Characterization and Assessment of Organic Pollution at a Fumaric Acid Chemical Brownfield Site in Northwestern China
Abstract
:1. Introduction
2. Materials and Methods
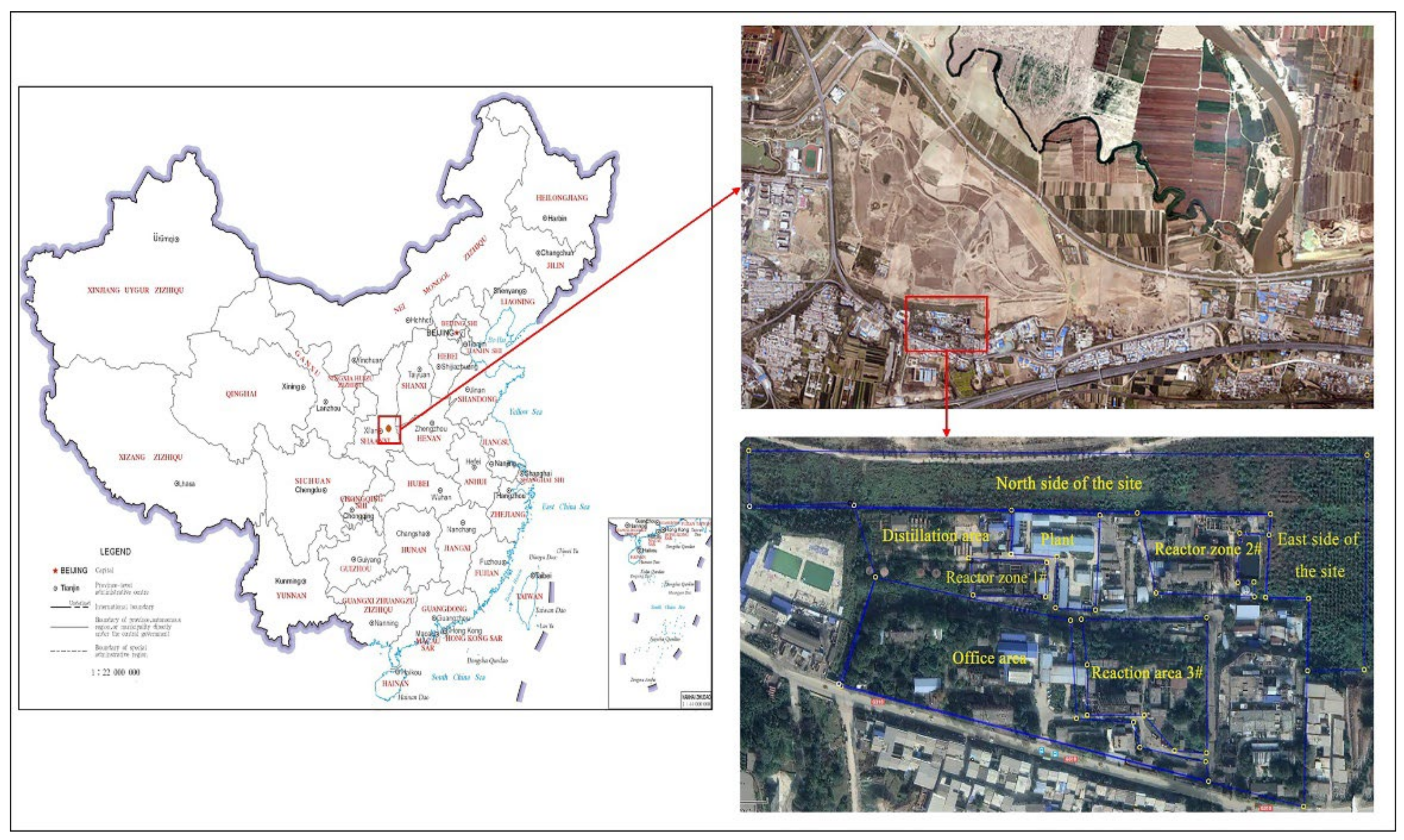
2.1. Overview of the Study Area
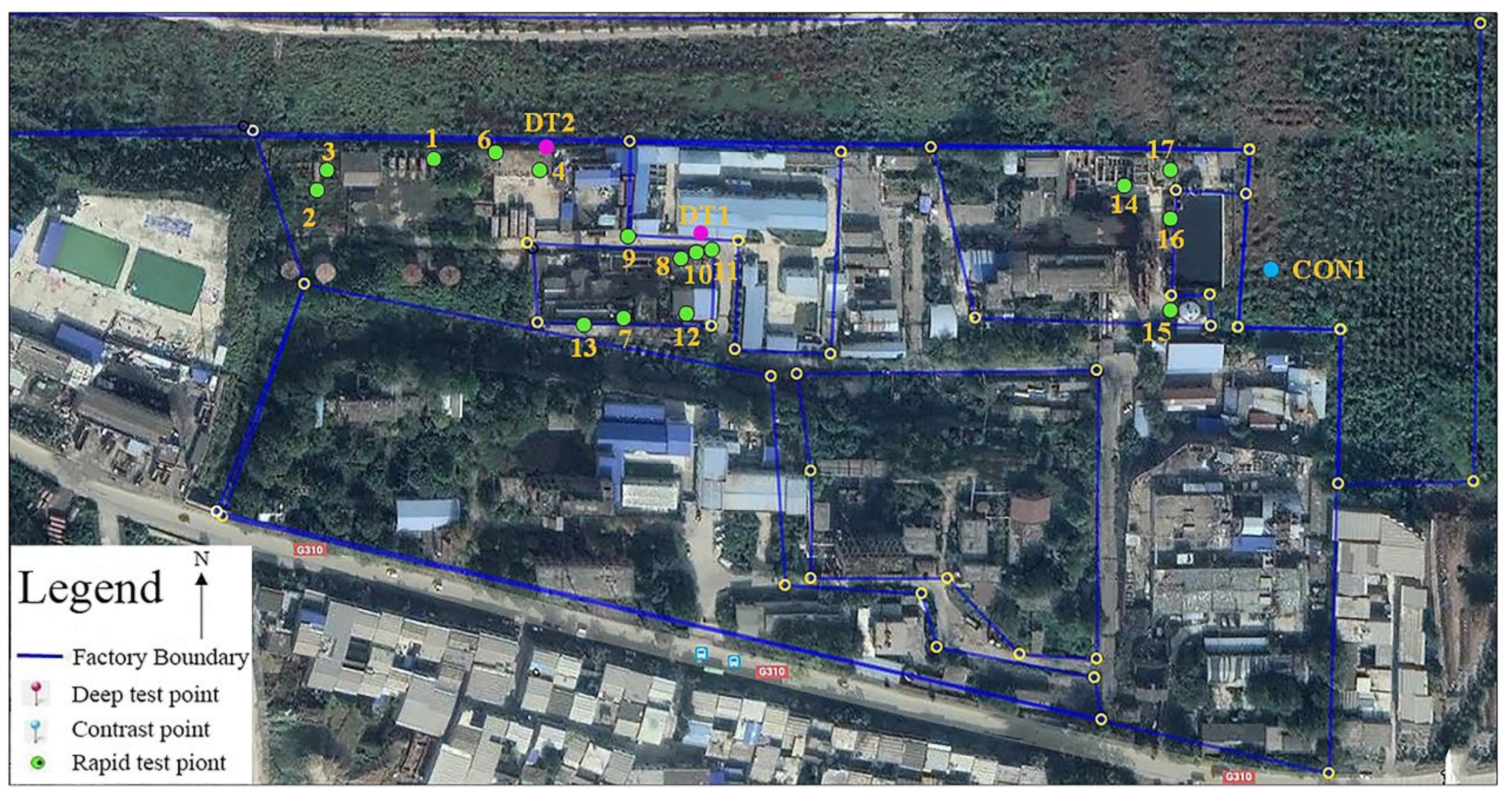
2.2. Distribution of Sampling Points and Sampling Methods
2.3. Testing Indicators and Analytical Methods
2.4. Evaluation Method of the Situation of Brownfield Contamination
3. Results and Discussion
3.1. Analysis of Physical and Chemical Properties
3.2. Analysis of Organic Pollution in the Atmosphere of Fumaric Acid Brownfield Sites
3.3. Analysis of Organic Pollution in the Soil of Fumaric Acid Brownfields
3.3.1. Phenological Properties of Soil
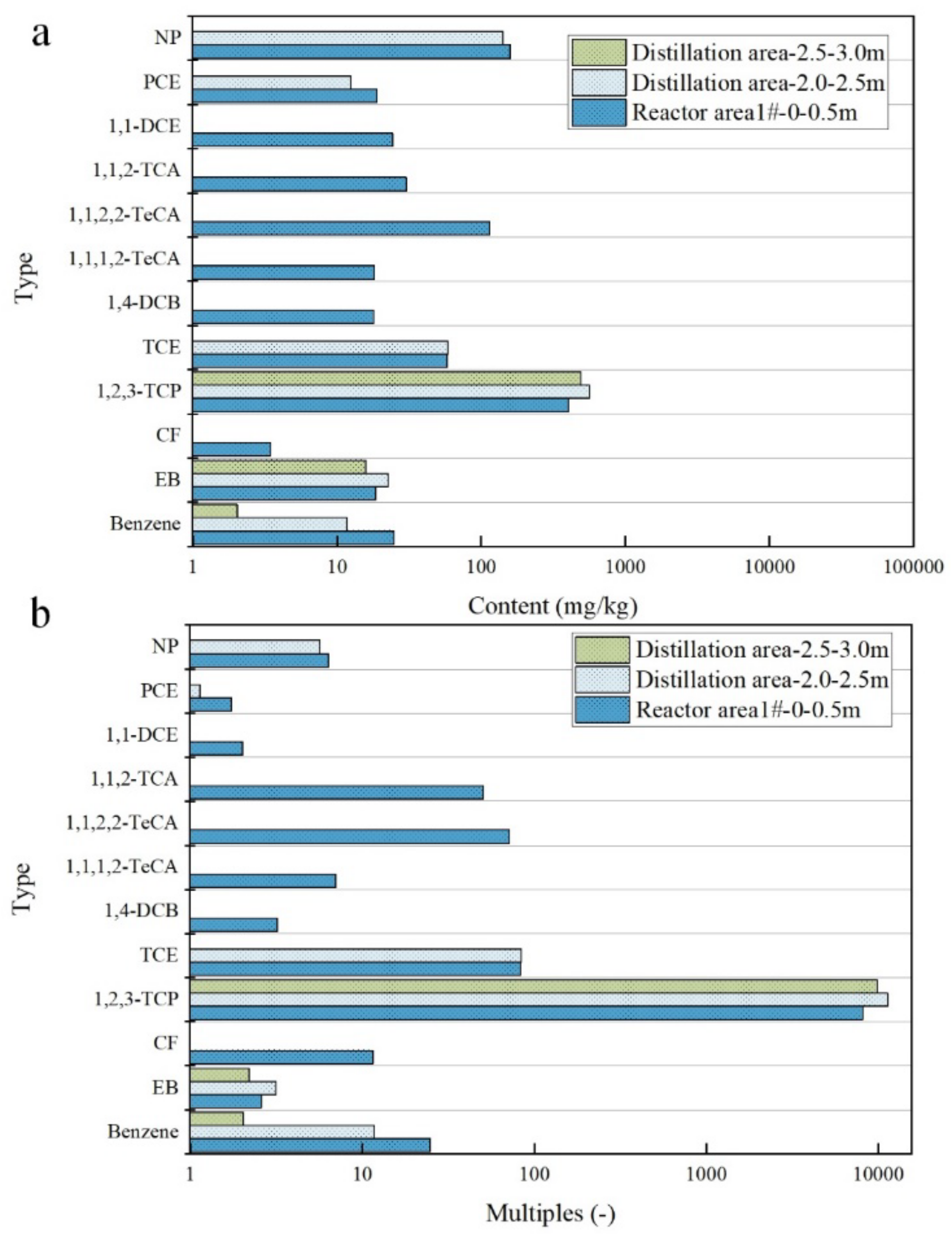
3.3.2. Distribution Characteristics of VOCs in Surface Soil
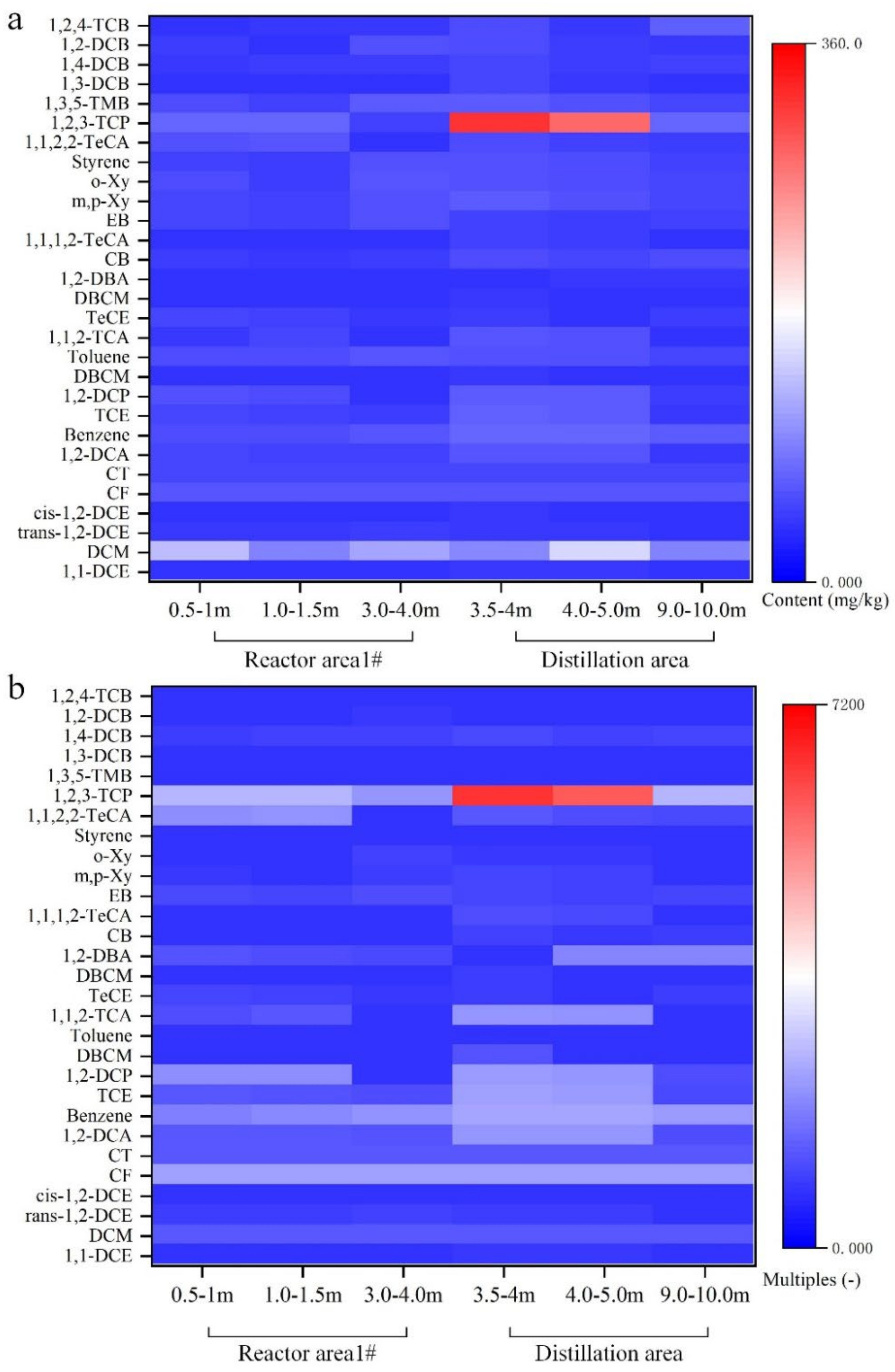
3.3.3. Distribution Characteristics of VOCs in Deep Soil
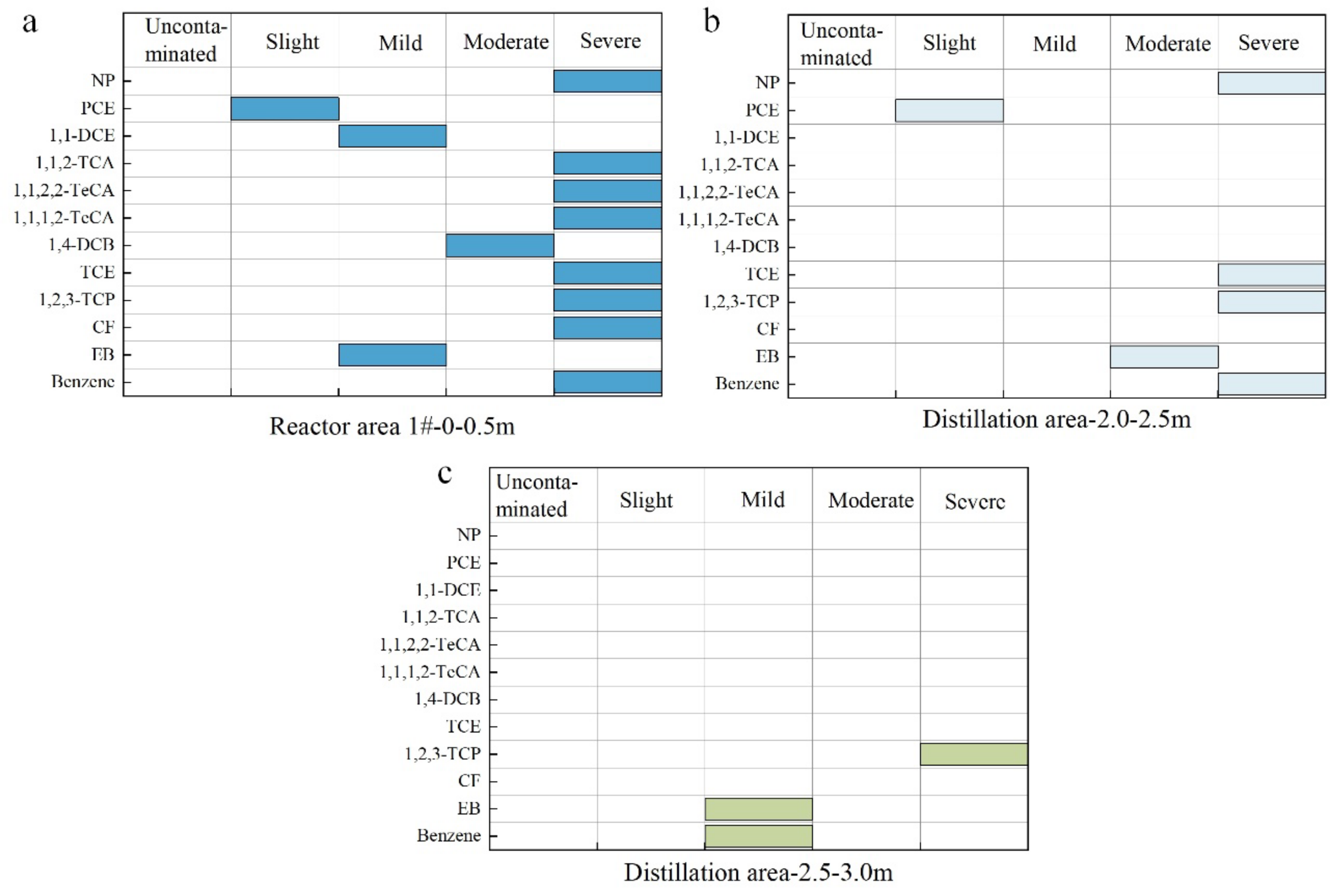
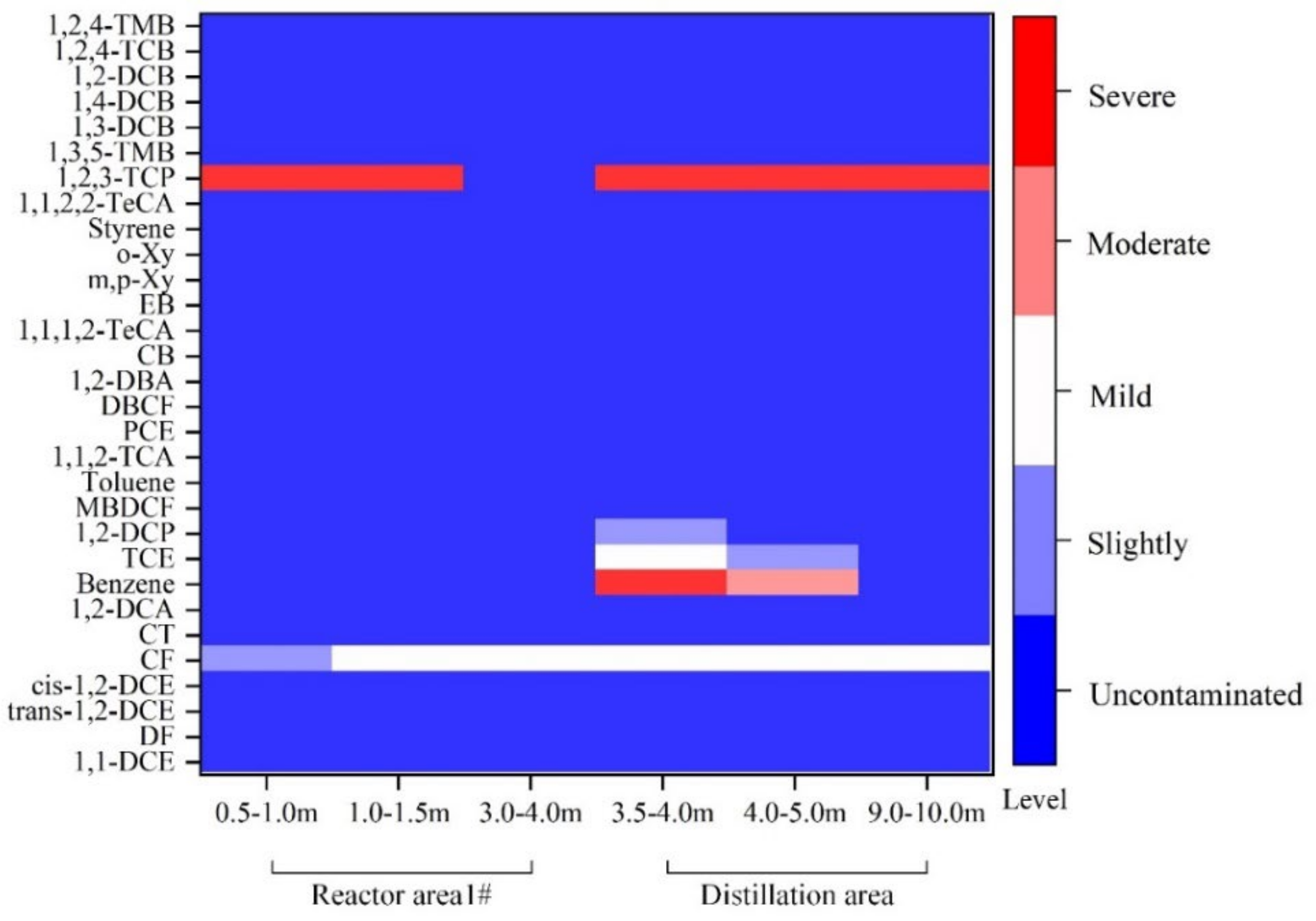
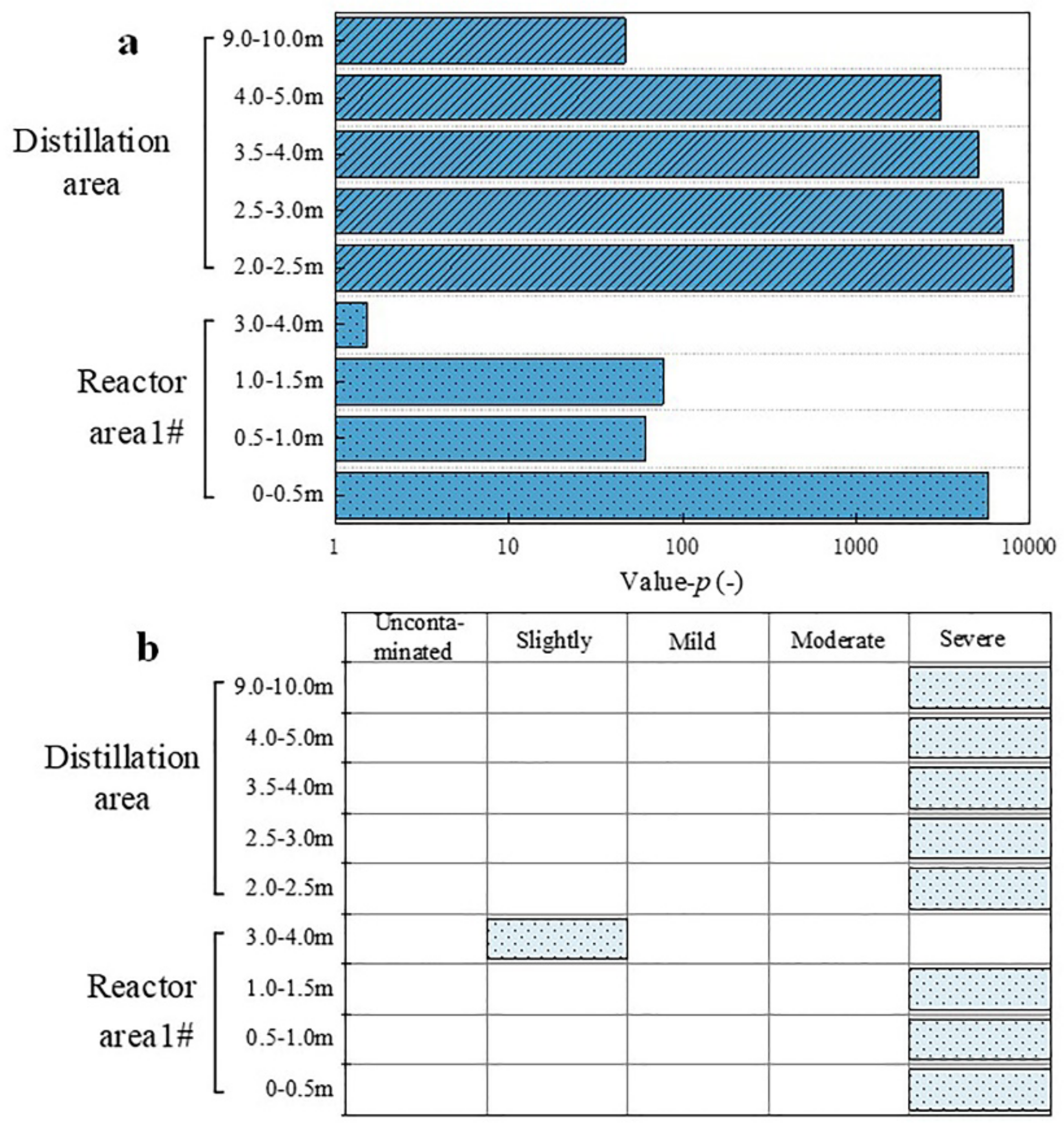
3.4. Evaluation of Organic Contamination of Fumaric Acid Brownfield Site
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Macgregor, C.J.; Bunting, M.J.; Deutz, P.; Bourn, N.A.D.; Roy, D.B.; Mayes, W.M. Brownfield sites promote biodiversity at a landscape scale. Sci. Total Environ. 2022, 804, 150162. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, J.; Tan, H.; Song, Q.; Zhang, J.; Zhong, X.; Zhou, J.; Wu, W.; Cai, X.; Zhang, W.; et al. Distributions of chlorinated paraffins and the effects on soil microbial community structure in a production plant brownfield site. Environ. Pollut. 2020, 262, 114328. [Google Scholar] [CrossRef] [PubMed]
