Vegetation Response to Goats Grazing Intensity in Semiarid Hilly Grassland of the Loess Plateau, Lanzhou, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Compilation
2.2.1. GI Obtained from GPS Tracking of Goats
2.2.2. Field Vegetation Sampling Data
2.2.3. NDVI
2.3. Data Analysis
2.3.1. Estimated AGB Using NDVI
2.3.2. Response of AGB and Growth Rate to Grazing
2.3.3. Response of Vegetation Community Structure to Grazing
3. Results
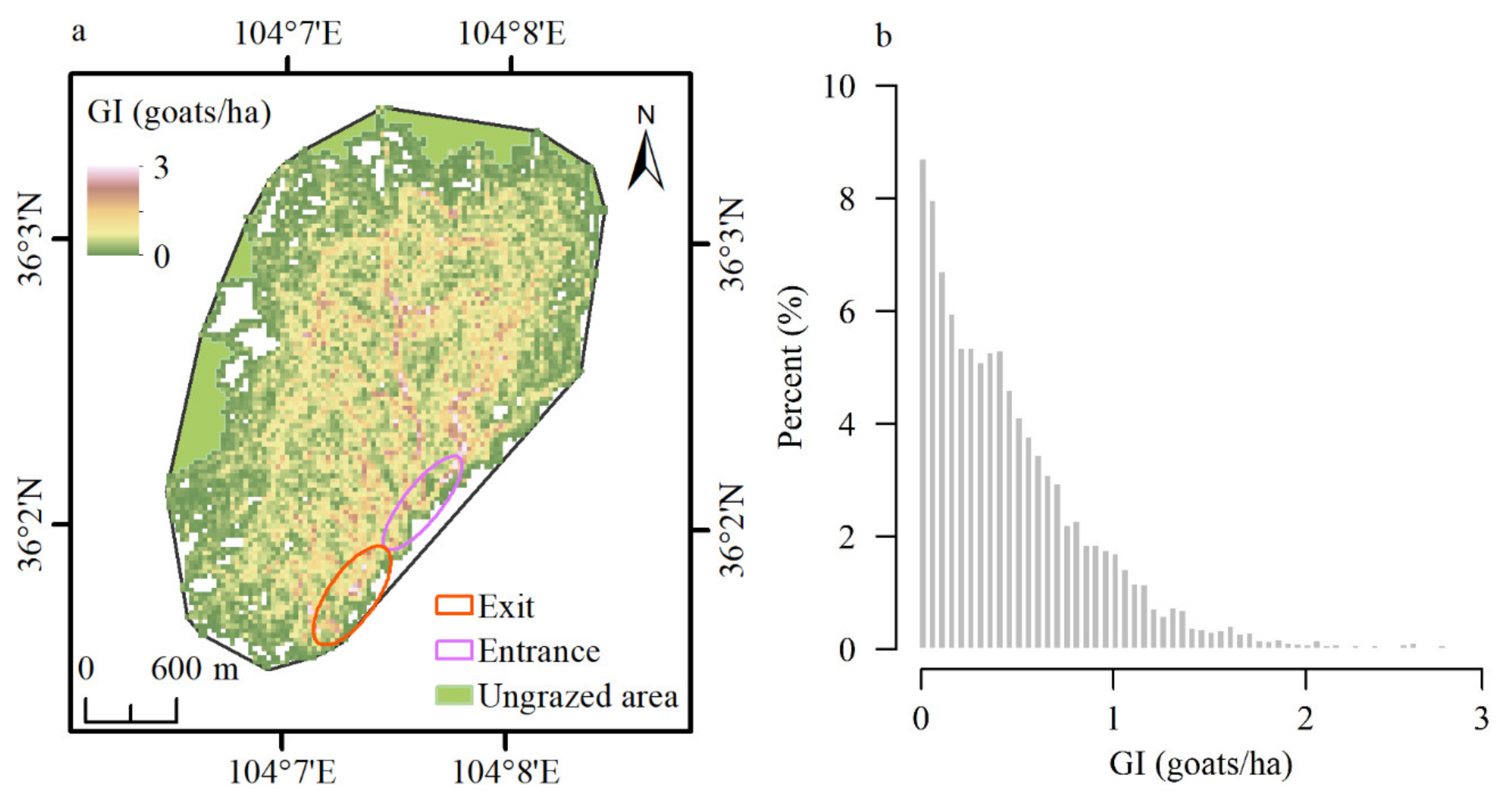
3.1. Grazing Intensity (GI)
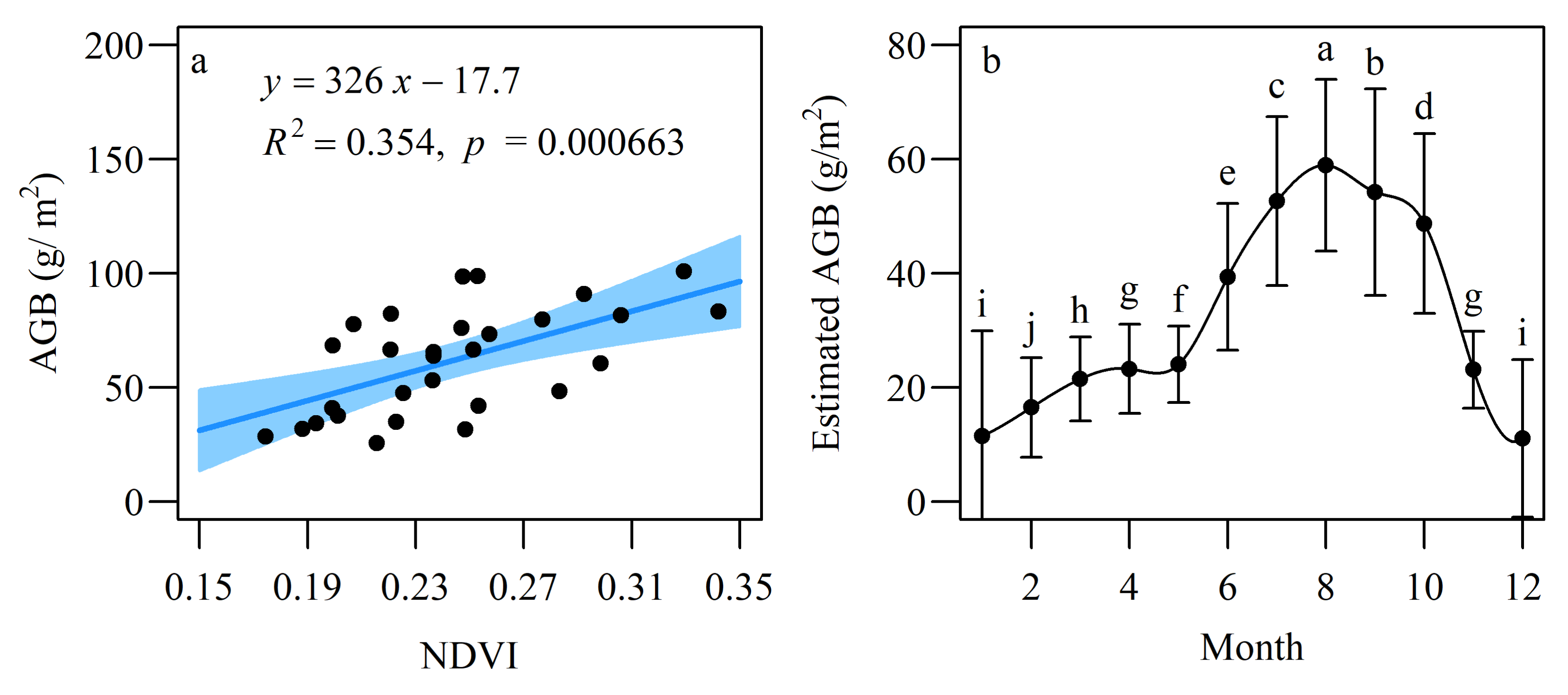
3.2. Estimated AGB Using NDVI
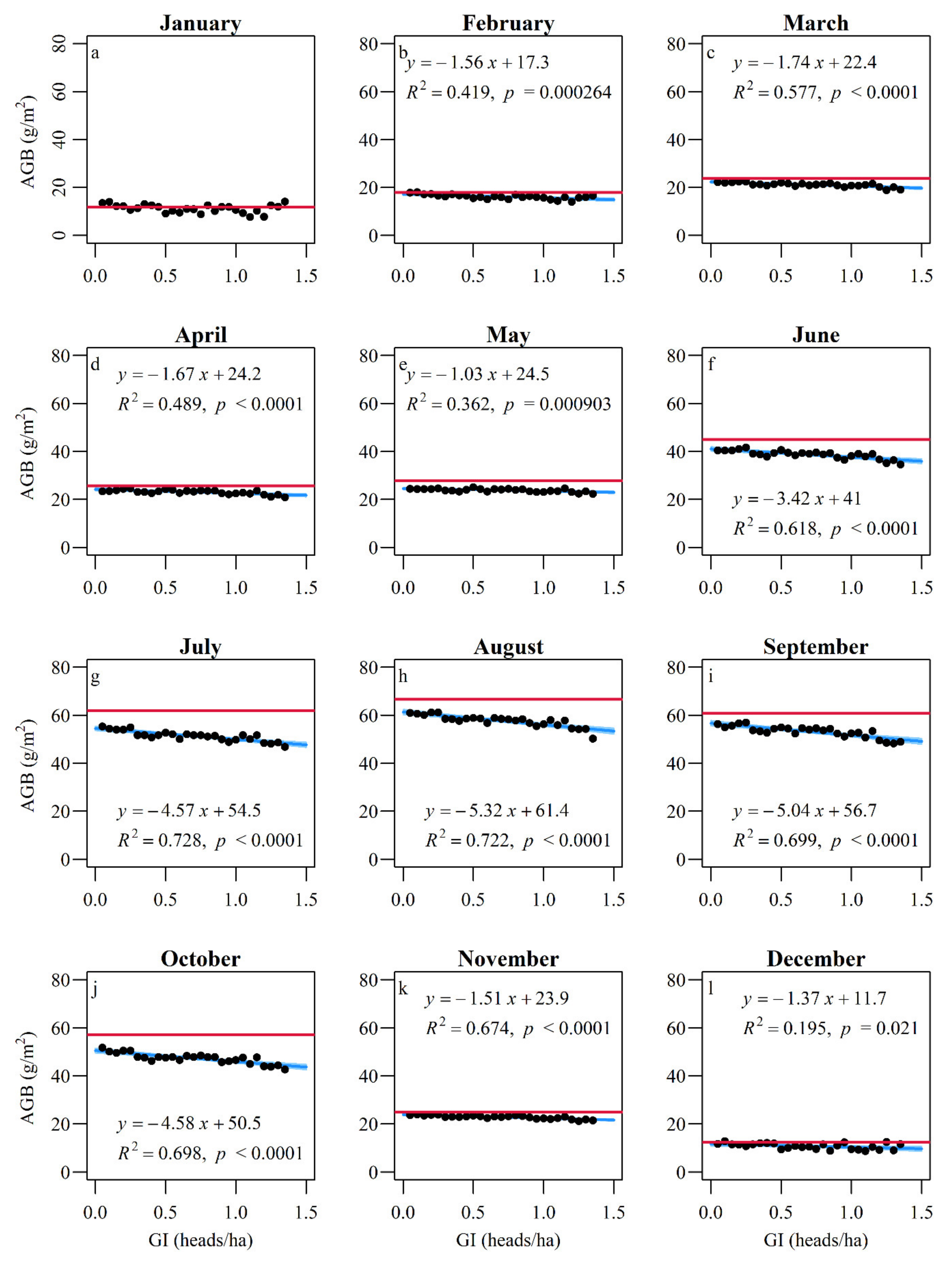
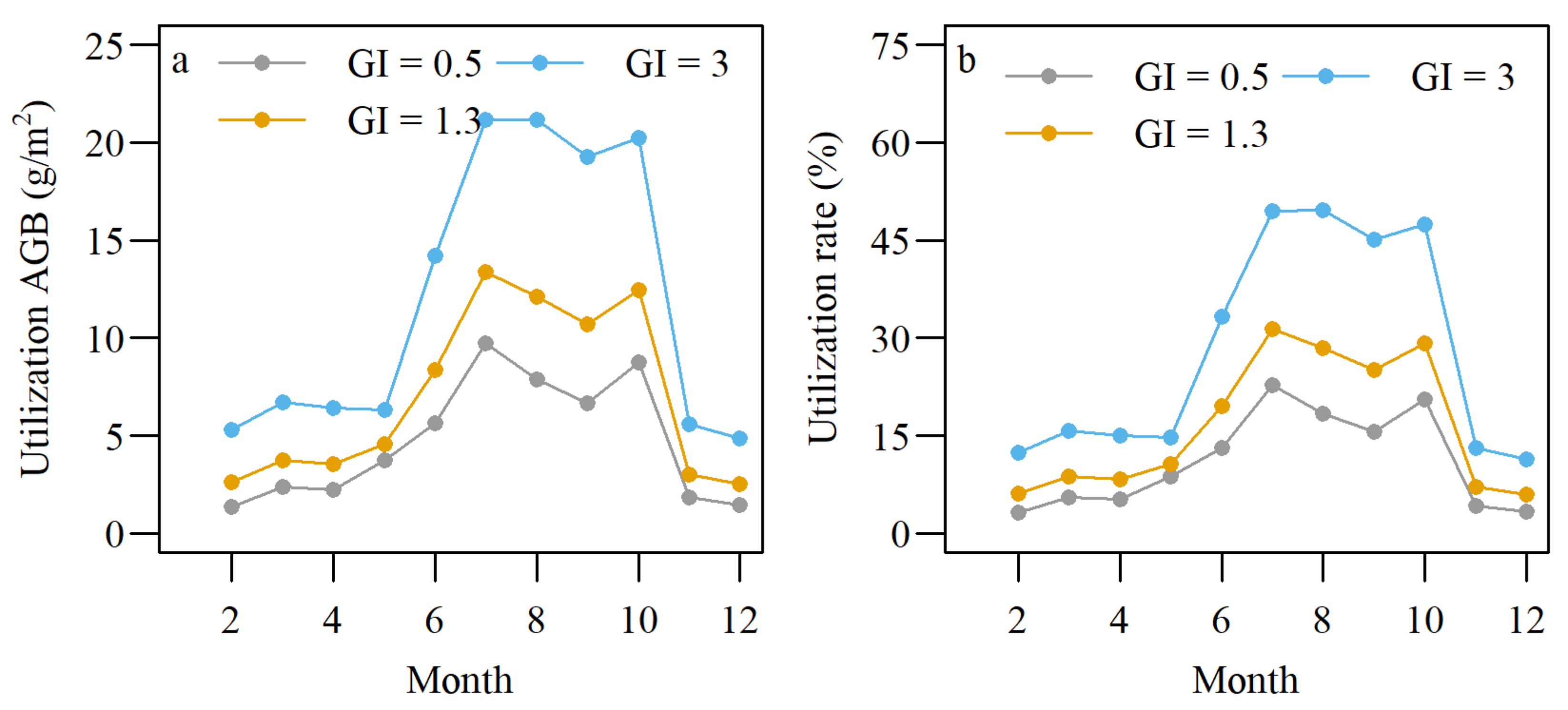
3.3. Response of AGB and Growth Rate to Grazing
3.4. Response of Vegetation Community Structure to Grazing
4. Discussion
4.1. Relationship between AGB and NDVI
4.2. Assessing the GI
4.3. Response of AGB to Grazing
4.4. Response of Species Diversity and Composition to Grazing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asner, G.P.; Elmore, A.J.; Olander, L.P.; Martin, R.E.; Harris, A.T. Grazing systems, ecosystem responses, and global change. Annu. Rev. Environ. Resour. 2004, 29, 261–299. [Google Scholar] [CrossRef]
- Kang, L.; Han, X.; Zhang, Z.; Sun, O.J. Grassland ecosystems in China: Review of current knowledge and research advancement. Philos. Trans. R. Soc. B 2007, 362, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, Q.; Liu, H.; Bai, Y.; Simmons, M.; Dittert, K.; Han, X. Plant responses following grazing removal at different stocking rates in an Inner Mongolia grassland ecosystem. Plant Soil 2011, 340, 199–213. [Google Scholar] [CrossRef]
- Perrino, E.V.; Musarella, C.M.; Magazzini, P. Management of grazing Italian river buffalo to preserve habitats defined by Directive 92/43/EEC in a protected wetland area on the Mediterranean coast: Palude Frattarolo, Apulia, Italy. Euro Mediterr. J. Environ. Integr. 2021, 6, 32. [Google Scholar] [CrossRef]
