Study of the Variability in Fatty Acids and Carotenoid Profiles: Laying the Ground for Tank Milk Authentication
Abstract
1. Introduction
2. Materials and Methods
2.1. Farms
2.2. Sample Design
2.3. Livestock Farms Included in the Study
2.4. Analytical Measurements
2.5. Statistical Analysis
3. Results and Discussion
3.1. Milk Production and Chemical Quality of the Milk Samples
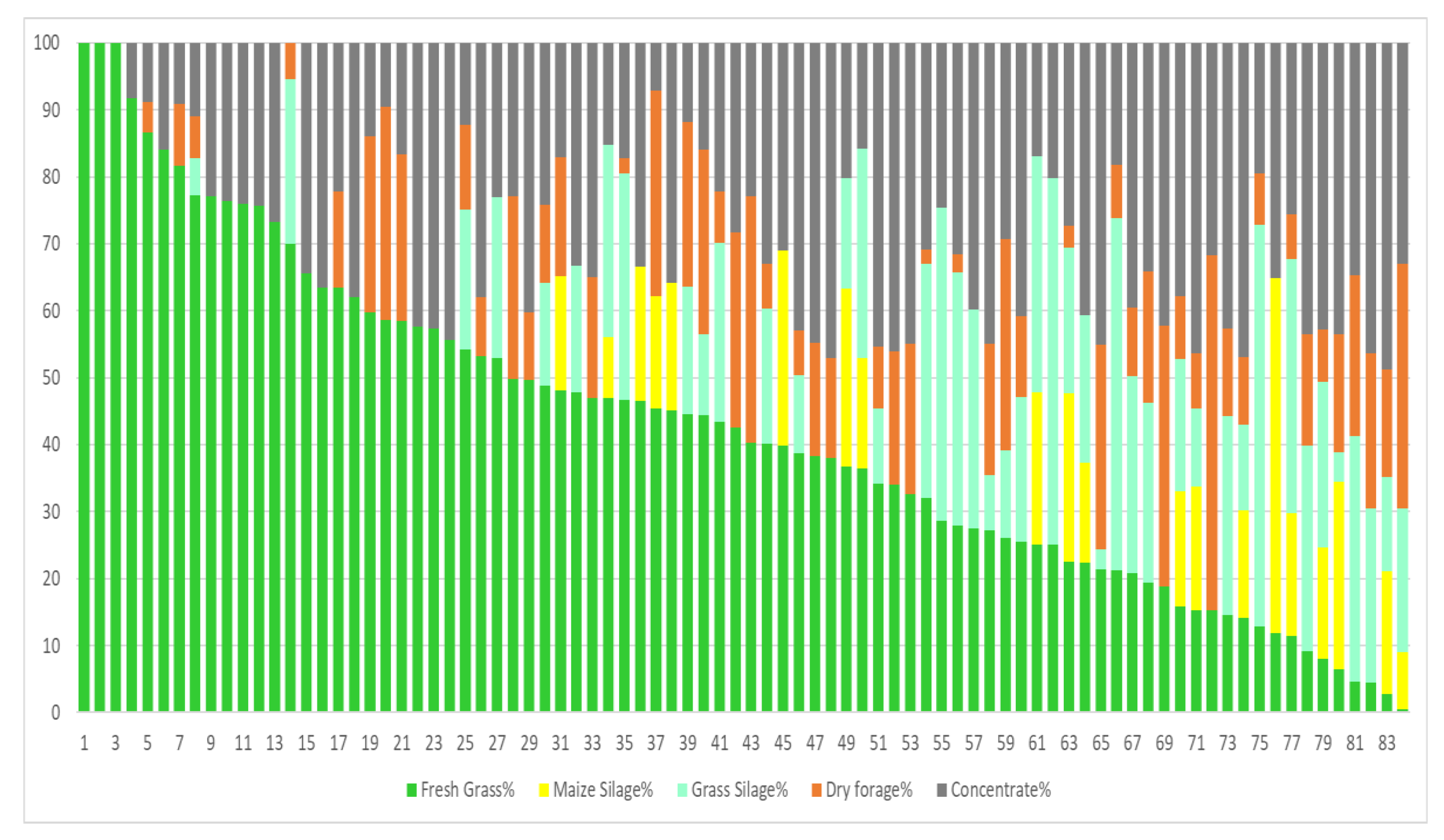
3.2. Diets
3.3. Fatty Acids Profile (FA)
3.4. Correlation between Diet Components and FA Profile of Milk Samples
3.5. Fat-Soluble Antioxidants Profile (FSA)
3.6. Correlations between Diet Components and Fat-Soluble Antioxidants Found in Milk
3.7. Correlations between Diet and Farm Characteristics
3.8. Correlations between FA and FSA Milk Content and Farm Characteristics
3.9. Differences in FA and FSA Profiles Depending on Whether or Not Fresh Forage Is Present in the Diet and How to Supply That Grass: Grazing vs. Cut and Carry (Zero-Grazed)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elgersma, A.; Tamminga, S.; Ellen, G. Comparison of the Effects of Grazing and zero-Grazing of Grass on Milk Fatty Acid Composition of Dairy Cows. In Optimal Forage Systems for Animal Production and the Environment, Proceedings of the 12th Symposium of the European Grassland Federation, Pleven, Bulgaria, 26–28 May 2003; pp. 271–274. Available online: https://books.google.com.hk/books/about/Optimal_Forage_Systems_for_Animal_Produc.html?id=UEfZAQAACAAJ&redir_esc=y (accessed on 16 April 2021).
