Durable Icephobic Slippery Liquid-Infused Porous Surfaces (SLIPS) Using Flame- and Cold-Spraying
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
2.2. Coating Manufacturing
2.2.1. Flame-Sprayed Coatings
2.2.2. Low-Pressure Cold-Sprayed Coatings
2.2.3. SLIPS Production
2.3. Characterization Methods
2.4. Water Contact Angle Measurements
2.5. Icing Tests
3. Results and Discussion
3.1. Feedstock Powders
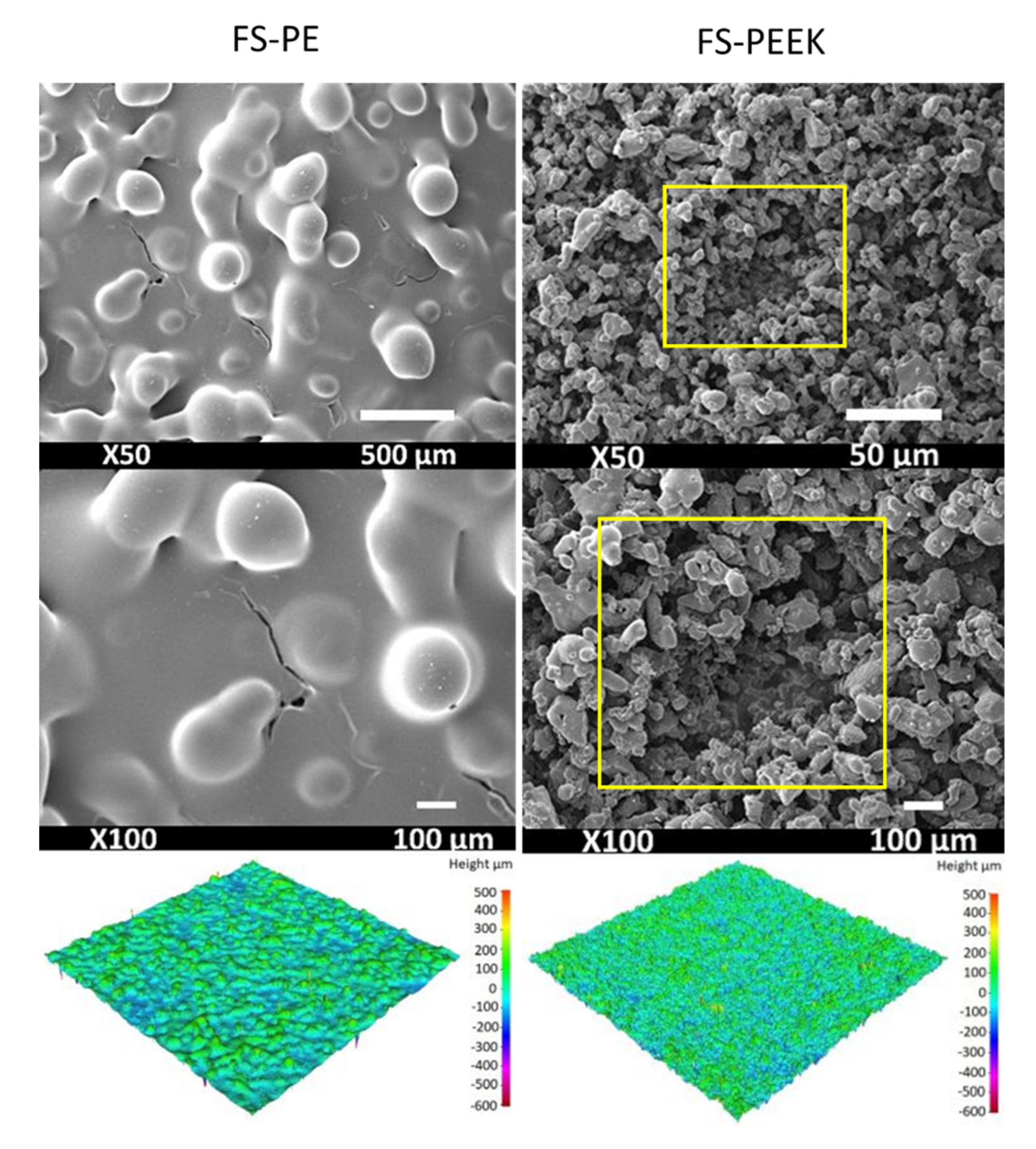
3.2. Structural Characteristics of the Coating
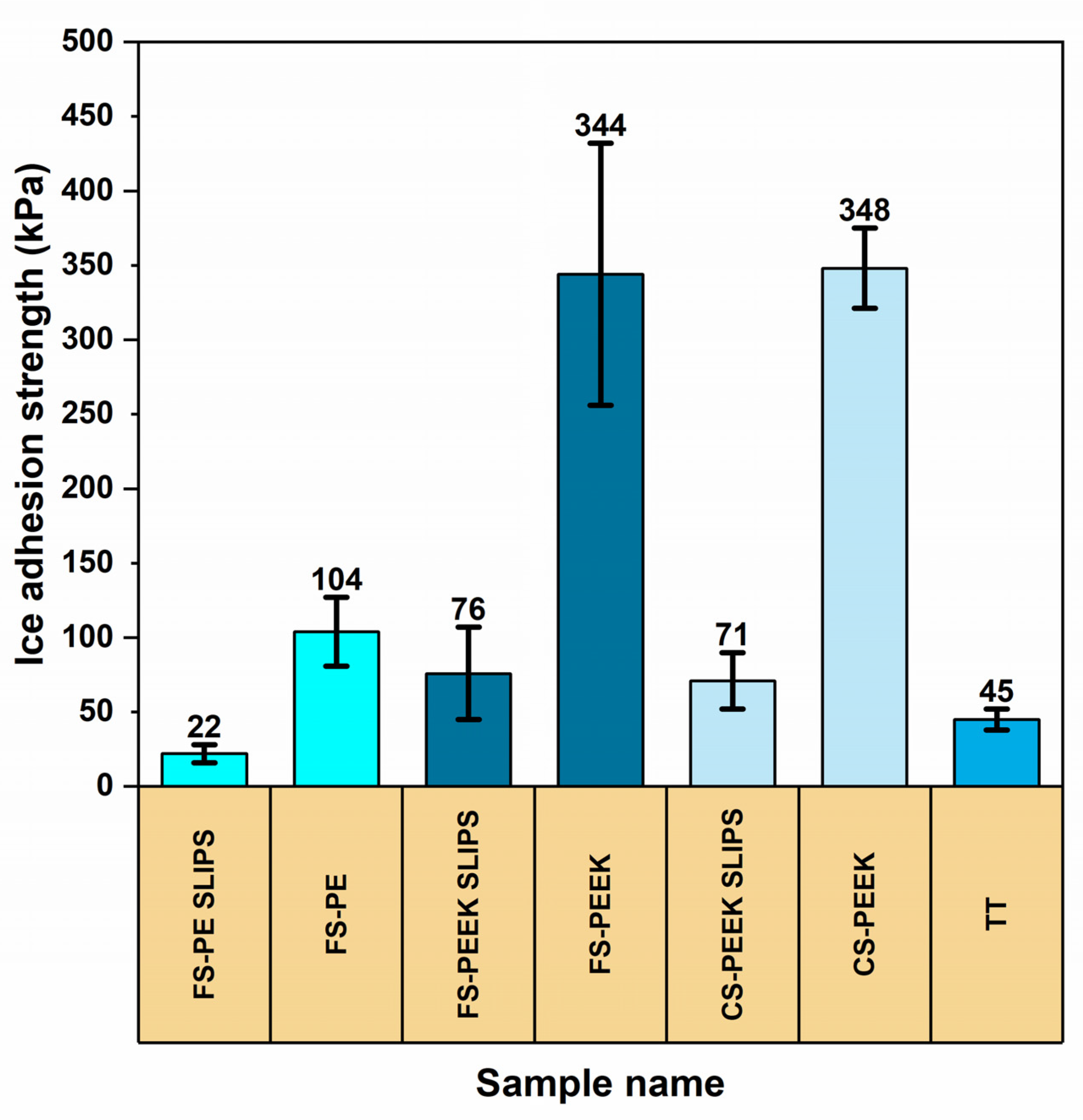
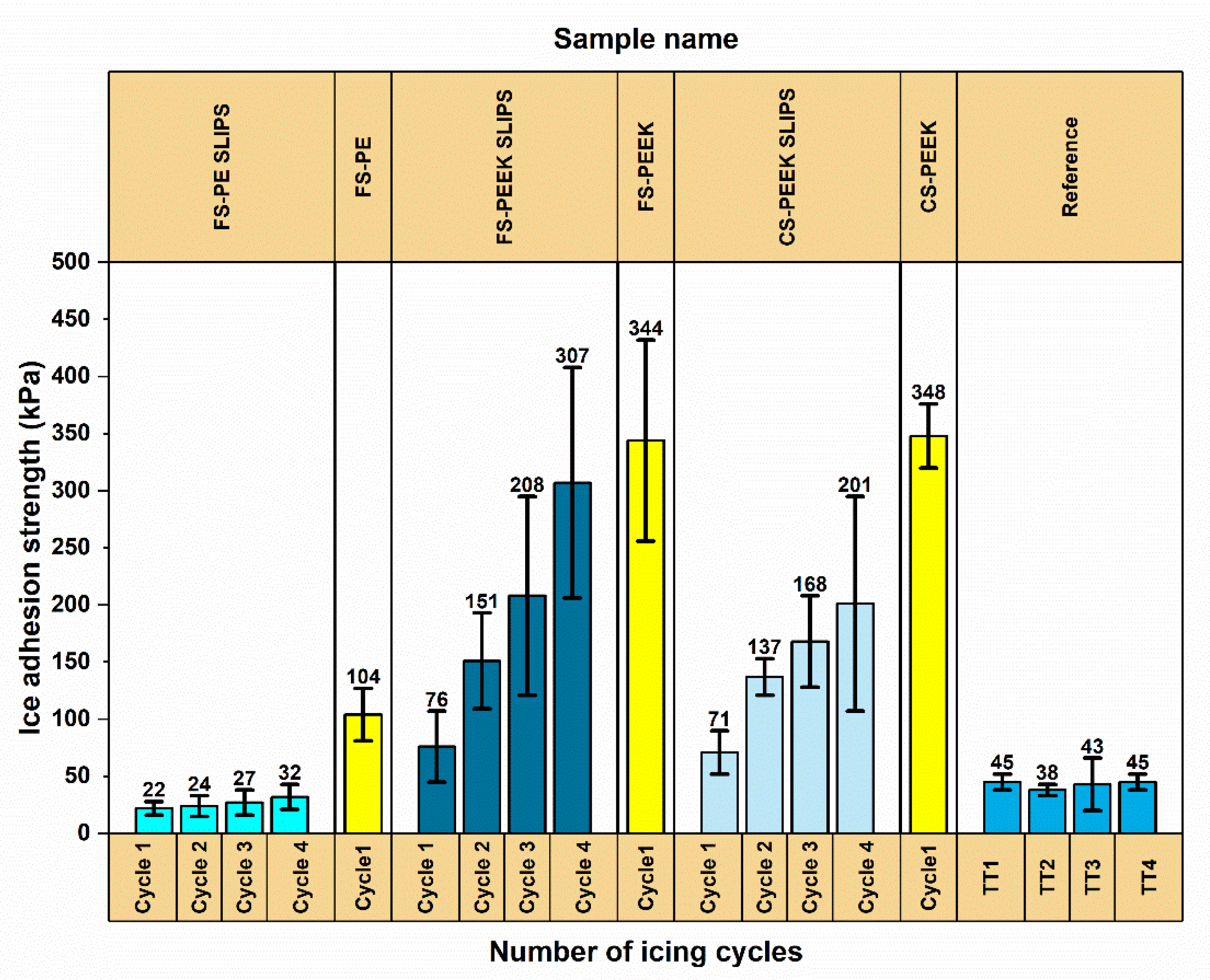
3.3. Ice Adhesion of the Coatings
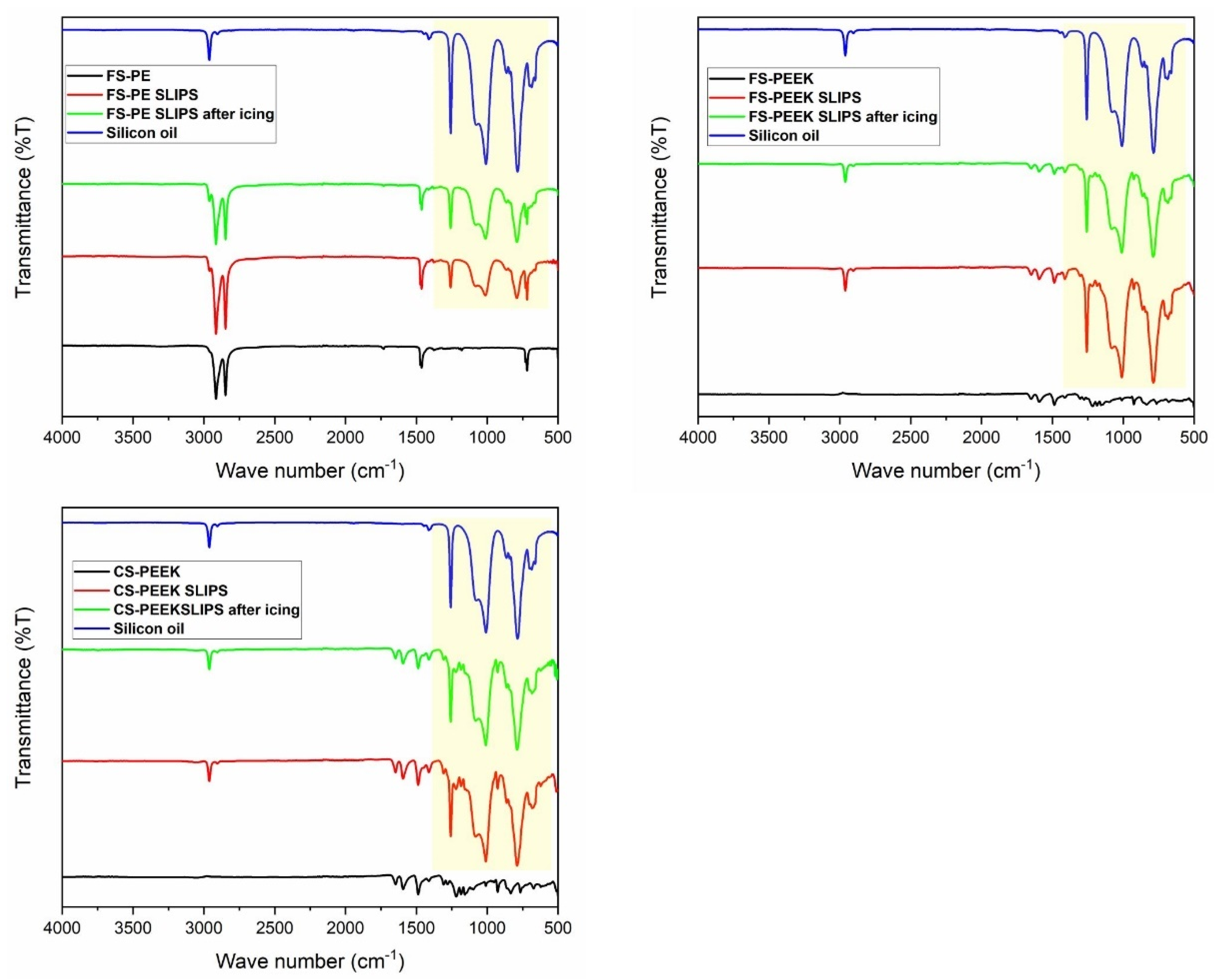
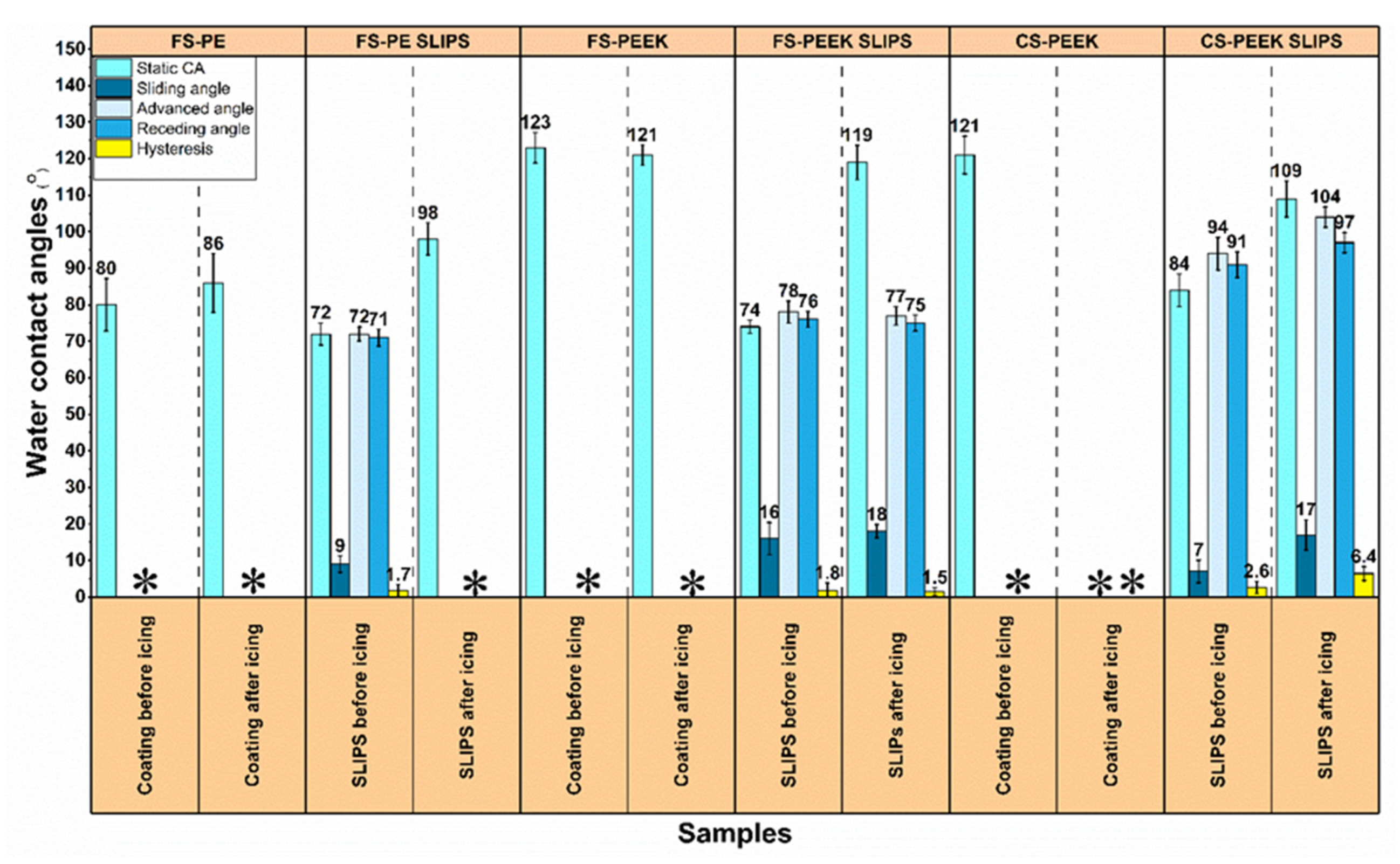
3.4. Wetting Properties of the Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fikke, S.M.; Kristjánsson, J.E.; Kringlebotn Nygaard, B.E. Modern meteorology and atmospheric icing. Atmos. Icing Power Netw. 2008, 1–29. [Google Scholar] [CrossRef]
- Carriveau, R.; Edrisy, A.; Cadieux, P.; Mailloux, R. Ice adhesion issues in renewable energy infrastructure. J. Adh. Sci. Technol. 2012, 26, 447–461. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Tian, Z.; Qiu, R.; Liu, Z. An Online Thermal Deicing Method for Urban Rail Transit Catenary. IEEE Trans. Transp. Electrif. 2021, 7, 870–882. [Google Scholar] [CrossRef]
- Shu, L.; Li, H.; Hu, Q.; Jiang, X.; Qiu, G.; McClure, G.; Yang, H. Study of ice accretion feature and power characteristics of wind turbines at natural icing environment. Cold Reg. Sci. Technol. 2018, 147, 45–54. [Google Scholar] [CrossRef]
- Rashid, T.; Abbas Khawaja, H.; Edvardsen, K. Review of marine icing and anti-/de-icing systems. J. Mar. Eng. Technol. 2016, 15, 79–87. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, W.; Wu, Z. Aircraft icing: An ongoing threat to aviation safety. Aerosp. Sci. Technol. 2018, 75, 353–385. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Huang, X.; Tepylo, N.; Pommier-Budinger, V.; Budinger, M.; Bonaccurso, E.; Villedieu, P.; Bennani, L. A survey of icephobic coatings and their potential use in a hybrid coating/active ice protection system for aerospace applications. Prog. Aerosp. Sci. 2019, 105, 74–97. [Google Scholar] [CrossRef] [Green Version]
- Sojoudi, H.; Wang, M.; Boscher, N.D.; McKinley, G.H.; Gleason, K.K. Durable and scalable icephobic surfaces: Similarities and distinctions from superhydrophobic surfaces. Soft Matter 2016, 12, 1938–1963. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zheng, Y.; Raji, A.R.O.; Li, Y.; Sikkema, W.K.A.; Tour, J.M. Passive Anti-Icing and Active Deicing Films. ACS Appl. Mater. Interfaces 2016, 8, 14169–14173. [Google Scholar] [CrossRef] [PubMed]
- Parent, O.; Ilinca, A. Anti-icing and de-icing techniques for wind turbines: Critical review. Cold Reg. Sci. Technol. 2011, 65, 88–96. [Google Scholar] [CrossRef]
- Han, S.; Wei, H.; Han, L.; Li, Q. Durability and Electrical Conductivity of Carbon Fiber Cloth/Ethylene Propylene Diene Monomer Rubber Composite for Active Deicing and Snow Melting. Polymers 2019, 11, 2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhu, X.; Li, L.; Ling, H.; Zhou, P.; Cheng, Z.; Su, A.; Du, Y. Prefabricated Flexible Conductive Composite Overlay for Active Deicing and Snow Melting. J. Mater. Civ. Eng. 2018, 30, 04018283. [Google Scholar] [CrossRef]