- Hellawell, E.E.; Hughes, S.J. Asbestos contamination on brownfield development sites in the UK. Environ. Res. 2021, 198, 110480. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lyu, Y.; Qian, S.; Zhang, X.; Lin, H.; Wang, S. Identifying urban candidate brownfield sites using multi-source data: The case of Changchun City, China. Land Use Policy 2022, 117, 106084. [Google Scholar] [CrossRef]
- Qiu, H.; Lou, Z.; Gu, X.; Sun, Y.; Wang, J.; Zhang, W.; Cao, X. Smart 6S roadmap for deciphering the migration and risk of heavy metals in soil and groundwater systems at brownfield sites nationwide in China. Sci. Bull. 2022, 67, 1295–1299. [Google Scholar] [CrossRef]
- Mahammedi, C.; Mahdjoubi, L.; Booth, C.A.; Butt, T.E. Framework for preliminary risk assessment of brownfield sites. Sci. Total Environ. 2022, 807, 151069. [Google Scholar] [CrossRef]
- Tonin, S.; Bonifaci, P. Assessment of brownfield redevelopment opportunities using a multi-tiered approach: A case in Italy. Socio-Econ. Plan. Sci. 2020, 71, 100812. [Google Scholar] [CrossRef]
- Lodge, E.K.; Guseh, N.S.; Martin, C.L.; Fry, R.C.; White, A.J.; Ward-Caviness, C.K.; Galea, S.; Aiello, A.E. The effect of residential proximity to brownfields, highways, and heavy traffic on serum metal levels in the Detroit Neighborhood Health Study. Environ. Adv. 2022, 9, 100278. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Lei, S.; Semple, K.T.; Coulon, F.; Hu, Q.; Gao, J.; Guo, G.; Gu, Q.; Jones, K.C. Redevelopment of urban brownfield sites in China: Motivation, history, policies and improved management. Eco-Environ. Health 2022, 1, 63–72. [Google Scholar] [CrossRef]
- Ahmad, N.; Zhu, Y.; Ullah, Z.; Iqbal, M.; Hussain, K.; Ahmed, R.I. Sustainable solutions to facilitate brownfield redevelopment projects in emerging countries—Pakistani scenario. Land Use Policy 2021, 109, 105727. [Google Scholar] [CrossRef]