- Couvreur, S.; Hurtaud, C.; Lopez, C.; Delaby, L.; Peyraud, J.L. The linear relationship between the proportion of fresh grass in the cow diet milk fatty acid composition and butter properties. J. Dairy Sci. 2006, 89, 1956–1969. [Google Scholar] [CrossRef]
- Slots, T.; Butle, G.; Leifert, C.; Kristensen, T.; Skibsted, L.H.; Nielsen, J.H. Potentials to differentiate milk composition by different feeding strategies. J. Dairy Sci. 2009, 92, 2057–2066. [Google Scholar] [CrossRef]
- Agabriel, C.; Cornu, A.; Journal, C.; Sibra, C.; Grolier, P.; Martin, B. Tanker milk variability according to farm feeding practices: Vitamins A and E carotenoids colour and terpenoids. J. Dairy Sci. 2007, 90, 4884–4896. [Google Scholar] [CrossRef]
- Benbrook, C.; Davis, D.R.; Bradley, J.H.; Baranski, M. Enhancing the fatty acid profile of milk through forage-based rations with nutrition modeling of diet outcomes. Food Sci. Nutr. 2018, 6, 682–700. [Google Scholar] [CrossRef] [PubMed]
- Elgersma, A.; Wever, A.C.; Nałęcz-Tarwacka, T. Grazing versus indoor feeding: Effects on Milk Quality. In Sustainable Grassland Productivity, Proceedings of the 21st General Meeting of the European Grassland Federation, Badajoz, Spain, 3–6 April 2006; Volume 11, pp. 419–427. Available online: https://books.google.com.hk/books/about/Sustainable_Grassland_Productivity.html?id=oEIjAQAAMAAJ&redir_esc=y (accessed on 16 April 2021).
- Elgersma, A. New developments in The Netherlands: Dairies reward grazing because of public perception. Grassl. Sci. Eur. 2015, 17, 420–422. [Google Scholar]
- Morales-Almaráz, E.; de la Roza Delgado, B.; González, A.; Soldado, A.; Rodríguez, M.L.; Peláez, M.; Vicente, F. Effect of feeding system on unsaturated fatty acid levels in milk of dairy cows. Renew. Agric. Food Syst. 2011, 224–229. [Google Scholar] [CrossRef]
- Villar, A.; Gradillas, G.; Fernández, C.; Gutiérrez, M.R.; Rodríguez-Loperena, M.A.; Barrachina, M.; García, J.A. Aspectos sanitarios y de calidad de la producción ecológica de leche: Infecciones mamarias y perfil de ácidos grasos. Tierras Castilla León Ganad. 2011, 179, 52–60. [Google Scholar]
- Flores, G.; Fernández-Lorenzo, B.; Dagnac, T.; Resch, C.; Pereira-Crespo, S.; Lorenzana, R.; González, L.; Agruña, M.J.; Barreal, M.; Veiga, M.; et al. Relación entre dieta y calidad de la leche en un panel de explotaciones lecheras gallegas. Rev. Afriga 2015, 118, 130–146. [Google Scholar]
- NRC-National Research Council. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- ISO-IDF (International Organization for Standardization-International Dairy Federation). Milk and Milk Products—Extraction Methods for Lipids and Fat-Soluble Compounds; ISO: Brussels, Belgium, 2001. [Google Scholar]
- ISO-IDF (International Organization for Standardization-International Dairy Federation). Milk Fat—Preparation of Fatty Acid Methyl Esters; ISO: Brussels, Belgium, 2002. [Google Scholar]
- Kramer, J.K.G.; Blackadar, C.B.; Zhou, J. Evaluation of Two GC Columns (60-m SUPELCOWAX 10 and 100-m CP Sil 88) for Analysis of Milkfat with Emphasis on CLA, 18:1, 18:2 and 18:3 Isomers, and Short- and Long-Chain FA. Lipids 2002, 37, 823–835. [Google Scholar] [CrossRef]