- Daniliuk, V.; Xu, Y.; Liu, R.; He, T.; Wang, X. Ultrasonic de-icing of wind turbine blades: Performance comparison of perspective transducers. Renew. Energy 2020, 145, 2005–2018. [Google Scholar] [CrossRef]
- Daniliuk, V.; Pamfilov, E.; Daniliuk, A.; Xu, Y. Feasibility Study of Ultrasonic De-Icing Technique for Aircraft Wing Ice Protection. Int. Conf. Aviamechanical Eng. Transp. 2019, 188, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, Y.; Lei, Y. An effect assessment and prediction method of ultrasonic de-icing for composite wind turbine blades. Renew. Energy 2018, 118, 1015–1023. [Google Scholar] [CrossRef]
- Zhou, F.; Zhu, J.; An, N.; Wang, C.; Liu, J.; Long, L. The anti-icing and deicing robot system for electricity transmission line based on external excitation resonant. IEEJ Trans. Electr. Electron. Eng. 2020, 15, 593–600. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Kuokkala, V.T.; Kokkonen, J.; Paajanen, H. Corrosion effects of runway de-icing chemicals on aircraft alloys and coatings. Mater. Chem. Phys. 2011, 126, 138–151. [Google Scholar] [CrossRef]
- Muthumani, A.; Fay, L.; Akin, M.; Wang, S.; Gong, J.; Shi, X. Correlating lab and field tests for evaluation of deicing and anti-icing chemicals: A review of potential approaches. Cold Reg. Sci. Technol. 2014, 97, 21–32. [Google Scholar] [CrossRef]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: Smooth, textured or slippery? Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-Icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef]
- Laturkar, S.V.; Mahanwar, P.A. Superhydrophobic coatings using nanomaterials for anti-frost applications—Review. Nanosyst. Phys. Chem. Math. 2016, 7, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Wang, N.; Xiong, D.; Deng, Y.; Shi, Y. Fabrication of superhydrophobic coating via spraying method and its applications in anti-icing and anti-corrosion. Appl. Surf. Sci. 2016, 389, 547–553. [Google Scholar] [CrossRef]
- Wu, X.; Silberschmidt, V.V.; Hu, Z.T.; Chen, Z. When superhydrophobic coatings are icephobic: Role of surface topology. Surf. Coat. Technol. 2019, 358, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Singh, A.V.; Rahman, A.; Sudhir Kumar, N.V.G.; Aditi, A.S.; Galluzzi, M.; Bovio, S.; Barozzi, S.; Montani, E.; Parazzoli, D. Bio-inspired approaches to design smart fabrics. Mater. Des. 2012, 36, 829–839. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; She, Z.; Chen, F.; Li, L. Low-cost and large-scale fabrication method for an environmentally-friendly superhydrophobic coating on magnesium alloy. J. Mater. Chem. 2012, 22, 4097–4105. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, K.; Bhattacharya, S. Superhydrophobic surfaces review: Functional application, fabrication techniques and limitations. J. Micromanuf. 2019, 2, 59–78. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Chen, J.; Gu, H.; Liu, J.; Wang, R.; Zhang, B.; Zhang, H.; Zhang, Q. Design and preparation of bioinspired slippery liquid-infused porous surfaces with anti-icing performance via delayed phase inversion process. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124384. [Google Scholar] [CrossRef]