- Li, H.; Ji, H. Chemical speciation, vertical profile and human health risk assessment of heavy metals in soils from coal-mine brownfield, Beijing, China. J. Geochem. Explor. 2017, 183, 22–32. [Google Scholar] [CrossRef]
- Hu, G.; Liu, H.; Chen, C.; He, P.; Li, J.; Hou, H. Selection of green remediation alternatives for chemical industrial sites: An integrated life cycle assessment and fuzzy synthetic evaluation approach. Sci. Total Environ. 2022, 845, 157211. [Google Scholar] [CrossRef]
- Sebastian, J.; Osorio-Gonzalez, C.; Rouissi, T.; Hegde, K.; Brar, S.K. Bioderived fumaric acid for sustainable production of key active pharmaceutical ingredients: Dimethyl fumarate and Monomethyl fumarate. Process Biochem. 2022, 120, 35–40. [Google Scholar] [CrossRef]
- Wei, P.; Ma, H.; Fu, H.; Xu, Z.; Qu, X. Efficient inhibition of cyanobacteria M. aeruginosa growth using commercial food-grade fumaric acid. Chemosphere 2022, 301, 134659. [Google Scholar] [CrossRef]
- Xu, Q.; Li, S.; Huang, H.; Wen, J. Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol. Adv. 2012, 30, 1685–1696. [Google Scholar] [CrossRef]
- Guo, F.; Wu, M.; Dai, Z.; Zhang, S.; Zhang, W.; Dong, W.; Zhou, J.; Jiang, M.; Xin, F. Current advances on biological production of fumaric acid. Biochem. Eng. J. 2020, 153, 107397. [Google Scholar] [CrossRef]
- Yang, S.; Ge, W.-y.; Chen, H.-h.; Xu, W.-l. Investigation of soil and groundwater environment in urban area during post-industrial era: A case study of brownfield in Zhenjiang, Jiangsu Province, China. China Geol. 2019, 2, 501–511. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, H.; Duan, L. Pollution characteristics and population health risks of polycyclic aromatic hydrocarbons (PAHs) in coking contaminated soils. Environ. Chall. 2022, 9, 100613. [Google Scholar] [CrossRef]
