.C.; Garé, L.M.; Abrantes, F.L.; Rodrigues, R.A.F. Do cover crops improve the productivity and industrial quality of upland rice? Agron. J. 2020, 112, 327–343. [Google Scholar] [CrossRef]
- Kuo, S.; Huang, B.; Bembenek, R. Effect of winter cover crops on soil nitrogen availability, corn yield, and nitrate leaching. Sci. World J. 2001, 1 (Suppl. S2), 22–29. [Google Scholar] [CrossRef] [PubMed]
- Jahanzad, E.; Barker, A.V.; Hashemi, M.; Eaton, T.; Sadeghpour, A.; Weis, S.A. Nitrogen release dynamics and decomposition of buried and surface cover crop residue. Agron. J. 2016, 108, 1735–1741. [Google Scholar] [CrossRef]
- Lynch, M.J.; Mulvaney, M.J.; Hodges, S.C.; Thompson, T.L.; Thomason, W.E. Decomposition, nitrogen and carbon mineralization from food and cover crop residues in the central plateau of Haiti. Springerplus 2016, 5, 973–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, C.M.; Finney, D.M.; Kemanian, A.R.; Kaye, J.P. A model-data fusion approach for predicting cover crop nitrogen supply to corn. Agron. J. 2016, 108, 2527–2540. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, B.; Ghimire, R.; VanLeeuwen, D.; Mesbah, A. Cover crop residue amount and quality effects on soil organic carbon mineralization. Sustainability 2017, 9, 2316. [Google Scholar] [CrossRef] [Green Version]
- Thorup-Kristensen, K.; Dresbøll, D.B. Incorporation time of nitrogen catch crops influences the N effect for the succeeding crop. Soil Use Manag. 2010, 26, 27–35. [Google Scholar] [CrossRef]
- Vigil, M.F.; Kissel, D.E. Rate of nitrogen mineralized from incorporated crop residues as influenced by temperature. Soil Sci. Soc. Am. J. 1995, 59, 1636–1644. [Google Scholar] [CrossRef]
- Chaves, B.; De Neve, S.; Hofman, G.; Boeckx, P.; Van Cleemput, O. Nitrogen mineralization of vegetable root residues and greenmanures as related to their (bio)chemical composition. Eur. J. Agron. 2004, 21, 161–170. [Google Scholar] [CrossRef]
- Frankenberger, W.T., Jr.; Abdelmagid, H.M. Kinetic parameters of nitrogen mineralization rates from leguminous crops incorporated into soil. Plant Soil 1985, 87, 257–271. [Google Scholar] [CrossRef]
- Kuo, S.; Sainju, U.M. Nitrogen mineralization and availability of mixed leguminous and non-leguminous cover crop residues in soil. Biol. Fertil. Soil 1998, 26, 346–353. [Google Scholar] [CrossRef]
- Melkonian, J.; Poffenbarger, H.J.; Mirsky, S.B.; Ryan, M.R.; Moebius-Clune, B.N. Estimating nitrogen mineralization from cover crop mixtures using the precision nitrogen management model. Agron. J. 2017, 109, 1944–1959. [Google Scholar] [CrossRef] [Green Version]
- Breland, T.A. Enhanced mineralization and denitrification as a result of heterogeneous distribution of clover residues in soil. Plant Soil 1994, 166, 1–12. [Google Scholar] [CrossRef]
- Dou, F.; Zhou, X.; McClung, A.; Storlien, J.; Lang, Y.; Torbert, A.; Hons, F.; Wards, B.; Kresovich, S.; Wight, J. Cover crop, Soil amendments, and variety effects on organic rice production in Texas. In Proceedings of the 35th The Rice Technical Working Group Meeting, New Orleans, LA, USA, 18–21 February 2014; p. 141. [Google Scholar]
- Dou, F.; Anderson, D.; Kresovich, S.; McClung, A.; Ward, B.; Zhou, X. Improving Soil Quality to Increase Yield and Reduce Diseases in Organic Rice Production; Final Report for USDA SARE Grant LS12-249; USDA: College Park, MD, USA, 2015. Available online: https://projects.sare.org/project-reports/ls12-249/ (accessed on 20 September 2020).