- Juuti, P.; Haapanen, J.; Stenroos, C.; Niemelä-Anttonen, H.; Harra, J.; Koivuluoto, H.; Teisala, H.; Lahti, J.; Tuominen, M.; Kuusipalo, J.; et al. Achieving a slippery, liquid-infused porous surface with anti-icing properties by direct deposition of flame synthesized aerosol nanoparticles on a thermally fragile substrate. Appl. Phys. Lett. 2017, 110, 161603. [Google Scholar] [CrossRef]
- Wilson, P.W.; Lu, W.; Xu, H.; Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Inhibition of ice nucleation by slippery liquid-infused porous surfaces (SLIPS). Phys. Chem. Chem. Phys. 2013, 15, 581–585. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Song, D.; Liu, P.; Gao, L.; Wang, J. Long-Term Stability of a Liquid-Infused Coating with Anti-Corrosion and Anti-Icing Potentials on Al Alloy. ChemElectroChem 2019, 6, 3911–3919. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, J.; Chen, R.; Liu, Q.; Liu, J.; Song, D.; Liu, P.; Gao, L.; Wang, J. Highly transparent and robust slippery lubricant-infused porous surfaces with anti-icing and anti-fouling performances. J. Alloys Compd. 2019, 803, 51–60. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Liu, X.; Zhou, Z. Slippery liquid-infused substrates: A versatile preparation, unique anti-wetting and drag-reduction effect on water. J. Mater. Chem. A 2016, 4, 2524–2529. [Google Scholar] [CrossRef]
- Huang, C.; Guo, Z. Fabrications and Applications of Slippery Liquid-infused Porous Surfaces Inspired from Nature: A Review. J. Bionic Eng. 2019, 16, 769–793. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Bhosale, A.K.; Nagappan, S.; Ha, C.S.; Sadasivuni, K.K.; Liu, S.; Xing, R. Recent developments in air-trapped superhydrophobic and liquid-infused slippery surfaces for anti-icing application. Prog. Org. Coat. 2019, 137, 105373. [Google Scholar] [CrossRef]
- Long, Y.; Yin, X.; Mu, P.; Wang, Q.; Hu, J.; Li, J. Slippery liquid-infused porous surface (SLIPS) with superior liquid repellency, anti-corrosion, anti-icing and intensified durability for protecting substrates. Chem. Eng. J. 2020, 401, 126137. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, L.; Liu, G.; Liao, R. Fabrication of ultralow ice-adhesion slippery liquid infused porous surfaces on aluminum alloy (7075-t651). Coatings 2020, 10, 1025. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Lu, C.; Liu, Y.; Feng, S.; Liu, Y. Robust Slippery Liquid-Infused Porous Network Surfaces for Enhanced Anti-icing/Deicing Performance. ACS Appl. Mater. Interfaces 2020, 12, 25471–25477. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Y.; Huang, M.; Zhou, Y.; Liu, Y.; Liang, X. Durability of a lubricant-infused Electrospray Silicon Rubber surface as an anti-icing coating. Appl. Surf. Sci. 2015, 346, 68–76. [Google Scholar] [CrossRef]
- Subramanyam, S.B.; Rykaczewski, K.; Varanasi, K.K. Ice adhesion on lubricant-impregnated textured surfaces. Langmuir 2013, 29, 13414–13418. [Google Scholar] [CrossRef]