- Ma, B.; Zeng, W.; Xie, Y.; Wang, Z.; Hu, G.; Li, Q.; Cao, R.; Zhuo, Y.; Zhang, T. Boundary delineation and grading functional zoning of Sanjiangyuan National Park based on biodiversity importance evaluations. Sci. Total Environ. 2022, 825, 154068. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Yan, X.; Liang, T.; Yang, X.; El-Naggar, A.; Liu, J.; Chen, H. Evaluation of potential ecological risks in potential toxic elements contaminated agricultural soils: Correlations between soil contamination and polymetallic mining activity. J. Environ. Manag. 2021, 300, 113679. [Google Scholar] [CrossRef]
- Yang, Z.B.; Qin, Z.H.; Wang, G.C.; Li, C.L. Environmental Effects of Water Product from Coalbed Methane Wells: A Case Study of the Songhe Well Group, Western Guizhou, China. Nat. Resour. Res. 2021, 30, 3747–3760. [Google Scholar] [CrossRef]
- Lin, C.; Chen, J.; Xu, J.; Li, Y.; Liu, Y.; Lin, H. Distribution and risk assessment of heavy metals in the economic fish of the Southern Fujian Province. Environ. Toxicol. Pharmacol. 2022, 92, 103834. [Google Scholar] [CrossRef]
- Miao, F.; Zhang, Y.; Li, Y.; Liang, X.; Lin, Q.; Zhou, Y. Establishing a weighted methodology for human health risk assessment of cadmium based on its equilibrium speciation in groundwater. J. Clean. Prod. 2021, 322, 129053. [Google Scholar] [CrossRef]
- Wu, J.; Jia, R.T.; Xuan, H.; Zhang, D.S.; Zhang, G.M.; Xiao, Y.T. Priority Soil Pollution Management of Contaminated Site Based on Human Health Risk Assessment: A Case Study in Southwest China. Sustainability 2022, 14, 3663. [Google Scholar] [CrossRef]
- Sun, J.; Pan, L.; Tsang, D.C.W.; Zhan, Y.; Zhu, L.; Li, X. Organic contamination and remediation in the agricultural soils of China: A critical review. Sci. Total Environ. 2018, 615, 724–740. [Google Scholar] [CrossRef]