- Silva, V.; Pinto-Cruz, C.; Espírito-Santo, M.D. Temporary ponds and hygrophilous grasslands plant communities in Monfurado site of community importance. Lazaroa 2009, 30, 81–88. [Google Scholar]
- Wang, X.; Zhang, W.; Sun, Y.; Han, G. Yuzhong County Annals; GanSu People’s Publishing House: Lanzhou, China, 2001; pp. 3–4. [Google Scholar]
- Hu, Z.; Zhao, Z.; Zhang, Y.; Jing, H.; Gao, S.; Fang, J. Does ‘Forage-Livestock Balance’ policy impact ecological efficiency of grasslands in China? J. Clean. Prod. 2019, 207, 343–349. [Google Scholar] [CrossRef]
- Belsky, A.J. Does herbivory benefit plants? A review of the evidence. Am. Nat. 1986, 127, 870–892. [Google Scholar] [CrossRef]
- McNaughton, S. Compensatory plant growth as a response to herbivory. Oikos 1983, 40, 329–336. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Lauenroth, W.K. Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol. Monogr. 1993, 63, 327–366. [Google Scholar] [CrossRef]
- Ren, H.; Schönbach, P.; Wan, H.; Gierus, M.; Taube, F. Effects of grazing intensity and environmental factors on species composition and diversity in typical steppe of Inner Mongolia, China. PLoS ONE 2012, 7, e52180. [Google Scholar] [CrossRef] [Green Version]
- Schönbach, P.; Wan, H.; Gierus, M.; Bai, Y.; Müller, K.; Lin, L.; Susenbeth, A.; Taube, F. Grassland responses to grazing: Effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant Soil 2011, 340, 103–115. [Google Scholar] [CrossRef]
- Raynor, E.J.; Derner, J.D.; Baldwin, T.; Ritten, J.P.; Augustine, D.J. Multidecadal directional shift in shortgrass stocking rates. Rangel. Ecol. Manag. 2021, 74, 72–80. [Google Scholar] [CrossRef]
- Stephenson, M.B.; Volesky, J.D.; Schacht, W.H.; Lawrence, N.C.; Soper, J.; Milby, J. Influence of precipitation on plant production at different topographic positions in the nebraska sandhills. Rangel. Ecol. Manag. 2019, 72, 103–111. [Google Scholar] [CrossRef]
- Ma, H.; Xie, Y. Plant compensatory growth under different grazing intensities in desert steppe. Sci. Agric. Sin. 2008, 41, 3645–3650. [Google Scholar]