- Gentili, A.; Caretti, F.; Bellante, S.; Ventura, S.; Canepari, S.; Curini, R. Comprehensive Profiling of Carotenoids and Fat-Soluble Vitamins in Milk from Different Animal Species by LC-DAD-MS/MS Hyphenation. J. Agric. Food Chem. 2013, 61, 1628–1639. [Google Scholar] [CrossRef]
- Chauveau-Duriot, B.; Doreau, M.; Noziere, P.; Graulet, B. Simultaneous quantification of carotenoids retinol and tocopherols in forages bovine plasma and milk: Validation of a novel UPLC method. Anal. Bioanal. Chem. 2010, 397, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, A.; Agabriel, C.; Sibra, C.; Journal, C.; Martin, B.; Chilliard, Y. Tanker milk variability in fatty acids according to farm feeding and husbandry practices in a French semi-mountain area. Dairy Sci. Technol. 2008, 88, 193–215. [Google Scholar] [CrossRef]
- Capuano, E.; Van der Veer, G.; Boerrigter-Eenling, R.; Elgersma, A.; Rademaker, J.; Sterian, A.; Van Ruth, S.M. Verification of fresh grass feeding, pasture grazing and organic farming by cows farm milk fatty acid profile. Food Chem. 2014, 164, 234–241. [Google Scholar] [CrossRef]
- Vicente, F.; Santiago, S.; Jiménez-Calderón, J.D.; Martínez-Fernández, A. Capacity of milk composition to identify the feeding system on dairy cows. J. Dairy Res. 2017, 84, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Morales-Almaráz, E.; de la Roza-Delgado, B.; Soldado, A.; Martínez-Fernández, A.; González, A.; Domínguez-Vara, I.A.; Vicente, F. Parity and grazing-time effects on milk fatty acid profile in dairy cows. Anim. Prod. Sci. 2018, 58, 1233–1238. [Google Scholar] [CrossRef]
- Dhiman, T.R.; Anand, G.R.; Satter, L.D.; Pariza, M.W. Conjugated linoleic acid content of milk from cows fed different diets. J. Dairy Sci. 1999, 82, 2146–2156. [Google Scholar] [CrossRef]
- Chin, S.F.; Liu, W.; Storkson, J.M.; Ha, Y.L.; Pariza, M.W. Dietary sources of conjugated dienoic isomers of linoleic acid a newly recognized class of anticarcinogens. J. Food Compost. Anal. 1992, 5, 185–197. [Google Scholar] [CrossRef]
- Bauman, D.E.; Corl, B.A.; Peterson, G.P. The Biology of Conjugated Linoleic Acids in Ruminants; Advances in Conjugated Linoleic Acid Research; Sébédio, J.-L., Christie, W.W., Adlof, R., Eds.; AOCS Press: Champaign, IL, USA, 2003; Volume 2, pp. 146–173. [Google Scholar]
- Chion, A.R.; Tabacco, E.; Giaccone, D.; Peiretti, G.; Batelli, G.; Borreani, G. Variation of fatty acid and terpene profiles in mountain milk and “Toma piemontese” cheese as affected by diet composition in different seasons. Food Chem. 2010, 121, 393–399. [Google Scholar] [CrossRef]
- Griinari, J.M.; Bauman, D.E. Update on Theories of Diet-Induced Milk Fat Depression and Potential Applications. In Recent Advances in Animal Nutrition; Garnsworthy, P.C., Wiseman, J., Eds.; Nottingham University Press: Nottingham, UK, 2003; pp. 115–156. [Google Scholar]
- Borreani, G.; Coppa, M.; Revello-Chion, A.; Comino, L.; Giaccone, D.; Ferlay, A.; Tabacco, E. Effect of different feeding strategies in intensive dairy farming systems on milk fatty acid profiles, and implications on feeding costs in Italy. J. Dairy Sci. 2013, 96, 6840–6855. [Google Scholar] [CrossRef]
