Study on Phosphorus Removal Pathway in Constructed Wetlands with Thermally Modified Sepiolite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of SEP
2.2.1. Purification of SEP
2.2.2. Thermal Modification
2.3. Characterization of T-SEP
2.4. Adsorption Experiments
2.5. Design of Constructed Wetlands
2.6. Samples Analysis
2.7. Statistical Analysis
3. Results and Discussion
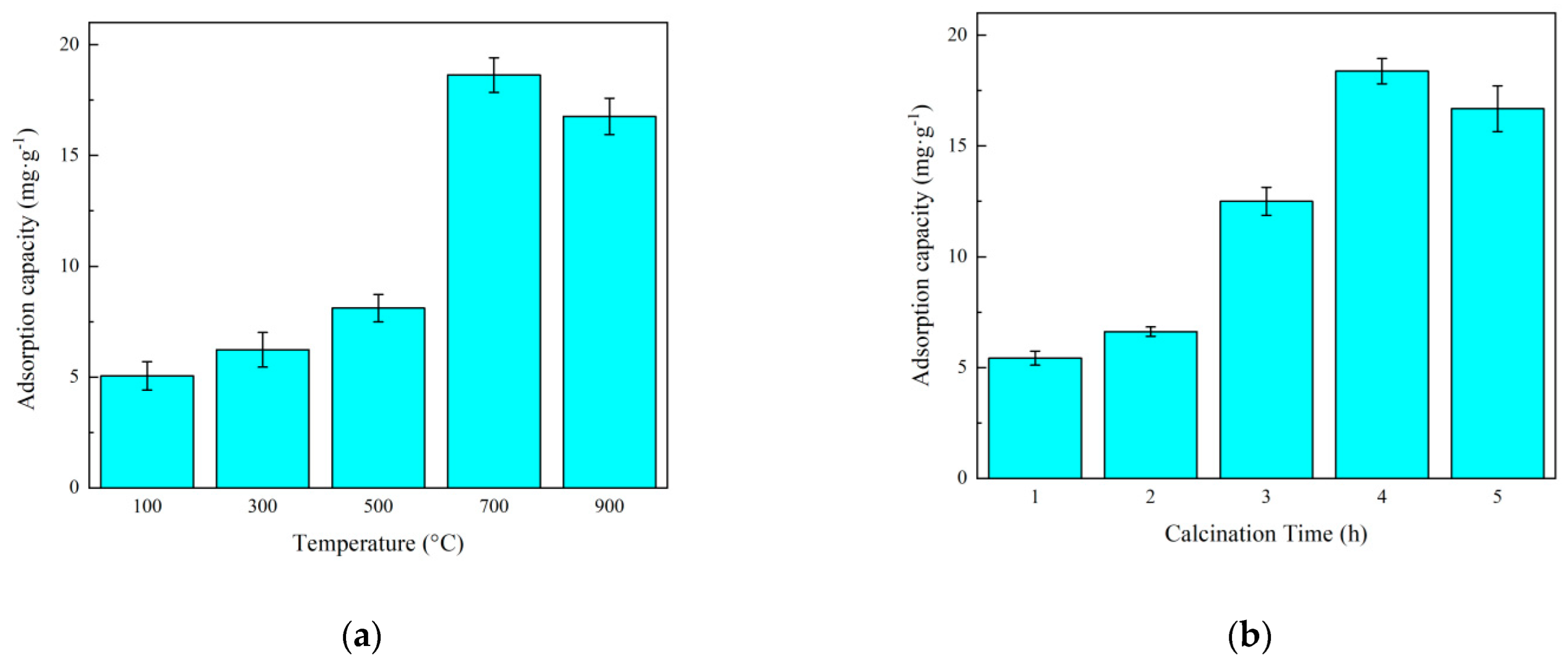
3.1. Optimum Parameters for Preparation of T-SEP
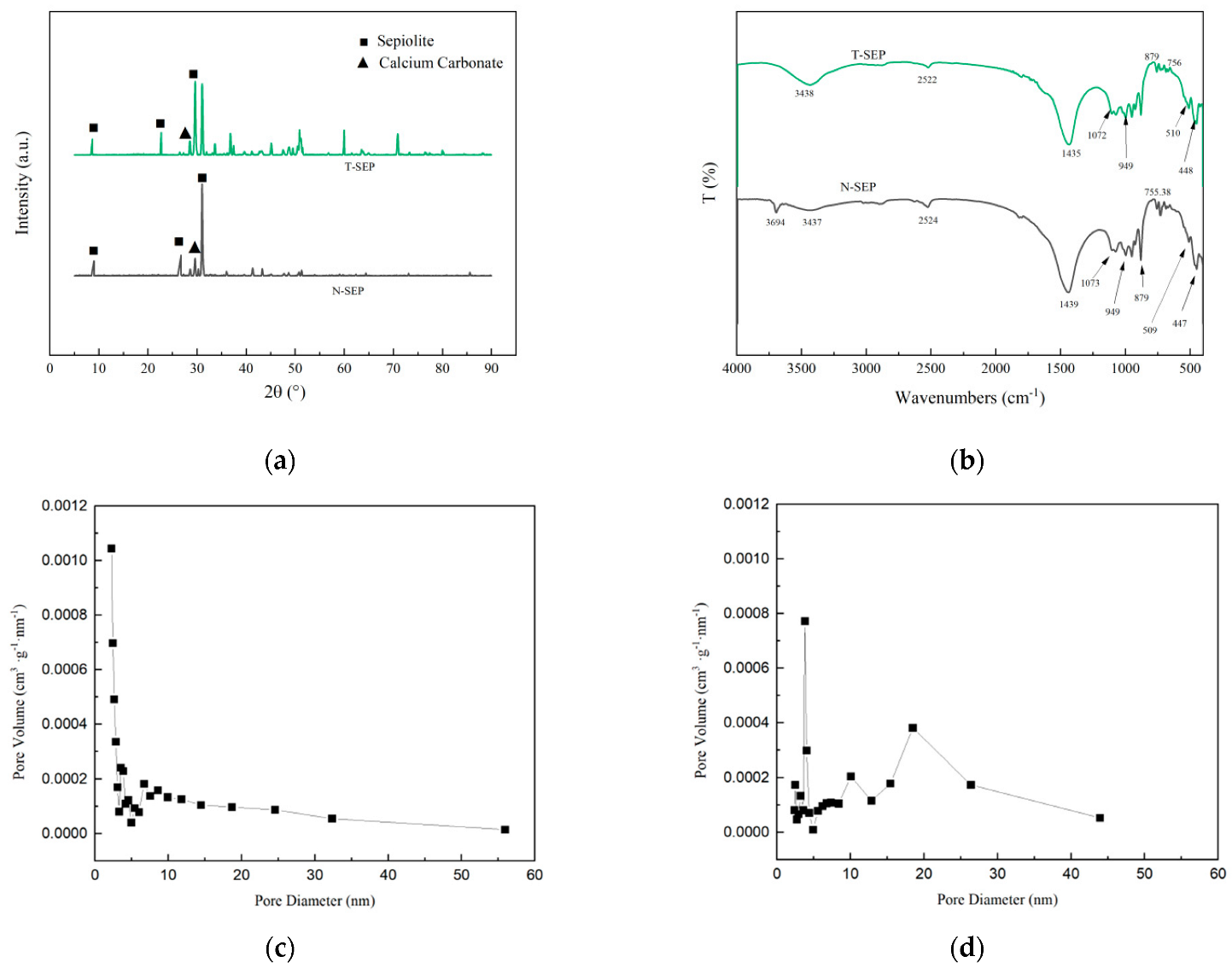
3.2. Characterization of T-SEP
3.3. Adsorption Properties of Phosphorus by T-SEP
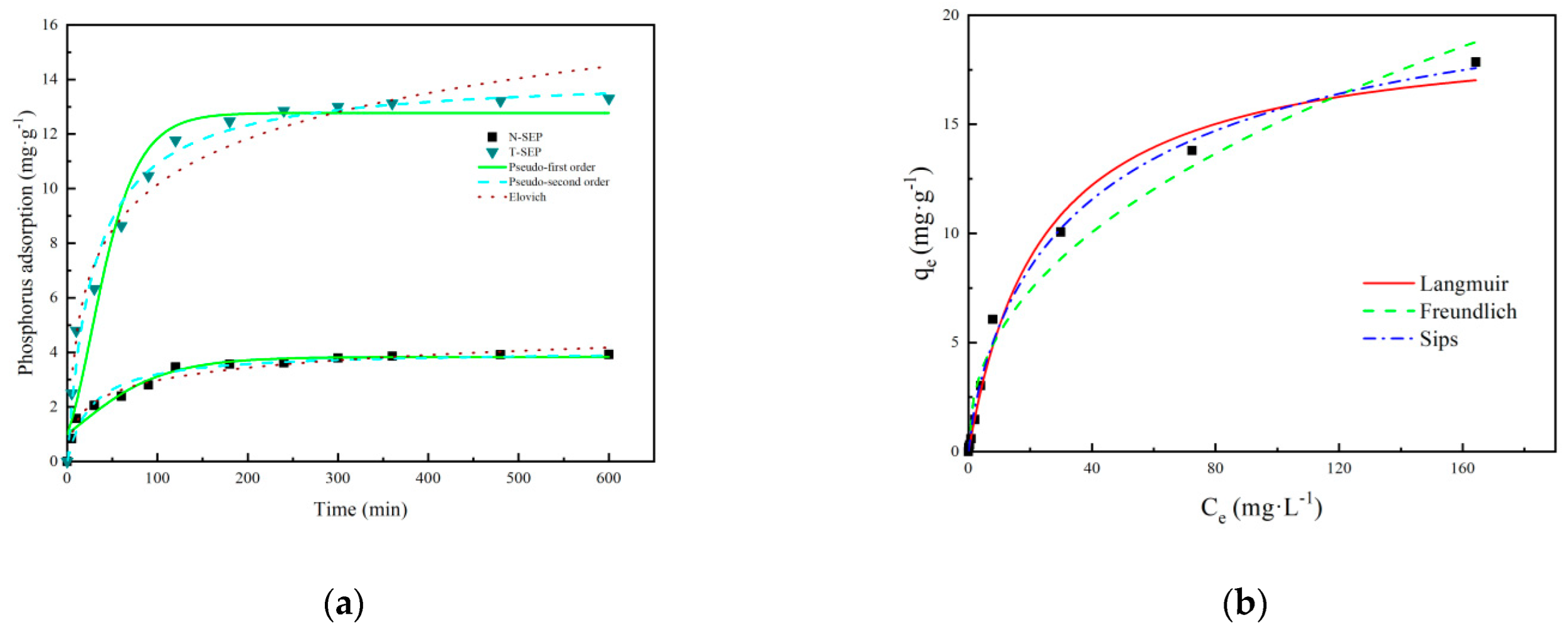
3.3.1. Adsorption Kinetics
3.3.2. Adsorption Isotherm
3.4. Phosphorus Removal Effect of T-SEP Substrate Constructed Wetlands
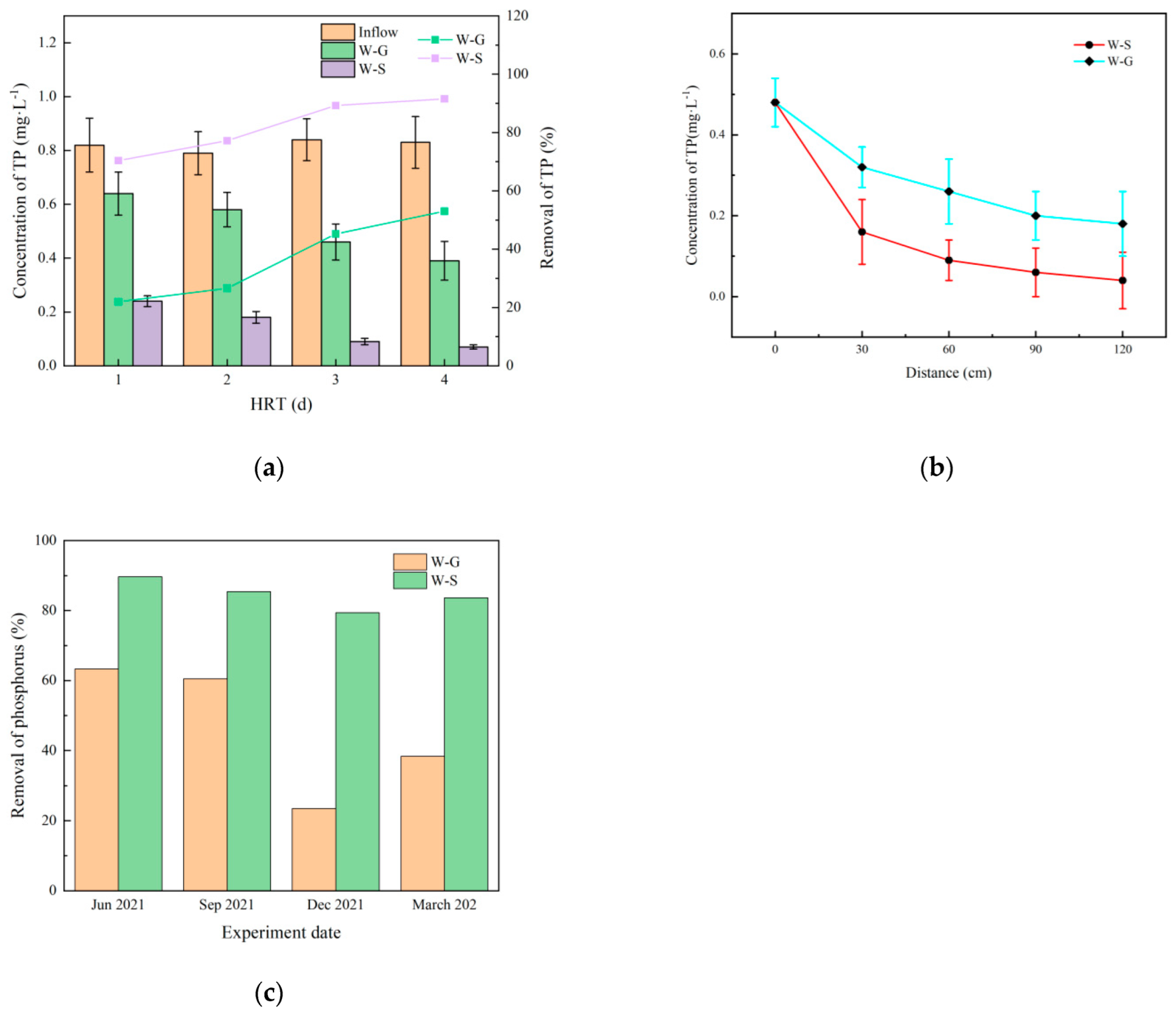
3.4.1. Effect of Hydraulic Retention Time on Total Phosphorus Concentration
3.4.2. Variation of Total Phosphorus along the Way
3.4.3. Variation of Phosphorus Removal in Four Seasons
3.5. Analysis of Phosphorus Removal Pathway in Constructed Wetland
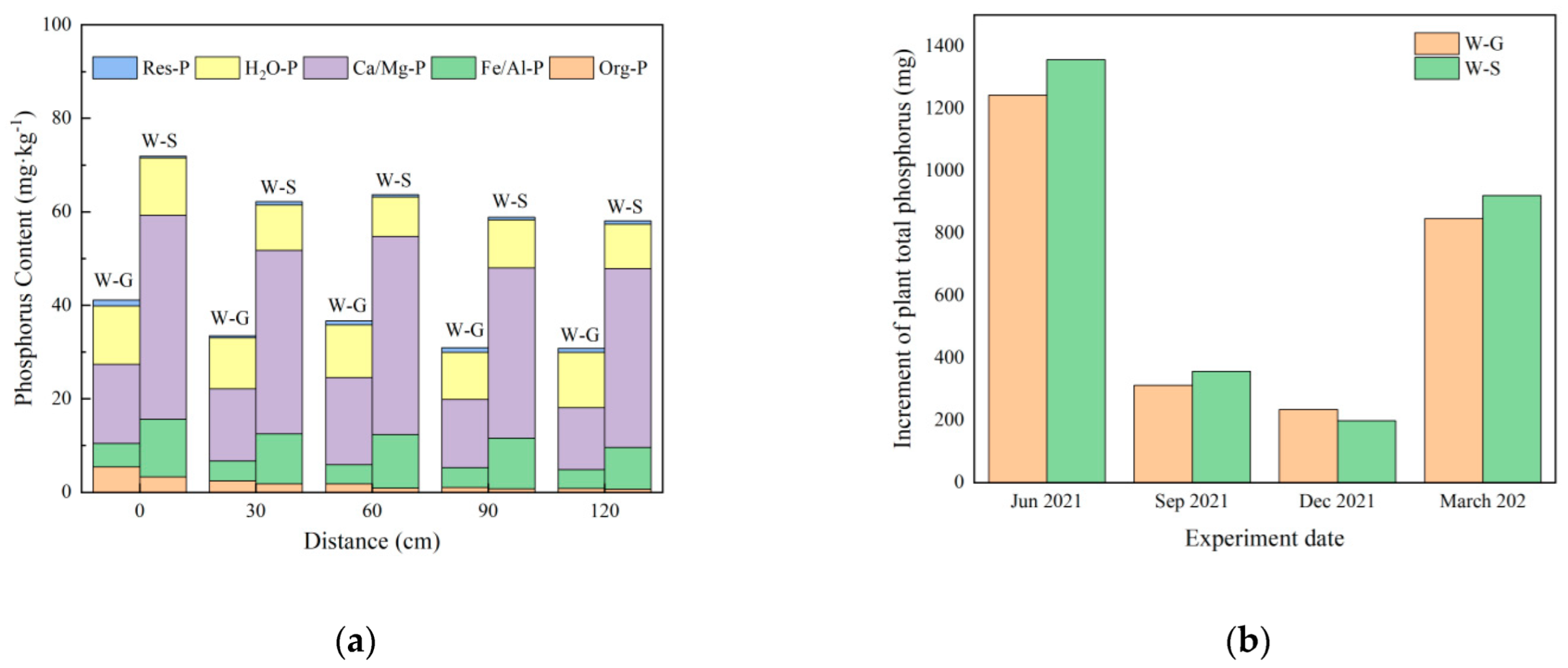
3.5.1. Spatial Distribution and Form of Phosphorus in Substrate
3.5.2. Analysis of Phosphorus Content in Plants
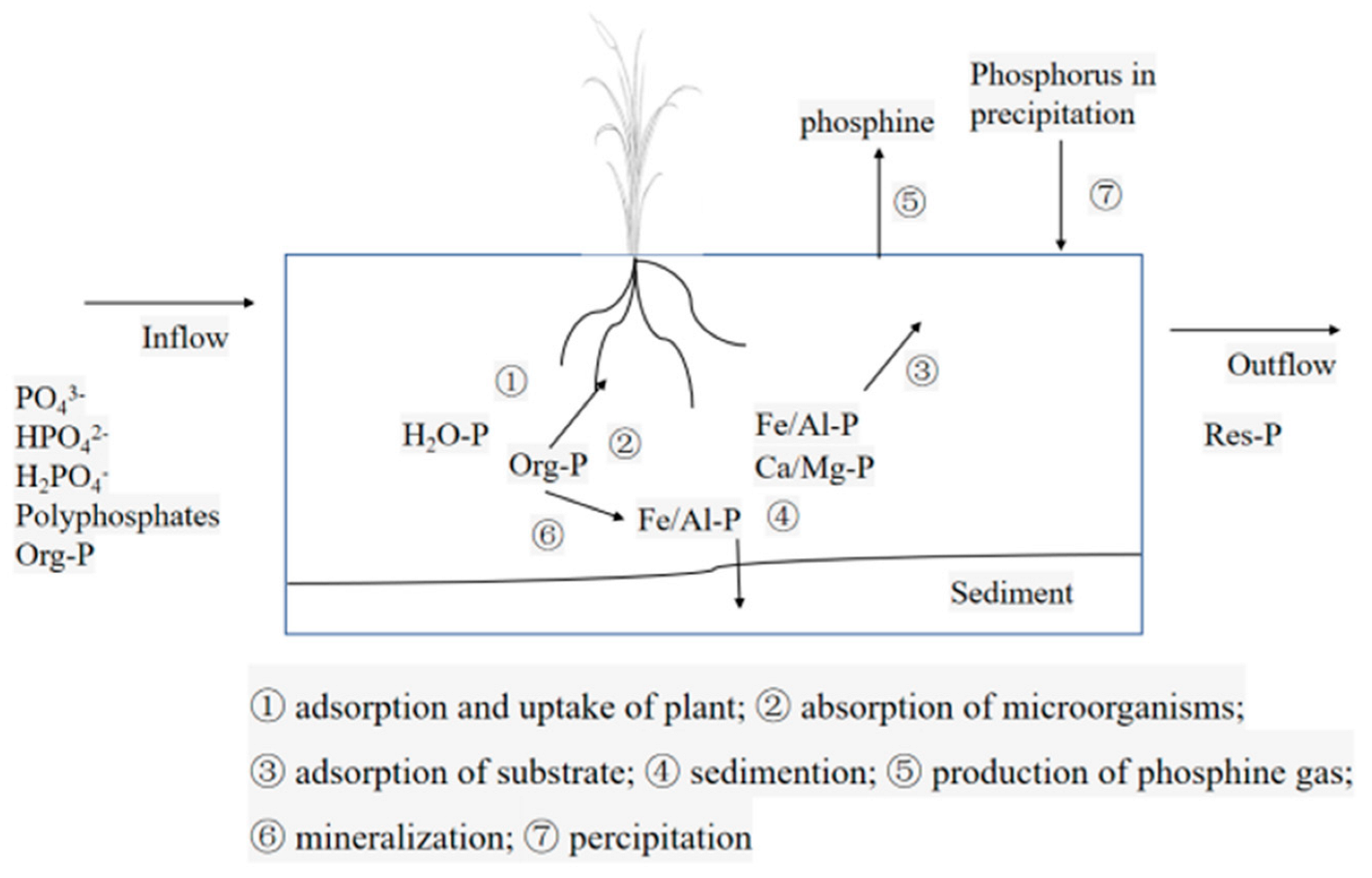
3.5.3. Analysis of Phosphorus Removal Pathway in Constructed Wetland
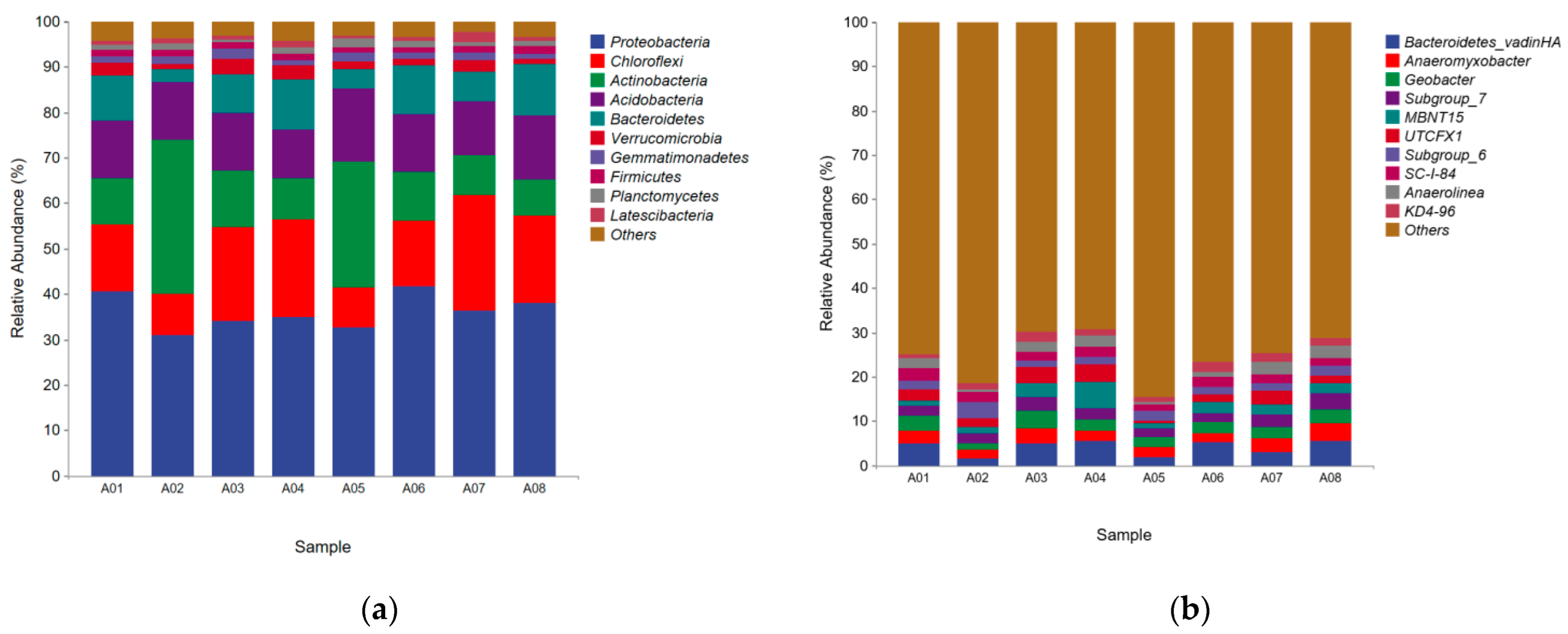
3.5.4. Microbial Community Structure of CW