- O’Connell, S.; Shi, W.; Grossman, J.M.; Hoyt, G.D.; Fager, K.L.; Creamer, N.G. Short-term nitrogen mineralization from warm-season cover crops in organic farming systems. Plant Soil 2015, 396, 353–367. [Google Scholar] [CrossRef]
- Zibilske, L.M.; Bradford, J.M. Oxygen effects on carbon, polyphenols, and nitrogen mineralization potential in soil. Soil Sci. Soc. Am. J. 2007, 71, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wan, S.; Xing, X.; Zhang, L.; Han, X. Temperature and soil moisture interactively affected soil net N mineralization in temperate grassland in Northern China. Soil Biol. Biochem. 2006, 38, 1101–1110. [Google Scholar] [CrossRef]
- Pold, G.; Domeignoz-Horta, L.A.; Morrison, E.W.; Frey, S.D.; Sistla, S.A.; DeAngelis, K.M. Carbon use efficiency and its temperature sensitivity covary in soil bacteria. mBio 2020, 11, e02293-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.V.; de Imperial, R.M.; Calvo, R.; Garcia, M.C.; Leon-Cofreces, C.; Delgado, M.M. Carbon mineralization kinetics of poultry manure in two soils. Soil Res. 2012, 50, 222–228. [Google Scholar] [CrossRef]
- Schofield, R.K.; Taylor, A.W. The measurement of soil pH. Soil Sci. Soc. Am. Proc. 1955, 19, 164–167. [Google Scholar] [CrossRef]
- Rhoades, J.D. Soluble salts. In Methods of Soil Analysis: Part 2. Agronomy Monograph 9, 2nd ed.; Page, A.L., Ed.; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1982; pp. 167–178. [Google Scholar]
- McGeehan, S.L.; Naylor, D.V. Automated instrumental analysis of carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plant Anal. 1988, 19, 493–505. [Google Scholar] [CrossRef]
- Mehlich, A. New extractant for soil test evaluation of phosphorus, potassium, magnesium, calcium, sodium, manganese, and zinc. Commun. Soil Sci. Plant Anal. 1978, 9, 477–492. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich-3 soil test extractant: A modification of Mehlich-2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Sweeney, R.A. Generic combustion method for determination of crude protein in feeds: Collaborative Study. J. Assoc. Off. Anal. Chem. 1989, 72, 770–774. [Google Scholar] [CrossRef]
- Dence, C.W. The determination of lignin. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Heidelberg, Germany, 1992; pp. 33–61. [Google Scholar]
- Isaac, R.A.; Johnson, W.C. Collaborative study of wet and dry ashing techniques for the elemental analysis of plant tissue by atomic absorption spectrophotometry. J. Assoc. Off. Anal. Chem. 1975, 58, 436–440. [Google Scholar] [CrossRef]
- Havlin, J.L.; Soltanpour, P.N. A nitric acid and plant digest method for use with inductively coupled plasma spectrometry. Commun. Soil Sci. Plant Anal. 1989, 14, 969–980. [Google Scholar] [CrossRef]
- Dorich, R.A.; Nelson, D.W. Direct colorimetric measurement of ammonium in potassium chloride extracts of soil. Soil Sci. Soc. Am. J. 1983, 47, 833–836. [Google Scholar] [CrossRef]
- Markus, D.K.; McKinnon, J.P.; Buccafuri, A.F. Automated analysis of nitrite, nitrate, and ammonium nitrogen in soils. Soil Sci. Soc. Am. J. 1985, 49, 1208–1215. [Google Scholar] [CrossRef]