- Yan, L.; Zhou, G.; Zhang, F. Effects of different grazing intensities on grassland production in China: A meta-analysis. PLoS ONE 2013, 8, e81466. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Jiao, F.; Li, Y.H.; Kallenbach, R.L. Anthropogenic disturbances are key to maintaining the biodiversity of grasslands. Sci. Rep. 2016, 6, 22132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milchunas, D.G.; Sala, O.E.; Lauenroth, W.K. A generalized model of the effects of grazing by large herbivores on grassland community structure. Am. Nat. 1988, 132, 87–106. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Wesche, K.; Chen, D.; Zhang, S.; Wu, G.; Du, G.; Comerford, N. Grazing depresses soil carbon storage through changing plant biomass and composition in a Tibetan alpine meadow. Plant Soil Environ. 2011, 57, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Jáuregui, C.; Oesterheld, M. Effects of grazing intensity on plant richness and diversity: A meta-analysis. Oikos 2018, 127, 757–766. [Google Scholar] [CrossRef]
- Proulx, M.; Mazumder, A. Reversal of grazing impact on plant species richness in nutrient-poor vs. nutrient-rich ecosystems. Ecology 1998, 79, 2581–2592. [Google Scholar] [CrossRef]
- Zhu, G.; Tang, Z.; Chen, L.; ShangGuan, Z.; Deng, L. Overgrazing depresses soil carbon stock through changing plant diversity in temperate grassland of the Loess Plateau. Plant Soil Environ. 2018, 64, 1–6. [Google Scholar]
- Deng, L.; Sweeney, S.; Shangguan, Z.P. Grassland responses to grazing disturbance: Plant diversity changes with grazing intensity in a desert steppe. Grass Forage Sci. 2014, 69, 524–533. [Google Scholar] [CrossRef]
- Zainelabdeen, Y.M.; Yan, R.; Xin, X.; Yan, Y.; Ahmed, A.I.; Hou, L.; Zhang, Y. The impact of grazing on the grass composition in temperate grassland. Agronomy 2020, 10, 1230. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Shangguan, Z.P.; Zhao, D. Modeling vegetation coverage and soil erosion in the Loess Plateau Area of China. Ecol. Model. 2006, 198, 263–268. [Google Scholar] [CrossRef]