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Huang, L.J.; Yun, L.J.; Tang, F.; Zhao, J.H.; Liu, Y.Q.; Zeng, X.; Luo, Q.F. Removal of nitrogen, phosphorus, and organic pollutants from water using seeding type immobilized microorganisms. Biomed. Environ. Sci. 2008, 21, 150–156. [Google Scholar] [CrossRef]
- Li, X.Q.; Liu, W.Z.; Wang, A.J.; Gao, X.Y.; Tao, Y.; Ge, X.Y.; Lin, Z. Enhanced treatment of oily ink wastewater using a modified degreaser by nano-Fe3O4/Na2S2O8: Efficient coagulation and sedimentation. J. Water Process Eng. 2022, 47, 102675. [Google Scholar] [CrossRef]
- Mousavi, I.; Darian, J.T.; Mokhtarani, B. Enhanced nitrogen adsorption capacity on Ca2+ ion-exchanged hierarchical X zeolite. Sep. Purif. Technol. 2021, 264, 118442. [Google Scholar] [CrossRef]
- Saabas, D.; Lee, J. Recovery of ammonia from simulated membrane contactor effluent using bipolar membrane electrodialysis. J. Membr. Sci. 2022, 644, 120081. [Google Scholar] [CrossRef]
- Max, W.; Craig, S.C.; Jörg, E.D.; Konrad, K. A proposed nomenclature for biological processes that remove nitrogen. J. Environ. Sci. Water Res. Technol. 2017, 3, 10–17. [Google Scholar]
- Gong, L.; Zhao, X.; Zhu, G. Pathways of Nitrogen and Phosphorus Utilization and Removal from Cyanobacteria Wastewater by Combining Constructed Wetlands with Aerobic Reactors. Sustainability 2022, 14, 8819. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, Y.; Zhang, T.; You, Z.; Shah, K.J.; Kim, H. Application of Reeds as Carbon Source for Enhancing Denitrification of Low C/N Micro-Polluted Water in Vertical-Flow Constructed Wetland. Appl. Sci. 2022, 12, 6756. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, L.; Lu, H.; Lu, S.; Zhao, X.; Liu, F. Effect of the Influent Substrate Concentration on Nitrogen Removal from Summer to Winter in Field Pilot-Scale Multistage Constructed Wetland–Pond Systems for Treating Low-C/N River Water. Sustainability 2021, 13, 12456. [Google Scholar] [CrossRef]
- Xu, M.; Liu, W.J.; Li, C.; Xiao, C.; Ding, L.L.; Xu, K.; Geng, J.J.; Ren, H.Q. Evaluation of the treatment performance and microbial communities of a combined constructed wetland used to treat industrial park wastewater. Environ. Sci. Pollut. Res. 2016, 23, 10990–11001. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.; Zhang, H.; Wu, H. Enhanced nitrogen removal of low C/N domestic wastewater using a biochar-amended aerated vertical flow constructed wetland. Bioresour. Technol. 2017, 241, 269–275. [Google Scholar] [CrossRef]
- Jakubaszek, A. Nitrogen and Phosphorus Accumulation in Horizontal Subsurface Flow Constructed Wetland. Agronomy 2021, 11, 1317. [Google Scholar] [CrossRef]
- Jamwalt, P.; Raj, A.V.; Raveendran, L.; Shirin, S.; Connelly, S.; Yeluripati, J.; Richards, S.; Rao, L.; Helliwell, R.; Tamburini, M. Evaluating the performance of horizontal sub-surface flow constructed wetlands: A case study from southern India. Ecol. Eng. 2021, 162, 106170. [Google Scholar] [CrossRef]
- Dong, S.C.; Ju, S.C.; Hong, J.L.; Heo, J.S. Phosphorus retention capacity of filter media for estimating the longevity of constructed wetland. Water Res. 2005, 39, 2445–2457. [Google Scholar]