- De La Torre-Santos, S.; Royo, L.J.; Martínez-Fernández, A.; Chocarro, C.; Vicente, F. The Mode of Grass Supply to Dairy Cows Impacts on Fatty Acid and Antioxidant Profile of Milk. Foods 2020, 9, 1256. [Google Scholar] [CrossRef]
- Coppa, M.; Ferlay, A.; Chassaing, C.; Agabriel, C.; Glasser, F.; Chilliard, Y.; Borreani, G.; Barcarolo, R.; Baars, T.; Kusche, D.; et al. Prediction of bulk milk fatty acid composition based on farming practices collected through on-farm surveys. J. Dairy Sci. 2013, 96, 4197–4211. [Google Scholar] [CrossRef]
- Larsen, M.K.; Nielsen, J.H.; Butler, G.; Leifert, C.; Slots, T.; Kristiansen, G.H.; Gustafsson, A.H. Milk quality as affected by feeding regimens in a country with climatic variation. J. Dairy Sci. 2010, 93, 2863–2873. [Google Scholar] [CrossRef]
- Ballet, N.; Robert, J.C.; Williams, P.E.V. Vitamins in Forages; Forage Evaluation in Ruminant Nutrition; Givens, D.I., Owen, E., Axford, R.F.E., Omed, H.M., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 341–399. [Google Scholar]
- Butler, G.; Nielsen, J.H.; Slots, T.; Seal, C.; Eyre, M.D.; Sanderson, R.; Leifert, C. Fatty acid and fat soluble antioxidant concentrations in milk from high-and low-input conventional and organic systems: Seasonal variation. J. Sci. Food Agric. 2008, 88, 1431–1441. [Google Scholar] [CrossRef]
- Havemose, M.S.; Weisbjerg, M.R.; Bredie, W.L.P.; Nielsen, J.H. Influence of feeding different types of roughage on the oxidative stability of milk. Int. Dairy J. 2004, 14, 563–570. [Google Scholar] [CrossRef]
- Lucas, A.; Agabriel, C.; Martin, B.; Ferlay, A.; Verdier-Metz, I.; Coulon, J.B.; Rock, E. Relationships between the conditions of cow’s milk production and the contents of components of nutritional interest in raw milk farmhouse cheese. Le Lait 2006, 86, 177–202. [Google Scholar] [CrossRef]
- Offer, N.W. Effects of Cutting and Ensiling Grass on Levels of CLA in Bovine Milk. In Proceedings of the XIIIth International Silage Conference, Auchincruive, Scottish Agricultural College, Scotland, UK, 11–13 September 2002; pp. 16–17. [Google Scholar]
- Kalač, P.; Samková, E. The effects of feeding various forages on fatty acid composition of bovine milk fat: A review. Czech. J. Anim. Sci. 2010, 55, 521–537. [Google Scholar] [CrossRef]
- Elgersma, A.; van der Hoeven, E.; Witkowska, I.M.; Smit, H.J. Effects of the grazed horizon in perennial ryegrass swards on the conjugated linoleic acid concentration in milk of dairy cows. Grassl. Sci. Eur. 2008, 13, 388–390. [Google Scholar]

| N (1) | Minimum | Maximum | Mean | SD (2) | |
|---|---|---|---|---|---|
| Useful Agricultural Area Total field area (ha) | 88 | 6.00 | 190.00 | 43.11 | 30.80 |
| Livestock units (3) | 88 | 12.80 | 611.80 | 110.27 | 98.58 |
| Livestock units per ha | 88 | 0.53 | 8.37 | 2.74 | 1.56 |
| Average milk yield (litres) | 89 | 42,700 | 5,150,000 | 713,075 | 780,498 |
| Production | N (1) | Minimum | Maximum | Mean | SD (2) |
|---|---|---|---|---|---|
| Milk delivered on the day of sampling (liters/day−1) | 173 | 140 | 14,440 | 2812 | 2727 |