- Kachurina, O.M.; Zhang, H.; Raun, W.R.; Krenzer, E.G. Simultaneous determination of soil aluminum, ammonium- and nitrate-nitrogen using 1 M potassium chloride extraction. Commun. Soil Sci. Plant Anal. 2000, 31, 893–903. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 2. Agronomy Monograph 9, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1982; pp. 643–687. [Google Scholar]
- Lavoie, M.; Bradley, R.L. Short-term increases in relative nitrification rates due to trenching in forest floor and mineral soil horizons of different forest types. Plant Soil 2003, 252, 367–384. [Google Scholar] [CrossRef]
- Tian, Y.; Toda, H. pH and substrate regulation of nitrogen and carbon dynamics in forest soils in a karst region of the upper Yangtze River basin, China. J. For. Res. 2013, 18, 228–237. [Google Scholar] [CrossRef]
- Beck, T.; Joergensen, R.G.; Kandeler, E.; Makeschin, F.; Nuss, E.; Oberholzer, H.R.; Scheu, S. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Brookes, P.C.; Kragt, J.F.; Powlson, D.S.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: The effects of fumigation time and temperature. Soil Biol. Biochem. 1985, 14, 831–835. [Google Scholar] [CrossRef]
- Schroeder, J.; Jannoura, R.; Beuschel, R.; Pfeiffer, B.; Dyckmans, J.; Murugan, R.; Chavannavar, S.; Wachendorf, C.; Joergensen, R.G. Carbon use efficiency and microbial functional diversity in a temperate Luvisol and a tropical Nitisol after millet litter and N addition. Biol. Fertil. Soils 2020, 56, 1139–1150. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Trinsoutrot, I.; Recous, S.; Bentz, B.; Lineres, M.; Cheneby, D.; Nicolardot, B. Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under nonlimiting nitrogen conditions. Soil Sci. Soc. Am. J. 2000, 64, 918–926. [Google Scholar] [CrossRef]
- McFarland, J.W.; Ruess, R.W.; Kielland, K.; Pregiter, K.; Hendrick, R. Glycine mineralization in situ closely correlates with soil carbon availability across six North American forest ecosystems. Biogeochemistry 2010, 99, 175–191. [Google Scholar] [CrossRef]
- Kirchmann, H.; Bergqvist, R. Carbon and nitrogen mineralization of white clover plants (Trifolium repens) of different age during aerobic incubation with soil. J. Soil Sci. Plant. Nutr. 1989, 152, 281–286. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.r-project.org/ (accessed on 12 August 2020).
- Huang, Y.; Zou, J.W.; Zheng, X.H.; Wang, Y.S.; Xu, X.K. Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Silva, E.C.D.; Muraoka, T.; Franzini, V.I.; Sakadevan, K.; Buzetti, S.; Arf, O.; Bendassolli, J.A.; Soares, F.A.L. Use of nitrogen from fertilizer and cover crops by upland rice in an Oxisol under no-tillage in the Cerrado. Pesqui. Agropecu. Bras. 2016, 51, 728–737. [Google Scholar] [CrossRef] [Green Version]
- Wagger, M.G. Time of desiccation effects on plant composition and subsequent nitrogen release from several winter annual cover crops. Agron. J. 1989, 81, 236–241. [Google Scholar] [CrossRef]
- Carvalho, A.M.; de Souza, L.L.P.; de Guimarães Júnior, R.; Alves, P.C.A.C.; Vivaldi, L.J. Cover plants with potential use for crop-livestock integrated systems in the Cerrado region. Pesqui. Agropecu. Bras. 2011, 46, 1200–1205. [Google Scholar] [CrossRef]