- Zhang, Y.; Liu, X.; Fu, C.; Li, X.; Yan, B.; Shi, T. Effect of Fe2+ addition on chemical oxygen demand and nitrogen removal in horizontal subsurface flow constructed wetlands. Chemosphere 2019, 220, 259–265. [Google Scholar] [CrossRef]
- Guo, X.; Cui, X.; Li, H. Effects of fillers combined with biosorbents on nutrient and heavy metal removal from biogas slurry in constructed wetlands. Sci. Total Environ. 2020, 703, 134778. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, J.; Liu, L.; Zhu, G.; Liu, C. Screening of phosphate-removing substrates for use in constructed wetlands treating swine wastewater. Ecol. Eng. 2013, 54, 57–65. [Google Scholar] [CrossRef]
- Wei, L.; Jian, Z.; Zhen, H.; Ji, M.; Yao, G. Phosphorus removal enhancement of magnesium modifiedconstructed wetland microcosm and its mechanism study. Chem. Eng. J. 2018, 335, 209–214. [Google Scholar]
- Marrakchi, F.; Khanday, W.A.; Asif, M.; Hameed, B.H. Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Int. J. Biol. Macromol. 2016, 93, 1231–1239. [Google Scholar] [CrossRef]
- Darder, M.; López-Blanco, M.; Aranda, P.; Antonio, J.A.; Bravo, J.; Ruiz-Hitzky, E. Micro fibrous chitosan sepiolite nanocomposites. Chem. Mater. 2006, 18, 1602–1610. [Google Scholar] [CrossRef]
- Suárez, M.; García-Romero, E. Variability of the surface properties of sepiolite. Appl. Clay Sci. 2012, 67, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Tartaglione, G.; Tabuani, D.; Camino, G. Thermal and morphological characterisation of organically modified sepiolite. Microporous Mesoporous Mater. 2008, 107, 161–168. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Q.; Su, Z.; Sheng, S.; Fu, J. Preparation, optimization, and application of sustainable ceramsite substrate from coal fly ash/waterworks sludge/oyster shell for phosphorus immobilization in constructed wetlands. J. Clean. Prod. 2018, 175, 572–581. [Google Scholar] [CrossRef]
- Song, N.; Hursthouse, A.; McLellan, I.; Wang, Z. Treatment of environmental contamination using sepiolite: Current approaches and future potential. Environ. Geochem. Health 2021, 43, 2679–2697. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Y.; Liu, R.; Morgan, D. Global development of various emerged substrates utilized in constructed wetlands. Bioresour. Technol. 2018, 261, 441–452. [Google Scholar] [CrossRef]
- Ajibade, F.O.; Wang, H.C.; Guadie, A.; Ajibade, T.F.; Fang, Y.K.; Sharif, H.M.A.; Liu, W.Z.; Wang, A.J. Total nitrogen removal in biochar amended non-aerated vertical flow constructed wetlands for secondary wastewater effluent with low C/N ratio: Microbial community structure and dissolved organic carbon release conditions. Bioresour. Technol. 2021, 322, 124430. [Google Scholar] [CrossRef]
- Forbes, M.G.; Dickson, K.L.; Saleh, F.; Waller, W.T.; Doyle, R.D.; Hudak, P. Recovery and Fractionation of Phosphorus Retained by Lightweight Expanded Shale and Masonry Sand Used as Media in Subsurface Flow Treatment Wetlands. Environ. Sci. Technol. 2005, 39, 4621–4627. [Google Scholar] [CrossRef]
- Kong, L.; Tian, Y.; Li, N.; Liu, Y.; Zhang, J.; Zhang, J.; Zuo, W. Highly-effective phosphate removal from aqueous solutions by calcined nano-porous palygorskite matrix with embedded lanthanum hydroxide. Appl. Clay Sci. 2018, 162, 507–517. [Google Scholar] [CrossRef]