| Estimated average milk production per cow (liters/cow−1/day−1) | 174 | 7.30 | 45.90 | 27.14 | 6.93 |
| Chemical quality of milk | |||||
| Fat (%) | 173 | 2.64 | 4.83 | 3.73 | 0.30 |
| Protein (%) | 174 | 2.87 | 3.56 | 3.24 | 0.15 |
| Lactose (%) | 173 | 4.01 | 4.94 | 4.74 | 0.12 |
| SNF (Solids Not Fat) (%) | 173 | 7.62 | 9.20 | 8.74 | 0.23 |
| Fatty Acids (g·100 g−1 of Total FA) (1) | Mean | SD (2) | Minimum | Maximum | CV (3) (%) |
|---|---|---|---|---|---|
| C4:0 | 5.81 | 0.65 | 4.27 | 7.55 | 11.2 |
| C6:0 | 2.49 | 0.25 | 1.77 | 3.04 | 10.0 |
| C8:0 | 1.25 | 0.14 | 0.88 | 1.57 | 11.2 |
| C10:0 | 2.81 | 0.40 | 1.74 | 3.79 | 14.2 |
| c9-C10:1 | 0.07 | 0.01 | 0.04 | 0.11 | 14.3 |
| C11:0 | 0.05 | 0.03 | 0.01 | 0.17 | 60.0 |
| C12:0 | 3.26 | 0.51 | 2.03 | 4.53 | 15.6 |
| C13:0 | 0.10 | 0.04 | 0.03 | 0.23 | 40.0 |
| C14:0-iso | 0.12 | 0.04 | 0.05 | 0.28 | 33.3 |
| C14:0 | 11.25 | 1.35 | 2.24 | 13.68 | 12.0 |
| C15:0-iso | 0.26 | 0.08 | 0.13 | 0.55 | 30.8 |
| c9-C14:1 | 1.03 | 0.17 | 0.69 | 1.51 | 16.5 |
| C15:0-anteiso | 0.53 | 0.10 | 0.33 | 0.81 | 18.9 |
| C15:0 | 1.16 | 0.17 | 0.71 | 1.70 | 14.7 |
| c10-C15:1 | 0.00 | 0.00 | 0.00 | 0.02 | |
| C16:0 | 30.18 | 3.45 | 21.44 | 41.83 | 11.4 |
| c9-16:1-n7 (5) | 1.79 | 0.36 | 0.02 | 2.48 | 20.1 |
| C17:0 | 0.58 | 0.09 | 0.39 | 0.97 | 15.5 |
| C18:0 | 9.37 | 1.42 | 6.22 | 12.70 | 15.2 |
| t6-t9-C18:1 | 0.51 | 0.15 | 0.30 | 1.11 | 29.4 |
| t10-C18:1 | 0.41 | 0.28 | 0.16 | 1.77 | 68.3 |
| t11-C:18:1 | 1.67 | 1.08 | 0.35 | 5.87 | 64.7 |
| t12-18:1 | 0.35 | 0.12 | 0.17 | 0.68 | 34.3 |
| c9-C18:1-n9 | 19.70 | 1.96 | 15.58 | 24.87 | 9.9 |
| c11-C18:1-n7 | 0.49 | 0.14 | 0.26 | 1.28 | 28.6 |
| c12-18:1 | 0.21 | 0.12 | 0.04 | 0.66 | 57.1 |
| tC18:2-n6 | 0.09 | 0.09 | 0.03 | 0.56 | 100.0 |
| cC18:2-n6 | 2.08 | 0.59 | 0.82 | 3.98 | 28.4 |
| C18:3-n6 | 0.04 | 0.01 | 0.02 | 0.06 | 25.0 |
| C18:3-n3 | 0.58 | 0.24 | 0.21 | 1.31 | 41.4 |
| C20:0 | 0.17 | 0.04 | 0.08 | 0.35 | 23.5 |
| c9,t11-CLA | 0.73 | 0.42 | 0.20 | 2.47 | 57.5 |
| CLA_isom_d (4) | 0.14 | 0.03 | 0.07 | 0.24 | 21.4 |
| c11-C20:1-n9 | 0.03 | 0.02 | 0.00 | 0.08 | 66.7 |
| C21:0 | 0.04 | 0.02 | 0.02 | 0.11 | 50.0 |
| C20:2-n6 | 0.02 | 0.01 | 0.00 | 0.04 | 50.0 |
| C20:3-n6 | 0.11 | 0.05 | 0.01 | 0.43 | 45.5 |
| C20:3-n3 | 0.18 | 0.05 | 0.01 | 0.32 | 27.8 |
| C22:1-n9 | 0.00 | 0.00 | 0.00 | 0.00 | |
| C22:0 | 0.04 | 0.02 | 0.01 | 0.11 | 50.0 |
| C20:4-n6 | 0.01 | 0.01 | 0.00 | 0.06 | 100.0 |
| C22:2-n6 | 0.07 | 0.04 | 0.01 | 0.20 | 57.1 |
| C23:0 | 0.03 | 0.02 | 0.00 | 0.09 | 66.7 |
| C20:5-n3 | 0.01 | 0.00 | 0.00 | 0.02 | 0.0 |
| C24:0 | 0.06 | 0.02 | 0.01 | 0.13 | 33.3 |
| C24:1-n9 | 0.01 | 0.01 | 0.00 | 0.03 | 100.0 |
| C22:5-n3 | 0.09 | 0.03 | 0.02 | 0.20 | 33.3 |
| C22:6-n3 | 0.01 | 0.01 | 0.00 | 0.08 | 100.0 |
| Main Relationships between FA (1) (2) (g 100 g Total FA) | Mean | SD (2) | Minimum | Maximum | CV (3) (%) |
|---|---|---|---|---|---|
| Total saturated FA (SFA) | 68.65 | 3.10 | 59.95 | 75.99 | 4.5 |
| Total branched FA (BCFA) | 0.91 | 0.18 | 0.59 | 1.51 | 19.8 |
| Total monounsaturated FA (MUFA) | 26.28 | 2.57 | 20.08 | 33.13 | 9.8 |
| c-MUFA | 23.34 | 1.87 | 18.65 | 28.65 | 8.0 |