- Simons, L.; Allsopp, N. Rehabilitation of rangelands in Paulshoek, Namaqualand: Understanding vegetation change using biophysical manipulations. J. Arid Environ. 2007, 70, 755–766. [Google Scholar] [CrossRef]
- Hunt, L.P. Safe pasture utilisation rates as a grazing management tool in extensively grazed tropical savannas of Northern Australia. Rangel. J. 2008, 30, 305–315. [Google Scholar] [CrossRef]
- Cowley, R.; McCosker, K.; MacDonald, R.; Hearnden, M. Optimal pasture utilisation rates for sustainable cattle production with a commercial Brahman herd in the Victoria River Downs region of the Northern Territory. In Proceedings of the Northern Beef Research Update Conference’, Townsville, Australia, 1 January 2007; pp. 34–44. [Google Scholar]
- Johnston, P.; McKeon, G.; Day, K. Objective ’safe’ grazing capacities for south-west Queensland Australia: Development of a model for individual properties. Rangel. J. 1996, 18, 244–258. [Google Scholar] [CrossRef]
- Kawamura, K.; Akiyama, T.; Yokota, H.-o.; Tsutsumi, M.; Yasuda, T.; Watanabe, O.; Wang, S. Quantifying grazing intensities using geographic information systems and satellite remote sensing in the Xilingol steppe region, Inner Mongolia, China. Agric. Ecosyst. Environ. 2005, 107, 83–93. [Google Scholar] [CrossRef]
- Akasbi, Z.; Oldeland, J.; Dengler, J.; Finckh, M. Analysis of GPS trajectories to assess goat grazing pattern and intensity in Southern Morocco. Rangel. J. 2012, 34, 415–427. [Google Scholar] [CrossRef]
- Zhang, T.H.; Zhao, H.L.; Li, S.G.; Zhou, R.L. Grassland changes under grazing stress in Horqin sandy grassland in Inner Mongolia, China. N. Z. J. Agric. Res. 2004, 47, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Mack, R.N.; Thompson, J.N. Evolution in steppe with few large, hooved mammals. Am. Nat. 1982, 119, 757–773. [Google Scholar] [CrossRef]
- Fortin, D.; Fryxell, J.M.; Pilote, R. The temporal scale of foraging decisions in bison. Ecology 2002, 83, 970–982. [Google Scholar] [CrossRef]