- Imre, D.; László, T.; Antonio, F.; János, B.N. The structure of acid treated sepiolites: Small-angle X-ray scattering and multi MAS-NMR investigations. Appl. Clay Sci. 1999, 14, 141–160. [Google Scholar]
- Hong, S.H.; Akem, M.N.; Yoo, S.C.; Lee, C.G.; Park, S.J. Use of calcined sepiolite in removing phosphate from water and returning phosphate to soil as phosphorus fertilizer. J. Environ. Manag. 2020, 270, 110817. [Google Scholar] [CrossRef]
- Lays, B.F.; Tiago, V.; Fernanda, H.T.; Gimenez, J.C.F.; José, A.S.C.; Sandra, A.C. Organically modified sepiolite: Thermal treatment and chemical and morphological properties. Appl. Clay Sci. 2019, 179, 105149. [Google Scholar]
- Jin, X.; Yu, B.; Chen, Z.; Arocena, J.M.; Thring, R.W. Adsorption of Orange II dye in aqueous solution onto surfactant-coated zeolite: Characterization, kinetic and thermodynamic studies. J. Colloid Interface Sci. 2014, 435, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kong, M.; Fan, C. Batch investigations on P immobilization from wastewaters and sediment using natural calcium rich sepiolite as a reactive material. Water Res. 2013, 47, 4247–4258. [Google Scholar] [CrossRef] [PubMed]
- Alshameri, A.; Yan, C.J.; Al-Ani, Y.; Dawood, A.S.; Ibrahim, A.; Zhou, C.Y.; Wang, H.Q. An investigation into the adsorption removal of ammonium by salt activated Chinese (Hulaodu) natural zeolite: Kinetics, isotherms, and thermodynamics. J. Taiwan Inst. Chem. Eng. 2014, 45, 554–564. [Google Scholar] [CrossRef]