- Hung, H.-W.; Daniel Sheng, G.; Lin, T.-F.; Su, Y.; Chiou, C.T. The organic contamination level based on the total soil mass is not a proper index of the soil contamination intensity. Environ. Pollut. 2009, 157, 2928–2932. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Wu, P.Y.; Chen, Y.C.; Chen, P.C.; Guo, Y.L.; Lin, Y.J.; Lin, P.P. An integrated strategy by using long-term monitoring data to identify volatile organic compounds of high concern near petrochemical industrial parks. Sci. Total Environ. 2022, 821, 153345. [Google Scholar] [CrossRef]
- Lei, R.R.; Sun, Y.M.; Zhu, S.; Jia, T.Q.; He, Y.C.; Deng, J.L.; Liu, W.B. Investigation on Distribution and Risk Assessment of Volatile Organic Compounds in Surface Water, Sediment, and Soil in a Chemical Industrial Park and Adjacent Area. Molecules 2021, 26, 5988. [Google Scholar] [CrossRef]
- Ran, H.; Deng, X.; Guo, Z.; Hu, Z.; An, Y.; Xiao, X.; Yi, L.; Xu, R. Pollution characteristics and environmental availability of toxic elements in soil from an abandoned arsenic-containing mine. Chemosphere 2022, 303, 135189. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Wang, X.; Ma, L.; Cheng, Z.; Su, L. Pollution characteristics, spatial distributions, and source apportionment of heavy metals in cultivated soil in Lanzhou, China. Ecol. Indic. 2021, 125, 107507. [Google Scholar] [CrossRef]
- Engel, C.A.R.; Straathof, A.J.J.; Zijlmans, T.W.; van Gulik, W.M.; van der Wielen, L.A.M. Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef]
- Rajabi, H.; Mosleh, M.H.; Mandal, P.; Lea-Langton, A.; Sedighi, M. Emissions of volatile organic compounds from crude oil processing—Global emission inventory and environmental release. Sci. Total Environ. 2020, 727, 138654. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Shen, Y.; Liu, X.; Zhang, Z.; Xu, J.; Zhang, Z. The effect of horizontal freezing on the characteristics of water migration and matric suction in unsaturated silt. Eng. Geol. 2021, 288, 106166. [Google Scholar] [CrossRef]