- van Beest, F.M.; Mysterud, A.; Loe, L.E.; Milner, J.M. Forage quantity, quality and depletion as scale-dependent mechanisms driving habitat selection of a large browsing herbivore. J. Anim. Ecol. 2010, 79, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Owen-Smith, N. Effects of temporal variability in resources on foraging behaviour. Res. Ecol. 2008, 23, 159–181. [Google Scholar]
- Huang, Y.; Wang, L.; Wang, D.; Zeng, D.-H.; Li, Y.; Liu, J.; Wang, Y. Foraging responses of sheep to plant spatial micro-patterns can cause diverse associational effects of focal plant at individual and population levels. J. Anim. Ecol. 2018, 87, 863–873. [Google Scholar] [CrossRef]
- Raynor, E.J.; Gersie, S.P.; Stephenson, M.B.; Clark, P.E.; Spiegal, S.A.; Boughton, R.K.; Bailey, D.W.; Cibils, A.; Smith, B.W.; Derner, J.D.; et al. Cattle grazing distribution patterns related to topography across diverse rangeland ecosystems of North America. Rangel. Ecol. Manag. 2021, 75, 91–103. [Google Scholar] [CrossRef]
- Junges, A.H.; Bremm, C.; Fontana, D.C.; Oliveira, C.A.O.d.; Schaparini, L.P.; Carvalho, P.C.d.F. Temporal profiles of vegetation indices for characterizing grazing intensity on natural grasslands in Pampa biome. Sci. Agric. 2016, 73, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO); The International Institute for Applied Systems Analysis (IIASA); International Soil Reference and Information Centre (ISRIC); Institute of Soil Science—Chinese Academy of Sciences (ISSCAS); Joint Research Centre of the European Commission (JRC). Harmonized World Soil Database (version 1.2); FAO; IIASA: Italy, Rome; Laxenburg, Austria, 2012. [Google Scholar]
- Holechek, J.L.; Gomes, H.D.S.; Molinar, F.; Galt, D. Grazing intensity: Critique and approach. Rangelands 1998, 20, 15–18. [Google Scholar]
- ESRI. ArcGIS; 10.2; Environmental Systems Research Institute: Redlands, CA, USA, 2013. [Google Scholar]