- Li, J.; Ueda, E.; Paulssen, D.; Levkin, P.A. Slippery Lubricant-Infused Surfaces: Properties and Emerging Applications. Adv. Funct. Mater. 2019, 29, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Coady, M.J.; Wood, M.; Wallace, G.Q.; Nielsen, K.E.; Kietzig, A.M.; Lagugné-Labarthet, F.; Ragogna, P.J. Icephobic Behavior of UV-Cured Polymer Networks Incorporated into Slippery Lubricant-Infused Porous Surfaces: Improving SLIPS Durability. ACS Appl. Mater. Interfaces 2018, 10, 2890–2896. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.J.; Lee, B.; Chun, J.M.; Patil, V.; Kim, Y.S. Durable ice-lubricating surfaces based on polydimethylsiloxane embedded silicone oil infused silica aerogel. Appl. Surf. Sci. 2020, 512, 145728. [Google Scholar] [CrossRef]
- Zhuo, Y.; Wang, F.; Xiao, S.; He, J.; Zhang, Z. One-Step Fabrication of Bioinspired Lubricant-Regenerable Icephobic Slippery Liquid-Infused Porous Surfaces. ACS Omega 2018, 3, 10139–10144. [Google Scholar] [CrossRef]
- Fender, T.D. Thermal spray high performance polymer coatings. Mater. Technol. 1996, 11, 16–20. [Google Scholar] [CrossRef]
- Ivosevic, M.; Coguill, S.L.; Galbraith, S.L. Polymer thermal spraying: A novel coating process. Proc. Int. Therm. Spray Conf. 2009, 1078–1083. [Google Scholar] [CrossRef]
- Petrovicova, E.; Schadler, L.S. Thermal spraying of polymers. Int. Mater. Rev. 2002, 47, 169–190. [Google Scholar] [CrossRef]
- Donadei, V.; Koivuluoto, H.; Sarlin, E.; Vuoristo, P. Icephobic Behaviour and Thermal Stability of Flame-Sprayed Polyethylene Coating: The Effect of Process Parameters. J. Therm. Spray Technol. 2020, 29, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Koivuluoto, H.; Hartikainen, E.; Niemelä-Anttonen, H. Thermally sprayed coatings: Novel surface engineering strategy towards icephobic solutions. Materials 2020, 13, 1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivuluoto, H.; Stenroos, C.; Kylmälahti, M.; Apostol, M.; Kiilakoski, J.; Vuoristo, P. Anti-icing Behavior of Thermally Sprayed Polymer Coatings. J. Therm. Spray Technol. 2017, 26, 150–160. [Google Scholar] [CrossRef]
- Donadei, V.; Koivuluoto, H.; Sarlin, E.; Vuoristo, P. Lubricated icephobic coatings prepared by flame spraying with hybrid feedstock injection. Surf. Coat. Technol. 2020, 403, 126396. [Google Scholar] [CrossRef]
- Rezvani Rad, M.; Mohammadian Bajgiran, M.; Moreau, C.; McDonald, A. Fabrication of thermally sprayed coating systems for mitigation of ice accumulation in carbon steel pipes and prevention of pipe bursting. Surf. Coat. Technol. 2020, 397, 126013. [Google Scholar] [CrossRef]
- Mora, J.; García, P.; Muelas, R.; Agüero, A. Hard quasicrystalline coatings deposited by hvof thermal spray to reduce ice accretion in aero-structures components. Coatings 2020, 10, 290. [Google Scholar] [CrossRef] [Green Version]
- Xi, N.; Liu, Y.; Zhang, X.; Liu, N.; Fu, H.; Hang, Z.; Yang, G.; Chen, H.; Gao, W. Steady anti-icing coatings on weathering steel fabricated by HVOF spraying. Appl. Surf. Sci. 2018, 444, 757–762. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Memon, H.; Fu, Y.; Barman, T.; Choi, K.S.; Hou, X. Hydrophobic/icephobic coatings based on thermal sprayed metallic layers with subsequent surface functionalization. Surf. Coat. Technol. 2019, 357, 267–272. [Google Scholar] [CrossRef]