- Balseiro-Romero, M.; Monterroso, C.; Casares, J.J. Environmental Fate of Petroleum Hydrocarbons in Soil: Review of Multiphase Transport, Mass Transfer, and Natural Attenuation Processes. Pedosphere 2018, 28, 833–847. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, J.; Feng, S.; Xiao, Z.; Chen, C. Effects of ecohydrological interfaces on migrations and transformations of pollutants: A critical review. Sci. Total Environ. 2022, 804, 150140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Qin, A.; Li, L.; Mei, G.; Li, T. Coupled consolidation via vertical drains in unsaturated soils induced by time-varying loading based on continuous permeable boundary. Geotext. Geomembr. 2022, 50, 383–392. [Google Scholar] [CrossRef]
- Jia, H.; Gao, S.; Duan, Y.; Fu, Q.; Che, X.; Xu, H.; Wang, Z.; Cheng, J. Investigation of health risk assessment and odor pollution of volatile organic compounds from industrial activities in the Yangtze River Delta region, China. Ecotoxicol. Environ. Saf. 2021, 208, 111474. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.M.; Geng, S.Y.; Cao, W.; Zuo, R.; Teng, Y.G.; Ding, A.Z.; Fan, F.Q.; Dou, J.F. Vertical distribution characteristics and interactions of polycyclic aromatic compounds and bacterial communities in contaminated soil in oil storage tank areas. Chemosphere 2022, 301, 134695. [Google Scholar] [CrossRef]