- Staaf, H.; Berg, B. Accumulation and release of plant nutrients in decomposing scots pine needle litter—Long-term decomposition in a scots pine forest. Can. J. Bot. 1982, 60, 1561–1568. [Google Scholar] [CrossRef]
- Tian, G.; Brussaard, L.; Kang, B.T. An index for assessing the quality of plant residues and evaluating their effects on soil and crop in the (sub-) humid tropics. Appl. Soil Ecol. 1995, 2, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Kaschuk, G.; Alberton, O.; Hungria, M. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indications for improving sustainability. Soil Biol. Biochem. 2010, 42, 1–13. [Google Scholar] [CrossRef]
- Gentsch, N.; Boy, J.; Batalla, J.D.K.; Heuermann, D.; von Wirén, N.; Schweneker, D.; Feuerstein, U.; Groß, J.; Bauer, B.; Reinhold-Hurek, B.; et al. Catch crop diversity increases rhizosphere carbon input and soil microbial biomass. Biol. Fertil. Soils 2020, 56, 943–957. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Menachem, S. Lignin. In Biopolymers from Renewable Resources; Kaplan, D.L., Ed.; Springer: New York, NY, USA, 1998; pp. 292–322. [Google Scholar]
- Jensen, E.S. Mineralization-immobilization of nitrogen in soil amended with low C:N ratio plant residues with different particle sizes. Soil Biol. Biochem. 1994, 26, 519–521. [Google Scholar] [CrossRef]
- Woli, K.P.; Pantoja, J.L.; Sawyer, J.E. Soil inorganic nitrogen with incubation of rye cover crop biomass. Commun. Soil Sci. Plant Anal. 2016, 47, 2558–2572. [Google Scholar] [CrossRef]
- Quemada, M.; Carbrera, M.L. Carbon and nitrogen mineralized from leaves and stems of four cover crops. Soil Sci. Soc. Am. J. 1995, 59, 471–477. [Google Scholar] [CrossRef]
- Marstorp, H.; Kirchmann, H. Carbon and nitrogen mineralization and crop uptake of nitrogen from six green manure legume decomposing in soil. Acta Agric. Scand. 1991, 41, 243–252. [Google Scholar] [CrossRef]
- Abera, G.; Wolde-Meskel, E.; Bakken, L.R. Carbon and nitrogen mineralization dynamics in different soils of the tropics amended with legume residues and contrasting soil moisture contents. Biol. Fertil. Soils 2012, 48, 51–66. [Google Scholar] [CrossRef]
- Recous, S.; Machet, J.M.; Mary, B. The partitioning of fertilizer-N between soil and crop: Comparison of ammonium and nitrate applications. Plant Soil 1992, 144, 101–111. [Google Scholar] [CrossRef]


| pH | EC (μS/cm) | WHC (%) | Total C | Total N | DOC | DON | P | Ca | Mg | S | Na | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | ||||||||||||||
| Background Soil | 6.3 | 118 | 51.2 | 11.9 | 2.5 | 82.3 | 4.4 | 16.16 | 0.01 | 0.02 | 41 | 1891 | 211 | 18 | 55 |
| Cover Crop | Cover Crop Seeding Rate (kg ha−1) | Cover Crop Fresh Biomass (kg ha−1) | Cover Crop Moisture (%) | Cover Crop Dry Biomass (kg ha−1) | Organic Rice Yield (kg ha−1) |
|---|---|---|---|---|---|
| Fallow | 9 | 16,585 | 79.0 | 4020 | 7312 |
| Clover | 9 | 20,999 | 77.8 | 4642 | 7443 |
| Ryegrass | 9 | 25,400 | 75.8 | 5613 | 7292 |
| Cover Crop | Crude Protein (g kg−1) | Lignin (g kg−1) | Total C (g kg−1) | Total N (g kg−1) | P (g kg−1) | K (g kg−1) | Cover Crop Lignin:N Ratio | Cover Crop C:N Ratio | Soil Microbial Biomass C:N Ratio |
|---|---|---|---|---|---|---|---|---|---|