| t-MUFA | 2.94 | 1.11 | 1.26 | 7.13 | 37.8 |
| Ratio t11/t10-C18:1 | 5.65 | 5.13 | 0.42 | 25.08 | 90.8 |
| Total polyunsaturated FA (PUFA) | 4.16 | 0.68 | 2.81 | 6.60 | 16.3 |
| CLA isomers sum | 0.87 | 0.44 | 0.28 | 2.69 | 50.6 |
| n6 isomers sum (5) | 2.42 | 0.59 | 1.16 | 4.25 | 24.4 |
| n3 isomers sum | 0.87 | 0.24 | 0.47 | 1.57 | 27.6 |
| PUFA/SFA | 0.06 | 0.01 | 0.04 | 0.11 | 16.7 |
| n6/n3 ratio (4) | 3.02 | 1.12 | 0.92 | 6.48 | 37.1 |
| Total unsaturated FA (UFA)/SFA | 0.45 | 0.07 | 0.31 | 0.65 | 15.6 |
| Fatty Acids (g·100 g−1 of Total FA) (1) | % Ration DM | Kg DM Cow−1 Day−1 | Liters Cow−1 Day−1 | |||
|---|---|---|---|---|---|---|
| Fresh Forage (2) | Forage (3) | Maize Silage | Concentrate | |||
| FA | ||||||
| t11-C18:1 | 0.765 ** | 0.702 ** | −0.520 ** | −0.644 ** | −0.564 ** | −0.625 ** |
| c12-C18:1 | −0.547 ** | −0.689 ** | 0.512 ** | 0.596 ** | 0.505 ** | 0.567 ** |
| C18:3-n3 (5) | 0.757 ** | 0.732 ** | −0.572 ** | −0.653 ** | −0.513 ** | −0.559 ** |
| n3 sum | 0.740 ** | 0.675 ** | −0.543 ** | −0.600 ** | −0.533 ** | |
| c9,t11 CLA | 0.734 ** | 0.690 ** | −0.503 ** | −0.642 ** | −0.508 ** | −0.624 ** |
| CLA isomers sum | 0.727 ** | 0.690 ** | −0.510 ** | −0.633 ** | −0.557 ** | −0.626 ** |
| C22:0 | 0.559 ** | 0.582 ** | −0.511 ** | −0.516 ** | −0.507 ** | −0.576 ** |
| C22:2-n6 | 0.667 ** | 0.669 ** | −0.662 ** | −0.551 ** | ||
| C22:5-n3 | 0.636 ** | 0.624 ** | −0.627 ** | −0.578 ** | ||
| Main relationships between FA | ||||||
| Ratio t11/t10-C18:1 | 0.765 ** | 0.787 ** | −0.539 ** | −0.771 ** | −0.624 ** | −0.694 ** |
| n6/n3 ratio (4) | −0.668 ** | −0.726 ** | 0.546 ** | 0.675 ** | 0.540 ** | 0.614 ** |
| Branched FA | 0.627 ** | 0.649 ** | −0.548 ** | −0.556 ** | −0.590 ** | −0.684 ** |
| FSA (1) ppbv (ng/mL) | Mean Value | SD (2) | Min. | Max. | CV (3) (%) |
|---|---|---|---|---|---|
| Retinol | 939.01 | 275.53 | 397.02 | 1978.24 | 29.3 |
| α-Tocopherol | 1419.49 | 651.89 | 416.68 | 4374.00 | 45.9 |
| γ- Tocopherol | 44.34 | 21.89 | 9.85 | 137.83 | 49.4 |
| Lutein | 14.53 | 9.56 | 1.99 | 48.36 | 65.8 |
| Zeaxanthin | 1.07 | 0.64 | 0.17 | 3.97 | 59.8 |
| β-Cryptoxanthin | 1.92 | 0.92 | 0.00 | 5.36 | 47.9 |
| all-trans-β-Carotene | 184.01 | 97.14 | 20.28 | 544.79 | 52.8 |
| 9-cis-β-Carotene | 1.54 | 1.10 | 0.26 | 6.36 | 71.4 |
| 13-cis-β-Carotene | 6.94 | 4.00 | 0.78 | 23.75 | 57.6 |
| ppbv (ng/mL) | Ration DM % | Kg DM Cow−1 Day−1 | Liters Cow−1 Day−1 | |||
|---|---|---|---|---|---|---|
| Fresh Forage (2) | Forages (3) | Maize Silage | Concentrates | |||
| α-Tocopherol (Vit. E) | 0.426 ** | 0.506 ** | −0.468 ** | −0.458 ** | −502 ** | |
| Ɣ-Tocopherol | −0.479 ** | −0.502 ** | 0.480 ** | |||
| Lutein | 0.658 ** | 0.749 ** | −0.530 ** | −0.698 ** | −0.565 ** | −0.647 ** |
| Zeaxanthin | 0.476 ** | 0.548 ** | −0.536 ** | −0.449 ** | −0.495 ** | |
| β-Cryptoxanthin | −0.454 ** | |||||
| all-trans-β-Carotene | 0.456 ** | 0.645 ** | −0.600 ** | −0.475 ** | −0.543 ** | |
| 13-cis-β-Carotene | 0.467 ** | 0.662 ** | −0.423 ** | −0.602 ** | −0.473 ** | −0.547 ** |