- ENVI. Environment for Visualizing Images; 5.0; Exelis Visual Information Solutions: Boulder, CO, USA, 2013. [Google Scholar]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar] [CrossRef]
- The Ministry of Agriculture of the People’s Republic of China. Agriculture Industry Standard of the People’s Republic of China: Calculation of Rangeland Carrying Capacity; China Meteorological Press: Beijing, China, 2015; pp. 1–6. [Google Scholar]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.-M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; Deng, L.; Tang, Z.; Wang, K.; Sun, W.; Shangguan, Z. Prediction of aboveground grassland biomass on the Loess Plateau, China, using a random forest algorithm. Sci. Rep. 2017, 7, 6940. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yang, X.; Qiu, J.; Li, J.; Gao, T.; Wu, Q.; Zhao, F.; Ma, H.; Yu, H.; Xu, B. Remote sensing-based biomass estimation and its spatio-temporal variations in temperate grassland, Northern China. Remote Sens. 2014, 6, 1496–1513. [Google Scholar] [CrossRef] [Green Version]
- Adler, P.B.; Hall, S.A. The development of forage production and utilization gradients around livestock watering points. Landsc. Ecol. 2005, 20, 319–333. [Google Scholar] [CrossRef]
- Robles, A.B.; Passera, C.B. Native forage shrub species in south-eastern Spain: Forage species, forage phytomass, nutritive value and carrying capacity. J. Arid Environ. 1995, 30, 191–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, D.; Badgery, W.B.; Kemp, D.R.; Chen, W.; Wang, X.; Liu, N. Reduced grazing pressure delivers production and environmental benefits for the typical steppe of north China. Sci. Rep. 2015, 5, 16434. [Google Scholar] [CrossRef] [Green Version]
- Ash, A.; McIvor, J.; Mott, J.; Andrew, M. Building grass castles: Integrating ecology and management of Australia’s tropical tallgrass rangelands. Rangel. J. 1997, 19, 123–144. [Google Scholar] [CrossRef]