- Chen, L.; Han, L.; Feng, Y.; He, J.; Xing, B. Soil structures and immobilization of typical contaminants in soils in response to diverse microplastics. J. Hazard. Mater. 2022, 438, 129555. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Giustra, M.G.; Vagliasindi, F.G.A. Low-temperature thermal desorption of diesel polluted soil: Influence of temperature and soil texture on contaminant removal kinetics. J. Hazard. Mater. 2011, 185, 392–400. [Google Scholar] [CrossRef]
- Zhu, H.; Ye, S.; Wu, J.; Xu, H. Characteristics of soil lithology and pollutants in typical contanmination sites in Chins. Earth Sci. Front. 2021, 28, 026–034. [Google Scholar] [CrossRef]

| Name | Thickness (m) | Depth (m) | Bottom Relative Elevation (m) |
|---|---|---|---|
| Layer of plain filling (Q4ml) | 0.40–9.20 | 0.40–9.20 | 341.33–345.90 |
| Layer of silt (Q4al + pl) | 5.50–14.10 | 7.80–16.20 | 330.33–340.89 |
| Layer of fine sand (Q4al + pl) | 1.00–4.20 | 10.10–19.30 | 330.29–336.07 |
| Layer of silty clay (Q3al + pl) | 5.30–10.20 | 18.80–24.00 | 320.95–328.17 |
| Layer of medium sand (Q3al + pl) | 6.60–7.50 | 27.60–29.30 | 317.54–320.95 |
| Level | Pi | Evaluation Level |
|---|---|---|
| Ⅰ | Pi ≤ 1 | Uncontaminated |
| Ⅱ | 1 < Pi ≤ 2 | Slight |
| Ⅲ | 2 < Pi ≤ 3 | Mild |
| Ⅳ | 3 < Pi ≤ 5 | Moderate |
| Ⅴ | Pi > 5 | Severe |
| Level | P | Evaluation Level |
|---|---|---|
| Ⅰ | P ≤ 0.7 | Uncontaminated |
| Ⅱ | 0.7 < P ≤ 1 | Slight |
| Ⅲ | 1 < P ≤ 2 | Mild |
| Ⅳ | 2 < P ≤ 3 | Moderate |
| Ⅴ | P > 3 | Severe |
| Type | pH | Soil Texture |
|---|---|---|
| CON1 | 8.64 | Silt |
| DT1 | 8.96 | Silt loam soil |
| DT2 | 9.48 | Silty |
| Type | Color | Lithology | Moisture | Odor | ||||
|---|---|---|---|---|---|---|---|---|
| Depth (m) | Reactor Area 1 | Distillation Area | Reactor Area 1 | Distillation Area | Reactor Area 1 | Distillation Area | Reactor Area 1 | Distillation Area |
| 0–1.0 | tan | / | silt | / | drier | / | slightly pungent odor | / |
| 1.1–2.0 | tan | / | silt | / | drier | / | slightly pungent odor | / |