| Composition of Diet of Lactating Dairy Cows | Livestock Units (3) | Livestock Units per ha | Annual Milk Production (Liters Year−1) | Estimated Average Milk Production per Cow (Liters Cow−1 Day−1) |
|---|---|---|---|---|
| kg DM (2) day−1 per cow | 0.441 ** | 0.836 ** | ||
| % ration DM | ||||
| %DM Fresh forage (4) | −0.402 ** | −0.486 ** | −0.414 ** | −0.622 ** |
| % Forage (5) | −490 ** | −0.489 ** | −0.502 ** | −0.735 ** |
| %DM Maize silage | 0.511 ** | 0.501 ** | 0.637 ** | |
| %DM Concentrate | 0.612 ** | |||
| Fatty Acid (g·100 g−1 of Total FA) (2) | Livestock Units (3) | Livestock Units per ha | Annual Milk Production (L Year−1) | Estimated Average Milk Production per Cow (L Cow−1 Day−1) |
|---|---|---|---|---|
| FA | ||||
| C10:0 | 0.488 ** | |||
| C11:0 | 0.412 ** | 0.443 ** | 0.556 ** | |
| C12:0 | 0.510 ** | |||
| C13:0 | 0.400 ** | 0.455 ** | ||
| C14:0-iso | −0.647 ** | |||
| C15:0-iso | −0.605 ** | |||
| C15:0-anteiso | −0.491 ** | |||
| t10-C18:1 | 0.402 ** | |||
| t11-C:18:1 | −0.446 ** | −0.625 ** | ||
| c11-C18:1-n7 | 0.542 ** | |||
| c12-18:1 | 0.486 ** | 0.414 ** | 0.500 ** | 0.567 ** |
| cC18:2-n6 (5) | 0.495 ** | |||
| C18:3-n3 | −0.452 ** | −0.559 ** | ||
| C20:0 | −0.429 ** | |||
| c9,t11-CLA | −0.449 ** | −0.624 ** | ||
| C20:3-n3 | 0.412 ** | 0.460 ** | ||
| C22:0 | −0.576 ** | |||
| C22:2-n6 | −0.551 ** | |||
| C23:0 | −0.540 ** | |||
| C24:0 | −0.584 ** | |||
| C22:5-n3 | −0.422 ** | −0.578 ** | ||
| Main relationships between FA | ||||
| Total saturated FA (SFA) | ||||
| Total branched FA (BCFA) | −0.428 ** | −0.438 ** | −0.684 ** | |
| Total trans mono-unsaturated FA (t-MUFA) | −0.436 ** | |||
| Ratio t11/t10-C18:1 | −0.450 ** | −0.694 ** | ||
| CLA isomers sum | −0.440 ** | −0.626 ** | ||
| n6 isomers sum | 0.495 ** | |||
| n3 isomers sum | −0.427 ** | −0.533 ** | ||
| n6/n3 ratio (6) | 0.404 ** | 0.469 ** | 0.422 ** | 0.614 ** |
| FSA (4) (ppbv (ng mL)) | ||||
| α-Tocopherol (Vit. E) | −0.502 ** | |||
| γ- Tocopherol | 0.429 ** | |||
| Lutein | −0.408 ** | −0.647 ** | ||
| Zeaxanthin | −0.495 ** | |||
| all-trans-β-Carotene | −0.543 ** | |||
| 13-cis-β-Carotene | −0.547 ** | |||
| Parameter | Milk Samples from Diets without Fresh Grass | Milks Samples from Diets with Fresh Grass (7) | Sig. (8) | |||
|---|---|---|---|---|---|---|
| Grazing | Zero-Grazed (Cut and Carry) | Both Ways | Total | |||
| N = 90 | N = 48 | N = 22 | N = 14 | N = 84 | ||
| FA (1) (g·100 g−1 of total FA) | ||||||
| C12:0 | 3.47 a | 3.08 b | 3.04 b | 2.90 b | 3.04 | *** |
| C14:0 | 11.47 | 10.98 | 11.15 | 10.92 | 11.02 | ns |
| C16:0 | 31.19 a | 28.62 b | 30.43 ab | 28.62 b | 29.09 | * |
| t10-C18:1 | 0.51 a | 0.32 b | 0.31 b | 0.30 b | 0.31 | * |
| t11-C:18:1 | 1.02 b | 2.54 a | 1.95 a | 2.38 a | 2.36 | ** |
| c12-18:1 | 0.28 a | 0.15 b | 0.16 b | 0.11 b | 0.09 | * |
| C18:3-n3 (9) | 0.43 c | 0.81 a | 0.59 b | 0.75 ab | 0.74 | ** |
| c9,t11-CLA | 0.49 b | 1.07 a | 0.84 a | 1.00 a | 1.00 | ** |
| C22:5-n3 | 0.07 c | 0.11 a | 0.09 b | 0.11 a | 0.11 | * |
| Main relationships between FA (1) | ||||||
| Total saturated FA (SFA) | 69.78 a | 67.24 b | 68.02 ab | 67.25 b | 67.45 | * |
| Total branched FA (BCFA) | 0.82 b | 1.01 a | 1.00 a | 1.05 a | 1.01 | ** |