- Wang, Z.; Johnson, D.; Rong, Y.; Wang, K. Grazing effects on soil characteristics and vegetation of grassland in northern China. Solid Earth 2016, 7, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Chai, L.; Hou, F.; Chang, S.; Ma, Y.; Tsunekawa, A.; Cheng, Y. Quantifying grazing intensity using remote sensing in alpine meadows on Qinghai-Tibetan plateau. Sustainability 2019, 11, 417. [Google Scholar] [CrossRef] [Green Version]
- Glindemann, T.; Wang, C.; Tas, B.M.; Schiborra, A.; Gierus, M.; Taube, F.; Susenbeth, A. Impact of grazing intensity on herbage intake, composition, and digestibility and on live weight gain of sheep on the Inner Mongolian steppe. Livest. Sci. 2009, 124, 142–147. [Google Scholar] [CrossRef]
- Ren, G.; Wang, C.; Dong, K.; Zhu, H.; Wang, Y.; Zhao, X. Effects of grazing exclusion on soil–vegetation relationships in a semiarid grassland on the Loess Plateau, China. Land Degrad. Dev. 2018, 29, 4071–4079. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Han, S.; Wang, X. Effect of grazing intensity on the regrowth capability in Leymus chinensis grassland. Acta Pratac. Sci. 2004, 13, 39–44. [Google Scholar]
- Fanselow, N.; Schönbach, P.; Gong, X.Y.; Lin, S.; Taube, F.; Loges, R.; Pan, Q.; Dittert, K. Short-term regrowth responses of four steppe grassland species to grazing intensity, water and nitrogen in Inner Mongolia. Plant Soil 2011, 340, 279–289. [Google Scholar] [CrossRef]
- Mackey, R.L.; Currie, D.J. The diversity–disturbance relationship: Is it generally strong and peaked? Ecology 2001, 82, 3479–3492. [Google Scholar]
- Wang, C.; Tang, Y. A global meta-analyses of the response of multi-taxa diversity to grazing intensity in grasslands. Environ. Res. Lett. 2019, 14, 114003. [Google Scholar] [CrossRef] [Green Version]
- Jia, S. Forage Floras of China; Agriculture Press: Beijing, China, 1987; Volume 1, pp. 191–193. [Google Scholar]
- Dumont, B.; Carrère, P.; D’Hour, P. Foraging in patchy grasslands: Diet selection by sheep and cattle is affected by the abundance and spatial distribution of preferred species. Anim. Res. 2002, 51, 367–381. [Google Scholar] [CrossRef] [Green Version]