- Sharifi, N.; Dolatabadi, A.; Pugh, M.; Moreau, C. Anti-icing performance and durability of suspension plasma sprayed TiO2 coatings. Cold Reg. Sci. Technol. 2019, 159, 1–12. [Google Scholar] [CrossRef]
- Niemelä-Anttonen, H.; Koivuluoto, H.; Kylmälahti, M.; Laakso, J.; Vuoristo, P. Thermally sprayed slippery and icephobic surfaces. Proc. Int. Therm. Spray Conf. 2018, 2018, 380–384. [Google Scholar]
- Espallargas, N. Introduction to thermal spray coatings. In Future Development of Thermal Spray Coatings; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–13. [Google Scholar] [CrossRef]
- Tucker, R.C. Introduction to Thermal Spray Technology. Therm. Spray Technol. 2018, 5, 3–9. [Google Scholar] [CrossRef]
- AlMangour, B. Fundamentals of cold spray processing: Evolution and future perspectives. In Cold-Spray Coatings; Springer: Cham, Switzerland, 2018; pp. 3–24. [Google Scholar] [CrossRef]
- Maev, R.G.; Leshchynsky, V. Low-Pressure Cold Spray (LPCS); Cavaliere, P., Ed.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319671833. [Google Scholar]
- Bush, T.B.; Khalkhali, Z.; Champagne, V.; Schmidt, D.P.; Rothstein, J.P. Optimization of Cold Spray Deposition of High-Density Polyethylene Powders. J. Therm. Spray Technol. 2017, 26, 1548–1564. [Google Scholar] [CrossRef]
- Sanpo, N.; Tan, M.L.; Cheang, P.; Khor, K.A. Antibacterial property of cold-sprayed HA-Ag/PEEK coating. J. Therm. Spray Technol. 2009, 18, 10–15. [Google Scholar] [CrossRef]
- Xu, Y.; Hutchings, I.M. Cold spray deposition of thermoplastic powder. Surf. Coat. Technol. 2006, 201, 3044–3050. [Google Scholar] [CrossRef]
- Khalkhali, Z.; Rothstein, J.P. Characterization of the cold spray deposition of a wide variety of polymeric powders. Surf. Coat. Technol. 2020, 383, 125251. [Google Scholar] [CrossRef]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Dao, M. Cold spray coating: Review of material systems and future perspectives. Surf. Eng. 2014, 30, 369–395. [Google Scholar] [CrossRef]
- Chebbi, A.; Stokes, J. Thermal spraying of bioactive polymer coatings for orthopaedic applications. J. Therm. Spray Technol. 2012, 21, 719–730. [Google Scholar] [CrossRef]
- Yin, S.; Suo, X.; Xie, Y.; Li, W.; Lupoi, R.; Liao, H. Effect of substrate temperature on interfacial bonding for cold spray of Ni onto Cu. J. Mater. Sci. 2015, 50, 7448–7457. [Google Scholar] [CrossRef]
- Xie, Y.; Planche, M.P.; Raoelison, R.; Liao, H.; Suo, X.; Hervé, P. Effect of Substrate Preheating on Adhesive Strength of SS 316L Cold Spray Coatings. J. Therm. Spray Technol. 2016, 25, 123–130. [Google Scholar] [CrossRef]
- Goldbaum, D.; Shockley, J.M.; Chromik, R.R.; Rezaeian, A.; Yue, S.; Legoux, J.G.; Irissou, E. The effect of deposition conditions on adhesion strength of Ti and Ti6Al4V cold spray splats. J. Therm. Spray Technol. 2012, 21, 288–303. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yoshida, C.; Atsumi, K.; Yamada, M.; Fukumoto, M. Influence of Substrate Temperature on Adhesion Strength of Cold-Sprayed Coatings. J. Therm. Spray Technol. 2014, 24, 86–91. [Google Scholar] [CrossRef]
- Xie, Y.; Yin, S.; Chen, C.; Planche, M.P.; Liao, H.; Lupoi, R. New insights into the coating/substrate interfacial bonding mechanism in cold spray. Scr. Mater. 2016, 125, 1–4. [Google Scholar] [CrossRef]