| Total mono-unsaturated FA (MUFA) | 25.46 b | 27.21 a | 26.79 ab | 27.48 ab | 27.15 | ** |
| Ratio t11/t10- C18:1 | 2.60 b | 9.54 a | 6.91 a | 9.94 a | 8.92 | ** |
| Total poly-unsaturated FA (PUFA) | 3.93 b | 4.54 a | 4.19 ab | 4.22 ab | 4.39 | * |
| CLA isomers sum | 0.61 b | 1.21 a | 0.99 a | 1.14 a | 1.14 | ** |
| n6 isomers sum | 2.60 a | 2.23 ab | 2.31 ab | 2.04 b | 2.22 | * |
| n3 isomers sum | 0.72 c | 1.09 a | 0.89 b | 1.03 ab | 1.03 | * |
| PUFA/SFA | 0.06 b | 0.07 a | 0.06 ab | 0.06 ab | 0.07 | * |
| n6/n3 ratio (2) | 3.69 a | 2.15 c | 2.77 b | 2.14 c | 2.31 | * |
| Total unsaturated FA (UFA)/SFA | 0.42 b | 0.48 a | 0.46 ab | 0.47 a | 0.47 | * |
| FSA (3) (ppbv (ng/mL)) | ||||||
| Retinol | 883.26 | 997.04 | 1039.50 | 940.57 | 998.75 | ns |
| α-Tocopherol (Vit. E) | 1181.90 b | 1622.03 a | 1691.19 a | 1825.45 a | 1674.04 | * |
| γ-Tocopherol | 54.28 b | 32.23 a | 39.33 a | 29.89 a | 33.70 | * |
| Lutein | 8.64 b | 22.00 a | 17.16 a | 22.64 a | 20.84 | *** |
| Zeaxanthin | 0.82 b | 1.63 a | 0.86 b | 1.14 ab | 1.35 | ** |
| β-Cryptoxanthin | 1.66 b | 2.39 a | 1.73 b | 2.31 a | 2.20 | *** |
| all-trans-β-Carotene | 135.70 b | 243.60 a | 195.74 a | 271.81 a | 235.77 | ** |
| 9-cis-β-Carotene | 1.34 | 1.80 | 1.36 | 1.84 | 1.69 | ns |
| 13-cis-β-Carotene | 4.87 b | 9.19 a | 7.59 a | 11.03 a | 9.08 | ** |
| Average ration | ||||||
| Kg DM (4) day−1 per cow | 22.76 a | 19.93 b | 20.64 b | 19.55 b | 20.06 | * |
| % ration DM (5) | ||||||
| Fresh forage (6) | 0.00 a | 43.68 b | 34.03 b | 52.28 b | 42.59 | *** |
| Maize silage | 25.49 a | 6.37 b | 4.05 b | 1.20 b | 4.90 | *** |
| Grass silage | 21.23 a | 14.24 ab | 10.94 b | 11.94 b | 12.99 | * |
| Dry forage | 9.12 b | 10.03 ab | 13.13 a | 10.19 a | 10.87 | ns |
| Concentrate | 44.16 a | 25.68 c | 37.85 b | 24.39 c | 28.65 | * (7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villar, A.; Vázquez-González, I.; Vicente, F.; Salcedo, G.; González, L.; Botana, A.; Royo, L.J.; Eguinoa, P.; Busqué, J. Study of the Variability in Fatty Acids and Carotenoid Profiles: Laying the Ground for Tank Milk Authentication. Sustainability 2021, 13, 4506. https://doi.org/10.3390/su13084506
Villar A, Vázquez-González I, Vicente F, Salcedo G, González L, Botana A, Royo LJ, Eguinoa P, Busqué J. Study of the Variability in Fatty Acids and Carotenoid Profiles: Laying the Ground for Tank Milk Authentication. Sustainability. 2021; 13(8):4506. https://doi.org/10.3390/su13084506
Chicago/Turabian StyleVillar, Ana, Ibán Vázquez-González, Fernando Vicente, Gregorio Salcedo, Laura González, Adrián Botana, Luís José Royo, Paola Eguinoa, and Juan Busqué. 2021. "Study of the Variability in Fatty Acids and Carotenoid Profiles: Laying the Ground for Tank Milk Authentication" Sustainability 13, no. 8: 4506. https://doi.org/10.3390/su13084506
APA StyleVillar, A., Vázquez-González, I., Vicente, F., Salcedo, G., González, L., Botana, A., Royo, L. J., Eguinoa, P., & Busqué, J. (2021). Study of the Variability in Fatty Acids and Carotenoid Profiles: Laying the Ground for Tank Milk Authentication. Sustainability, 13(8), 4506. https://doi.org/10.3390/su13084506