| 2.1–3.0 | tan | black brown | silt | sandy soil | drier | drier | slightly pungent odor | pungent odor |
| 3.1–4.0 | black brown | black brown | mud | sandy soil | wetter | wet | slightly pungent odor | pungent odor |
| 4.1–5.0 | tan | light black-brown | sandy soil | sandy soil | wetter | wetter | slightly pungent odor | pungent odor |
| 5.1–6.0 | tan | light black-brown | sandy soil | sandy soil (with 2 cm silt) | wet (near muddy water) | wet | no pungent odor | pungent odor |
| 6.1–7.0 | / | light black-brown | / | sand and soil mixture | / | wetter | / | pungent odor |
| 7.1–8.0 | / | light black-brown | / | sand and soil mixture(more coarse sand) | / | wet | / | slightly pungent odor |
| 8.1–9.0 | / | light black-brown | / | sand and soil mixture(more silver sand) | / | wet | / | slightly pungent odor |
| 9.1–10.0 | / | light black-brown | / | silver sand | / | wet (like thin mud) | / | minimal pungent odor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, C.; Chen, Z.; Dong, Y.; Zhang, N.; Wei, Y.; Xi, H.; Wang, W. Characterization and Assessment of Organic Pollution at a Fumaric Acid Chemical Brownfield Site in Northwestern China. Sustainability 2022, 14, 12476. https://doi.org/10.3390/su141912476
Yang Y, Li C, Chen Z, Dong Y, Zhang N, Wei Y, Xi H, Wang W. Characterization and Assessment of Organic Pollution at a Fumaric Acid Chemical Brownfield Site in Northwestern China. Sustainability. 2022; 14(19):12476. https://doi.org/10.3390/su141912476
Chicago/Turabian StyleYang, Ye, Chaokun Li, Zhiwen Chen, Yingying Dong, Nan Zhang, Yulu Wei, Hui Xi, and Wendong Wang. 2022. "Characterization and Assessment of Organic Pollution at a Fumaric Acid Chemical Brownfield Site in Northwestern China" Sustainability 14, no. 19: 12476. https://doi.org/10.3390/su141912476