- Allred, B.W.; Fuhlendorf, S.D.; Engle, D.M.; Elmore, R.D. Ungulate preference for burned patches reveals strength of fire–grazing interaction. Ecol. Evol. 2011, 1, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi Kohyani, P.; Bossuyt, B.; Bonte, D.; Hoffmann, M. Differential herbivory tolerance of dominant and subordinate plant species along gradients of nutrient availability and competition. Plant Ecol. 2008, 201, 247–255. [Google Scholar]
- Augustine, D.J.; McNaughton, S.J. Ungulate effects on the functional species composition of plant communities: Herbivore selectivity and plant tolerance. J. Wildlife Manag. 1998, 62, 1165–1183. [Google Scholar] [CrossRef]



| Month | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | Total | |
| 1 | 15 | 15 | ||||
| 2 | 1 | 1 | ||||
| 3 | 6 | 6 | ||||
| 4 | 1 | 7 | 16 | 24 | ||
| 5 | 13 | 5 | 18 | |||
| 6 | 2 | 2 | ||||
| 7 | 1 | 18 | 19 | |||
| 8 | 1 | 2 | 5 | 8 | ||
| 9 | 7 | 13 | 20 | |||
| 10 | 2 | 9 | 15 | 26 | ||
| 11 | 6 | 0 | 6 | |||
| 12 | 4 | 23 | 9 | 36 | ||
| Total | 7 | 48 | 1 | 82 | 43 | 181 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Jin, B.; Luo, K.; Pei, J.; Zhang, X.; Zhang, Y.; Tang, J.; Yang, Q.; Sun, G. Vegetation Response to Goats Grazing Intensity in Semiarid Hilly Grassland of the Loess Plateau, Lanzhou, China. Sustainability 2021, 13, 3569. https://doi.org/10.3390/su13063569
Cheng H, Jin B, Luo K, Pei J, Zhang X, Zhang Y, Tang J, Yang Q, Sun G. Vegetation Response to Goats Grazing Intensity in Semiarid Hilly Grassland of the Loess Plateau, Lanzhou, China. Sustainability. 2021; 13(6):3569. https://doi.org/10.3390/su13063569
Chicago/Turabian StyleCheng, Hua, Baocheng Jin, Kai Luo, Jiuying Pei, Xueli Zhang, Yonghong Zhang, Jiaqi Tang, Qin Yang, and Guojun Sun. 2021. "Vegetation Response to Goats Grazing Intensity in Semiarid Hilly Grassland of the Loess Plateau, Lanzhou, China" Sustainability 13, no. 6: 3569. https://doi.org/10.3390/su13063569