- Uğurlu, M.; Karaoğlu, M.H. Adsorption of ammonium from an aqueous solution by fly ash and sepiolite: Isotherm, kinetic and thermodynamic analysis. Microporous Mesoporous Mater. 2011, 139, 73–78. [Google Scholar] [CrossRef]
- Ren, Z.J.; Jia, B.; Zhang, G.M.; Fu, X.L.; Wang, Z.X.; Wang, P.F.; Lv, L.L. Study on adsorption of ammonia nitrogen by iron-loaded activatedcarbon from low temperature wastewater. Chemosphere 2021, 262, 127895. [Google Scholar] [CrossRef]
- Qadiri, R.Z.Z.; Gani, K.M.; Zaid, A.; Aalam, T.; Kazmi, A.A.; Khalil, N. Comparative evaluation of the macrophytes in the constructed wetlands for the treatment of combined wastewater (greywater and septic tank effluent) in a sub-tropical region. Environ. Chall. 2021, 5, 100265. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zhang, W.; Wang, S.; Peng, H. Pollutants removal efficiency assessment of constructed subsurface flow wetlands in lakes with numerical models. J. Hydrol. 2021, 598, 126289. [Google Scholar] [CrossRef]


| Water Quality Index | Inlet Value Range |
|---|---|
| CODCr | 43.2~46.8 mg·L−1 |
| TN | 12.4~14.3 mg·L−1 |
| NH3-N | 3.6~4.2 mg·L−1 |
| TP | 0.6~1.0 mg·L−1 |
| SS | 12.3~23.4 mg·L−1 |
| DO | 6.2~8.3 mg·L−1 |
| pH | 6.5~8.1 |
| Temperature | 15.2~32.4 °C |
| Absorbent | SBET (m2·g−1) | Da (nm) | Vmicro (cm3·g−1) |
|---|---|---|---|
| N-SEP | 12.84 | 7.37 | 0.0083 |
| T-SEP | 30.41 | 12.65 | 0.0209 |
| Models | Parameters | Value |
|---|---|---|