- Peppou-Chapman, S.; Hong, J.K.; Waterhouse, A.; Neto, C. Life and death of liquid-infused surfaces: A review on the choice, analysis and fate of the infused liquid layer. Chem. Soc. Rev. 2020, 49, 3688–3715. [Google Scholar] [CrossRef] [PubMed]
- Koivuluoto, H.; Stenroos, C.; Ruohomaa, R.; Bolelli, G.; Lusvarghi, L.; Vuoristo, P. Research on icing behavior and ice adhe-sion testing of icephobic surfaces. In Proceedings of the 16th International Workshop on Atmospheric Icing of Structures (IWAIS), Uppsala, Sweden, 28 June–3 July 2015; pp. 1–14. [Google Scholar]
- Wei, C.; Jin, B.; Zhang, Q.; Zhan, X.; Chen, F. Anti-icing performance of super-wetting surfaces from icing-resistance to ice-phobic aspects: Robust hydrophobic or slippery surfaces. J. Alloys Compd. 2018, 765, 721–730. [Google Scholar] [CrossRef]
- Crawmer, D.E. Coating Structures, Properties, and Materials. Therm. Spray Technol. 2018, 5, 60–64. [Google Scholar] [CrossRef]
- Barthwal, S.; Lee, B.; Lim, S.H. Fabrication of robust and durable slippery anti-icing coating on textured superhydrophobic aluminum surfaces with infused silicone oil. Appl. Surf. Sci. 2019, 496, 143677. [Google Scholar] [CrossRef]
- Gupta, M.C.; Mulroney, A. Ice Adhesion and Anti-Icing Using Microtextured Surfaces. Ice Adhes. 2020, 389–415. [Google Scholar] [CrossRef]
- Rønneberg, S.; He, J.; Zhang, Z. The need for standards in low ice adhesion surface research: A critical review. J. Adhes. Sci. Technol. 2020, 34, 319–347. [Google Scholar] [CrossRef]
- Niemelä-Anttonen, H.; Koivuluoto, H.; Tuominen, M.; Teisala, H.; Juuti, P.; Haapanen, J.; Harra, J.; Stenroos, C.; Lahti, J.; Kuusipalo, J.; et al. Icephobicity of Slippery Liquid Infused Porous Surfaces under Multiple Freeze–Thaw and Ice Accretion–Detachment Cycles. Adv. Mater. Interfaces 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Tetteh, E.; Loth, E. Reducing static and impact ice adhesion with a self-lubricating icephobic coating (SLIC). Coatings 2020, 10, 262. [Google Scholar] [CrossRef] [Green Version]
- Hanh, V.T.H.; Truong, M.X.; Nguyen, T.B. Anti-icing approach on flexible slippery microstructure thin-film. Cold Reg. Sci. Technol. 2021, 186, 103280. [Google Scholar] [CrossRef]
- Manna, U.; Lynn, D.M. Fabrication of liquid-infused surfaces using reactive polymer multilayers: Principles for manipulating the behaviors and mobilities of aqueous fluids on slippery liquid interfaces. Adv. Mater. 2015, 27, 3007–3012. [Google Scholar] [CrossRef]
- Yeong, Y.H.; Wang, C.; Wynne, K.J.; Gupta, M.C. Oil-infused superhydrophobic silicone material for low ice adhesion with long-term infusion stability. ACS Appl. Mater. Interfaces 2016, 8, 32050–32059. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, J.; Wang, Y.; Chen, Q.; Ding, J.; Wang, Q. Ice-phobic coatings based on silicon-oil-infused polydimethylsiloxane. ACS Appl. Mater. Interfaces 2013, 5, 4053–4062. [Google Scholar] [CrossRef]