| Fallow | 110 | 120 | 341 | 17.6 | 3.2 | 28.6 | 6.8 | 19 | 14 |
| Clover | 159 | 40 | 398 | 25.4 | 1.5 | 10.8 | 1.6 | 16 | 27 |
| C7R3 * | 130 | 40 | 393 | 20.8 | 1.8 | 13.8 | 1.9 | 19 | 38 |
| C3R7 * | 92 | 40 | 385 | 14.6 | 2.2 | 17.7 | 2.7 | 26 | 43 |
| Ryegrass | 63 | 40 | 380 | 10.0 | 2.5 | 20.7 | 4 | 38 | 52 |
| Cumulative CO2 | TMN | DOC | DON | pH | EC | ||||
|---|---|---|---|---|---|---|---|---|---|
| CC Type | ≤0.001 *** | ≤0.001 *** | 0.222 | ≤0.001 *** | ≤0.001 *** | 0.010 * | ≤0.001 *** | ≤0.001 *** | ≤0.0001 **** |
| CC Rate | ≤0.001 *** | ≤0.001 *** | 0.804 | 0.017 * | 0.001 ** | ≤0.001 *** | ≤0.001 *** | 0.005 ** | ≤0.0001 **** |
| Incubation Time | ≤0.001 *** | ≤0.001 *** | 0.019 * | ≤0.001 *** | 0.011 * | 0.925 | ≤0.001 *** | ≤0.001 *** | ≤0.001 *** |
| CC Type × CC Rate | ≤0.001 *** | ≤0.001 *** | 0.437 | 0.053 | 0.024 * | 0.033 * | ≤0.001 *** | ≤0.001 *** | ≤0.001 *** |
| CC Type × Incubation Time | ≤0.001 *** | ≤0.001 *** | 0.238 | ≤0.001 *** | ≤0.001 *** | 0.670 | 0.450 | ≤0.001 *** | 0.853 |
| CC Rate × Incubation Time | ≤0.001 *** | ≤0.001 *** | 0.761 | ≤0.001 *** | ≤0.001 *** | 0.143 | 0.005 ** | 0.003 ** | 0.005 ** |
| CC Type × CC Rate × Incubation Time | ≤0.001 *** | ≤0.001 *** | 0.694 | 0.005 ** | 0.040 * | 0.564 | 0.717 | 0.003 ** | 0.718 |
| Model | Cover Crop | Residue Application Rate (%) | Mineralization Potential (C0, N0) (mg kg−1) | Mineralization Rate Constant (kC, kN) (day−1) | Initial Potential Mineralization Rate (C0kc, N0kN) (mg kg−1 day−1) | Half-Life of Mineralization (t1/2) (day) | Correlation Coefficient (r) |
|---|---|---|---|---|---|---|---|
| C Mineralization Model Cmin = C0 · (1 − exp(−kC · t)) | CK | 0 | 4281 | 0.040 | 172.9 | 17.2 | 0.987 |
| Fallow | 0.6 | 9069 | 0.145 | 1318.8 | 4.8 | 0.970 | |
| Clover | 0.6 | 8430 | 0.182 | 1534.6 | 3.8 | 0.991 | |
| Ryegrass | 0.6 | 8638 | 0.128 | 1102.6 | 5.5 | 0.970 | |
| C7R3 | 0.6 | 9228 | 0.155 | 1433.5 | 4.5 | 0.985 | |
| C3R7 | 0.6 | 9232 | 0.115 | 1063.7 | 6.0 | 0.987 | |
| N Mineralization Model Nmin = N0 · (1 − exp(−kN · t)) | CK | 0 | 911 | 0.040 | 36.8 | 17.2 | 0.993 |
| Fallow | 0.6 | 477 | 0.145 | 69.4 | 4.8 | 0.985 | |
| Clover | 0.6 | 527 | 0.182 | 95.9 | 3.8 | 0.995 | |
| Ryegrass | 0.6 | 227 | 0.128 | 29.0 | 5.4 | 0.985 | |
| C7R3 | 0.6 | 486 | 0.155 | 75.4 | 4.5 | 0.992 | |
| C3R7 | 0.6 | 355 | 0.115 | 40.9 | 6.0 | 0.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Tan, A.; Chen, K.; Pan, Y.; Gentry, T.; Dou, F. Effect of Cover Crop Type and Application Rate on Soil Nitrogen Mineralization and Availability in Organic Rice Production. Sustainability 2021, 13, 2866. https://doi.org/10.3390/su13052866
Li X, Tan A, Chen K, Pan Y, Gentry T, Dou F. Effect of Cover Crop Type and Application Rate on Soil Nitrogen Mineralization and Availability in Organic Rice Production. Sustainability. 2021; 13(5):2866. https://doi.org/10.3390/su13052866
Chicago/Turabian StyleLi, Xiufen, Andrew Tan, Kun Chen, Yeming Pan, Terry Gentry, and Fugen Dou. 2021. "Effect of Cover Crop Type and Application Rate on Soil Nitrogen Mineralization and Availability in Organic Rice Production" Sustainability 13, no. 5: 2866. https://doi.org/10.3390/su13052866