| Pseudo-first order | K1 (min−1) | 0.035 |
| qe (mg·g−1) | 12.776 | |
| R2 | 0.945 | |
| Pseudo-second order | K2 (g·mg−1·min−1) | 0.003 |
| qe (mg·g−1) | 14.15 | |
| R2 | 0.985 | |
| Elovich model | α (g·min−1) | 1.612 |
| β (g·mg−1·min−1) | 0.414 | |
| R2 | 0.974 |
| Isotherm | Parameters | Value |
|---|---|---|
| Langmuir | KL (L·mg−1) | 0.042 |
| qm (mg·g−1) | 19.494 | |
| R2 | 0.989 | |
| Freundlich | KF (L·mg−1) | 1.965 |
| 1/n | 0.442 | |
| R2 | 0.974 | |
| Sips | αs | 0.053 |
| 1/n | 0.782 | |
| qm (mg·g−1) | 23.695 | |
| R2 | 0.993 |
| Phosphorus Removal Way | W-G | W-S | ||
|---|---|---|---|---|
| Quality (mg) | Proportion (%) | Quality (mg) | Proportion (%) | |
| Phosphorus removal by substrate | 8316 | 67.2 | 14,385 | 78.2 |
| Phosphorus removal by plant | 2636 | 21.3 | 2833 | 15.4 |
| Phosphorus removal by other means | 1423 | 11.5 | 1177 | 6.4 |
| Total phosphorus removal | 12,375 | 100 | 18,396 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Zhang, C. Study on Phosphorus Removal Pathway in Constructed Wetlands with Thermally Modified Sepiolite. Sustainability 2022, 14, 12535. https://doi.org/10.3390/su141912535
Gao P, Zhang C. Study on Phosphorus Removal Pathway in Constructed Wetlands with Thermally Modified Sepiolite. Sustainability. 2022; 14(19):12535. https://doi.org/10.3390/su141912535
Chicago/Turabian StyleGao, Pan, and Chao Zhang. 2022. "Study on Phosphorus Removal Pathway in Constructed Wetlands with Thermally Modified Sepiolite" Sustainability 14, no. 19: 12535. https://doi.org/10.3390/su141912535