- Golovin, K.; Kobaku, S.P.R.; Lee, D.H.; DiLoreto, E.T.; Mabry, J.M.; Tuteja, A. Designing durable icephobic surfaces. Sci. Adv. 2016, 2, e1501496. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, B.; Tian, Y.; Wang, F.; Chen, Q.; Zhang, F.; Qian, H.; Ma, L. Facile one-step method to fabricate a slippery lubricant-infused surface (lis) with self-replenishment properties for anti-icing applications. Coatings 2020, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Yuan, Y.; Liao, R.; Wang, L.; Gao, X. Fabrication of a porous slippery icephobic surface and effect of lubricant viscosity on anti-icing properties and durability. Coatings 2020, 10, 896. [Google Scholar] [CrossRef]
- Chen, X.; Wen, G.; Guo, Z. What are the design principles, from the choice of lubricants and structures to the preparation method, for a stable slippery lubricant-infused porous surface? Mater. Horizons 2020, 7, 1697–1726. [Google Scholar] [CrossRef]
- De Gennes, P.; Brochard-Wyart, F.; Quere, D. Capillarity and Wetting Phenomena Drops, Bubbles, Pearls, Waves; Springer: New York, NY, USA, 2004. [Google Scholar]
- Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Hierarchical or not? Effect of the length scale and hierarchy of the surface roughness on omniphobicity of lubricant-infused substrates. Nano Lett. 2013, 13, 1793–1799. [Google Scholar] [CrossRef]
- Anand, S.; Paxson, A.T.; Dhiman, R.; Smith, J.D.; Varanasi, K.K. Enhanced condensation on lubricant-impregnated nanotextured surfaces. ACS Nano 2012, 6, 10122–10129. [Google Scholar] [CrossRef]
- Rykaczewski, K.; Anand, S.; Subramanyam, S.B.; Varanasi, K.K. Mechanism of frost formation on lubricant-impregnated surfaces. Langmuir 2013, 29, 5230–5238. [Google Scholar] [CrossRef]
- Vogel, N.; Belisle, R.A.; Hatton, B.; Wong, T.S.; Aizenberg, J. Transparency and damage tolerance of patternable omniphobic lubricated surfaces based on inverse colloidal monolayers. Nat. Commun. 2013, 4, 2176. [Google Scholar] [CrossRef] [Green Version]
- Van Oss, C.J.; Giese, R.F.; Wentzek, R.; Norris, J.; Chuvilin, E.M. Surface Tension Parameters of Ice Obtained From Contact Angle Data and From Positive and Negative Particle Adhesion to Advancing Freezing Fronts. J. Adhes. Sci. Technol. 1992, 6, 503–516. [Google Scholar] [CrossRef]
- Zou, M.; Beckford, S.; Wei, R.; Ellis, C.; Hatton, G.; Miller, M.A. Effects of surface roughness and energy on ice adhesion strength. Appl. Surf. Sci. 2011, 257, 3786–3792. [Google Scholar] [CrossRef]
- Bormashenko, E. General equation describing wetting of rough surfaces. J. Colloid Interface Sci. 2011, 360, 317–319. [Google Scholar] [CrossRef]
- Smith, J.D.; Dhiman, R.; Anand, S.; Reza-Garduno, E.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Droplet mobility on lubricant-impregnated surfaces. Soft Matter 2013, 9, 1772–1780. [Google Scholar] [CrossRef] [Green Version]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Villegas, M.; Zhang, Y.; Abu Jarad, N.; Soleymani, L.; Didar, T.F. Liquid-Infused Surfaces: A Review of Theory, Design, and Applications. ACS Nano 2019, 13, 8517–8536. [Google Scholar] [CrossRef]
- Schellenberger, F.; Xie, J.; Encinas, N.; Hardy, A.; Klapper, M.; Papadopoulos, P.; Butt, H.J.; Vollmer, D. Direct observation of drops on slippery lubricant-infused surfaces. Soft Matter 2015, 11, 7617–7626. [Google Scholar] [CrossRef] [Green Version]
- Boinovich, L.B.; Chulkova, E.V.; Emelyanenko, K.A.; Domantovsky, A.G.; Emelyanenko, A.M. The mechanisms of anti-icing properties degradation for slippery liquid-infused porous surfaces under shear stresses. J. Colloid Interface Sci. 2022, 609, 260–268. [Google Scholar] [CrossRef]
- Whyman, G.; Bormashenko, E.; Stein, T. The rigorous derivation of Young, Cassie-Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon. Chem. Phys. Lett. 2008, 450, 355–359. [Google Scholar] [CrossRef]
- Yoshimitsu, Z.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Effects of surface structure on the hydrophobicity and sliding behavior of water droplets. Langmuir 2002, 18, 5818–5822. [Google Scholar] [CrossRef]
- Tattelman, P. An objective method for measuring surface ice accretion. J. Appl. Meteorol. 1982, 21, 599–612. [Google Scholar] [CrossRef] [Green Version]
- Stenroos, C. Properties of Icephobic Surfaces in Different Icing Conditions. Master’s Thesis, Tampere University, Tampere, Finland, 2015. [Google Scholar]
- Donadei, V.; Koivuluoto, H.; Sarlin, E.; Niemelä, H.; Varis, T.; Vuoristo, P. The effect of mechanical and thermal stresses on the performance of lubricated icephobic coatings during cyclic icing / deicing tests. Prog. Org. Coat. 2022, 163, 10661. [Google Scholar] [CrossRef]

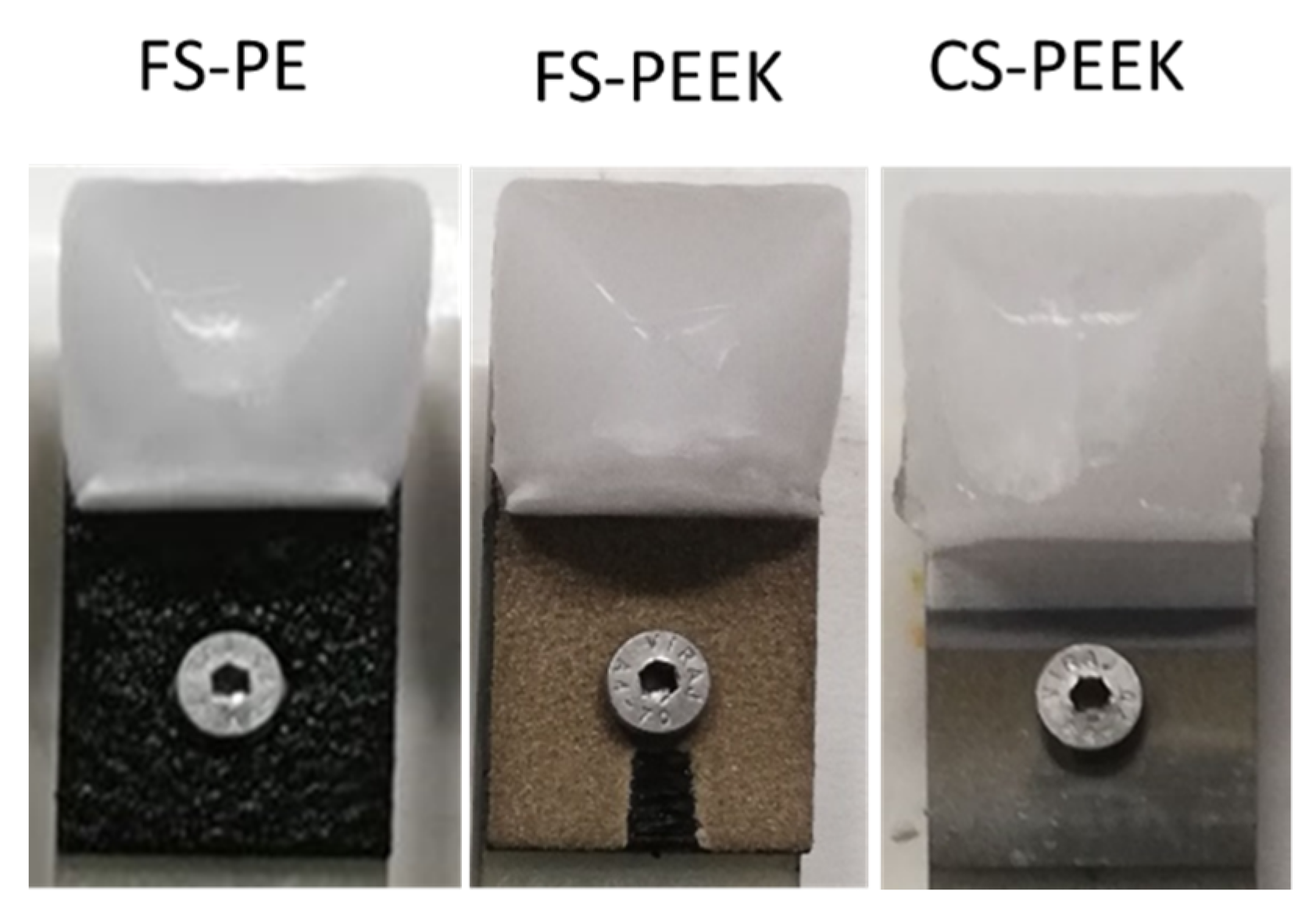
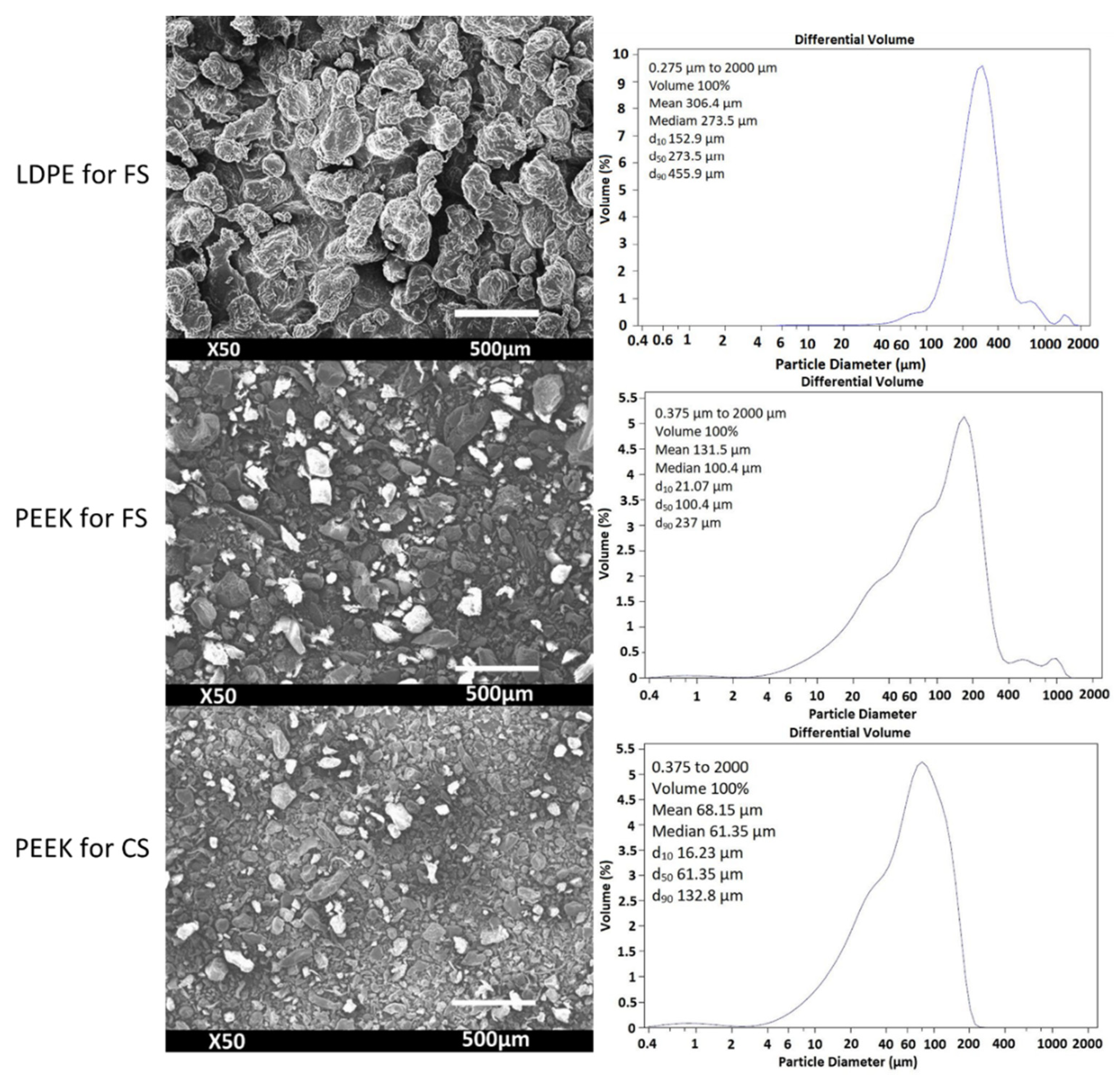
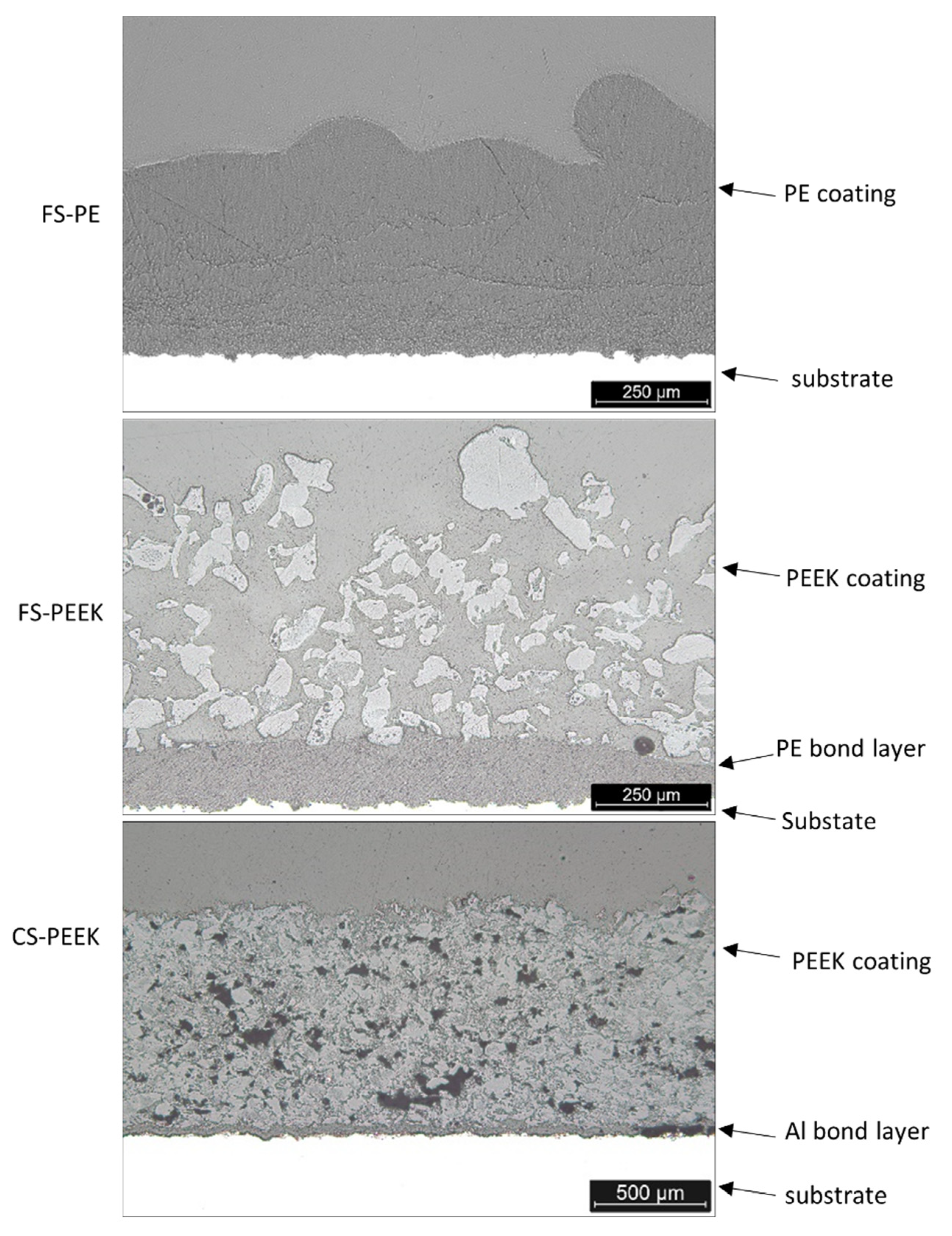
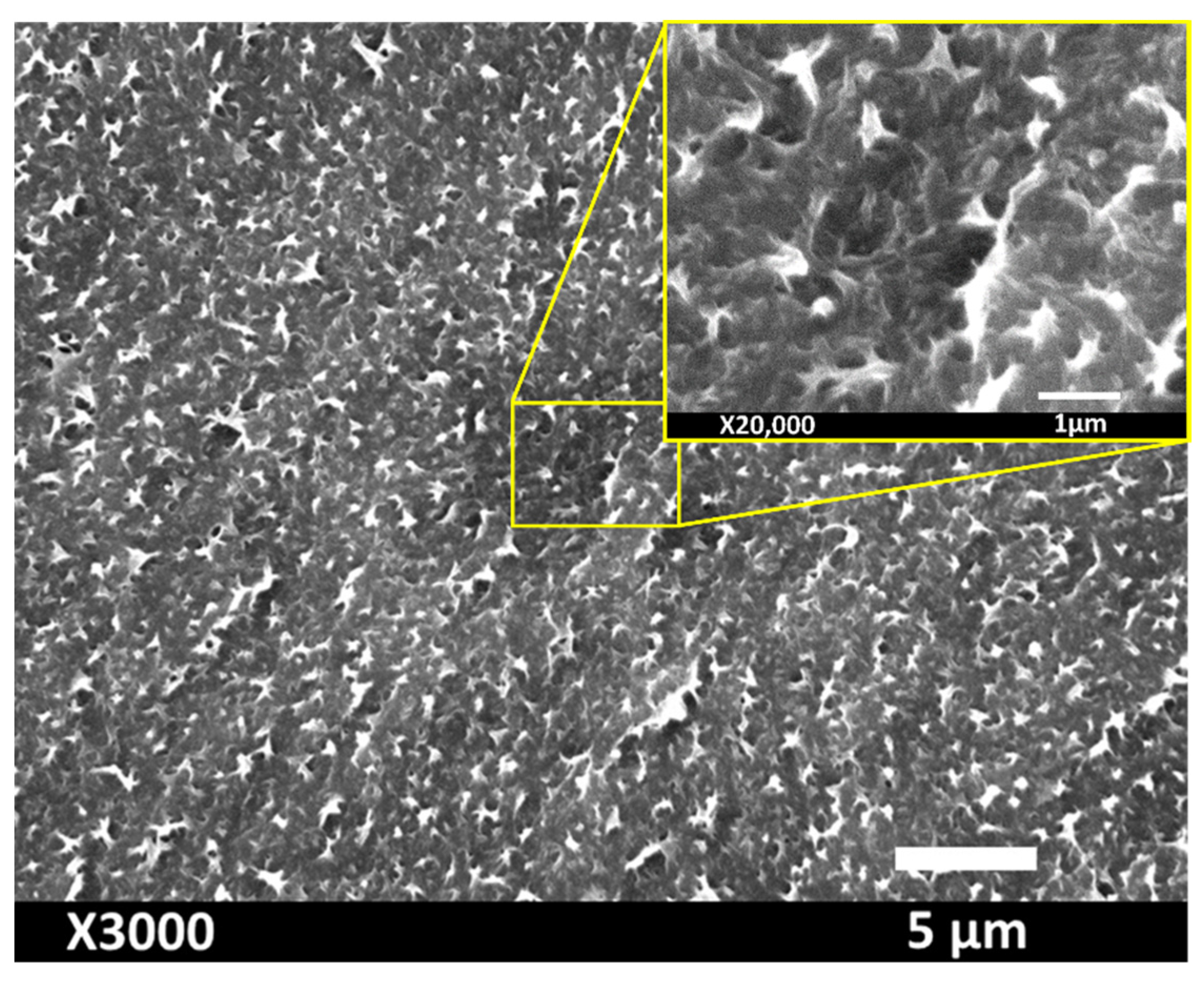
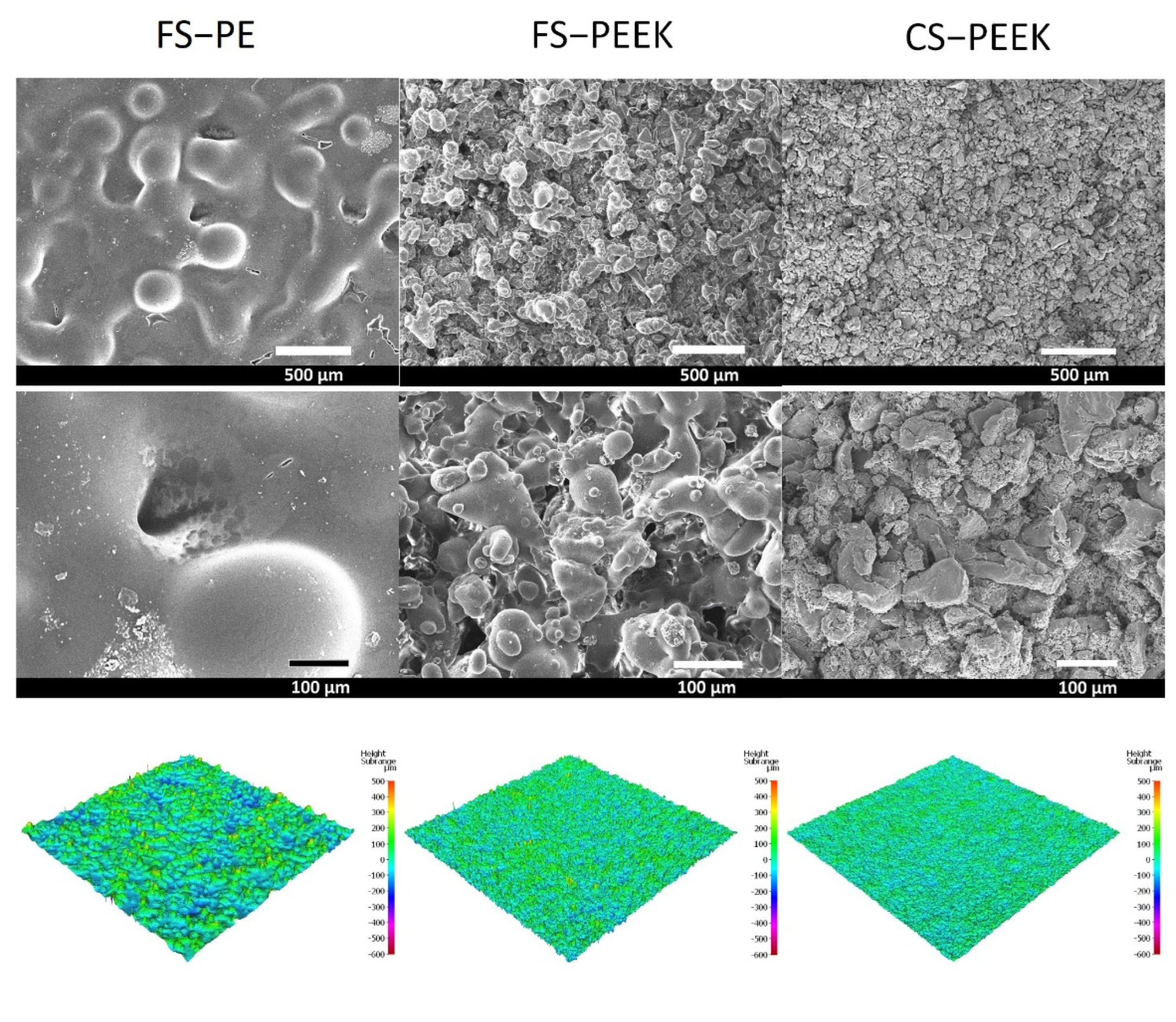

| Powder | Melting Point (°C) | Bulk Density (g/cm3) | Particle Size (µm) |
|---|---|---|---|
| LDPE PEEK | 107 | 0.36 | 300 |
| 340 | 1.3 | 110 for FS | |
| 55 for LPCS |
| Sample | Spray Nozzle Type | O2 Pressure (Bar) | C2H2 Pressure (Bar) | Spray Distance (mm) | Moving Speed (mm/s) | Step Size (mm) |
|---|---|---|---|---|---|---|
| FS-PE | M10 | 4.2 | 0.7 | 250 | 500 | 5 |
| FS-PEEK | M40 | 4.0 | 0.7 | 250 | 500 | 5 |
| PE bond layer | M10 | 4.2 | 0.7 | 250 | 500 | 5 |
| Sample | Air Heat Temperature (°C) | Air Pressure (Bar) | Spray Distance (mm) | Moving Speed (mm/s) | Step Size (mm) |
|---|---|---|---|---|---|
| CS-PEEK | 320 | 4.2 | 10 | 50 | 1 |
| Al bond layer | 540 | 6 | 10 | 50 | 1 |
| Temperature (°C) | Relative Humidity (%) | Wind Speed (m/s) | Ice Type |
|---|---|---|---|
| −10 | 83–86 | 25 | Mixed glaze |
| Before Icing Tests | After Icing Tests | |
|---|---|---|
| Coating | Sa (µm) | Sa (µm) |
| FS-PE | 65 | 47 |
| FS-PEEK | 53 | 55 |
| CS-PEEK | 31 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khammas, R.; Koivuluoto, H. Durable Icephobic Slippery Liquid-Infused Porous Surfaces (SLIPS) Using Flame- and Cold-Spraying. Sustainability 2022, 14, 8422. https://doi.org/10.3390/su14148422
Khammas R, Koivuluoto H. Durable Icephobic Slippery Liquid-Infused Porous Surfaces (SLIPS) Using Flame- and Cold-Spraying. Sustainability. 2022; 14(14):8422. https://doi.org/10.3390/su14148422
Chicago/Turabian StyleKhammas, Ruqaya, and Heli Koivuluoto. 2022. "Durable Icephobic Slippery Liquid-Infused Porous Surfaces (SLIPS) Using Flame- and Cold-Spraying" Sustainability 14, no. 14: 8422. https://doi.org/10.3390/su14148422






