Free and Encapsulated Phosphate-Solubilizing Bacteria for the Enhanced Dissolution of Swine Wastewater-Derived Struvite—An Attractive Approach for Green Phosphorus Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Cultures
2.2. Screening of Bacterial Capabilities for Struvite and Hydroxyapatite Dissolution
2.3. Struvite and Hydroxyapatite Dissolution by B. megaterium (TBRC 1396)
2.4. Struvite Precipitation from Swine Wastewater and Its Characterization
2.5. Dissolution of Swine Wastewater-Derived Struvite Using B. megaterium (TBRC 1396)
2.6. Applications of Free Cells and Encapsulated Cells for the Dissolution of Swine Wastewater-Derived Struvite
3. Results and Discussion
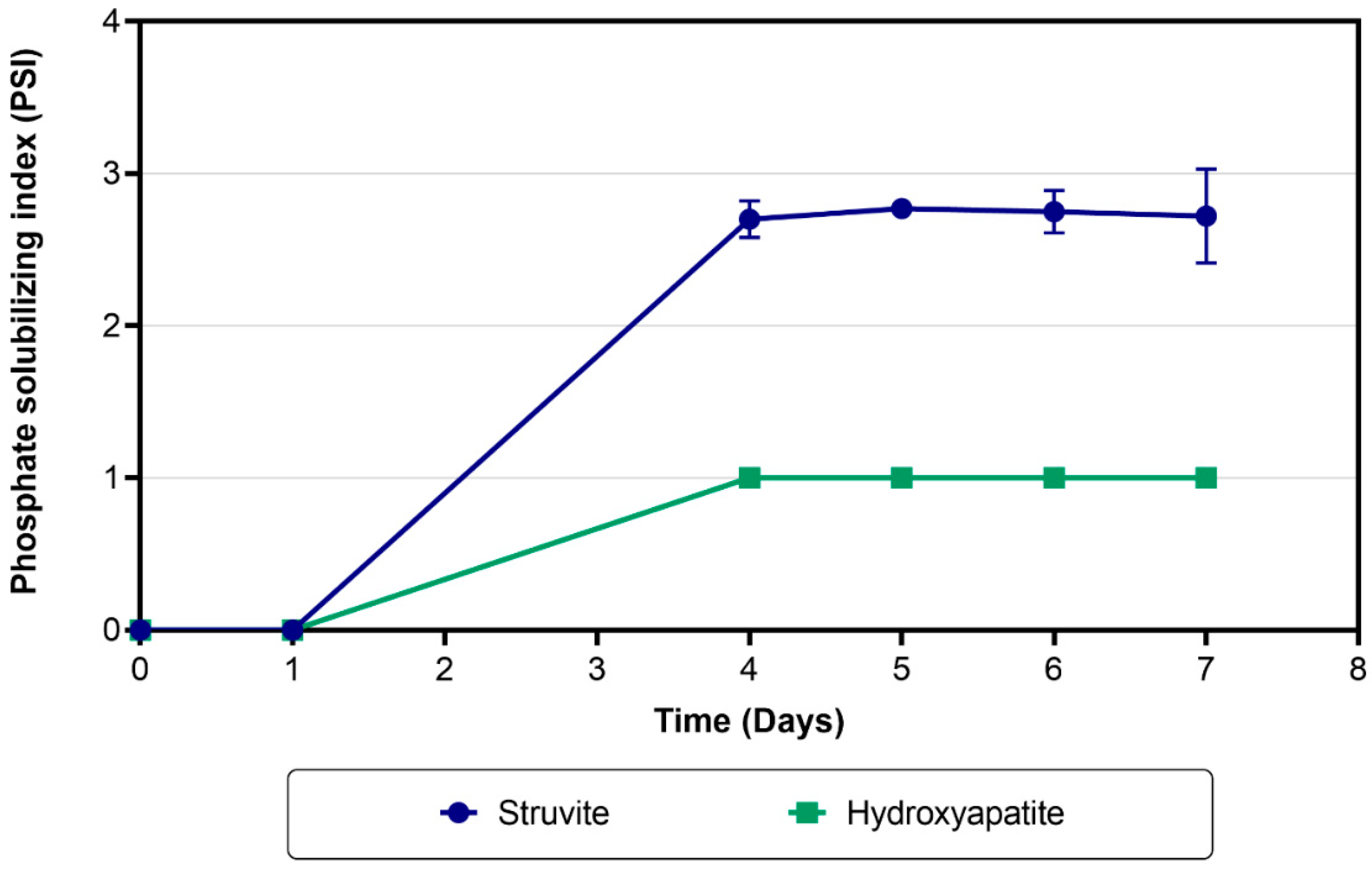
3.1. Screening of Bacterial Capabilities for Struvite and Hydroxyapatite Dissolution
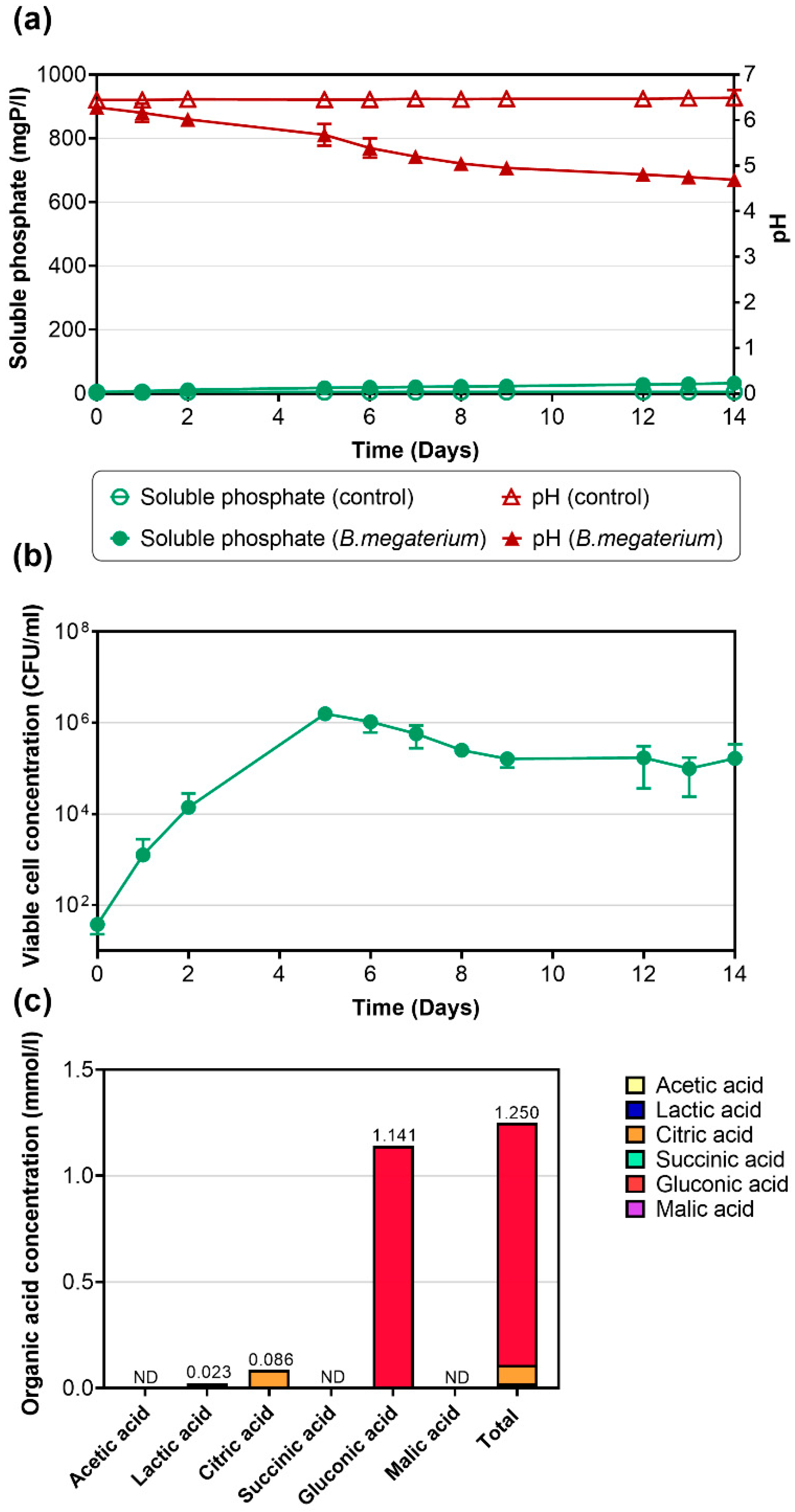
3.2. Struvite Dissolution by B. megaterium (TBRC 1396)
3.3. Hydroxyapatite Dissolution by B. megaterium (TBRC 1396)
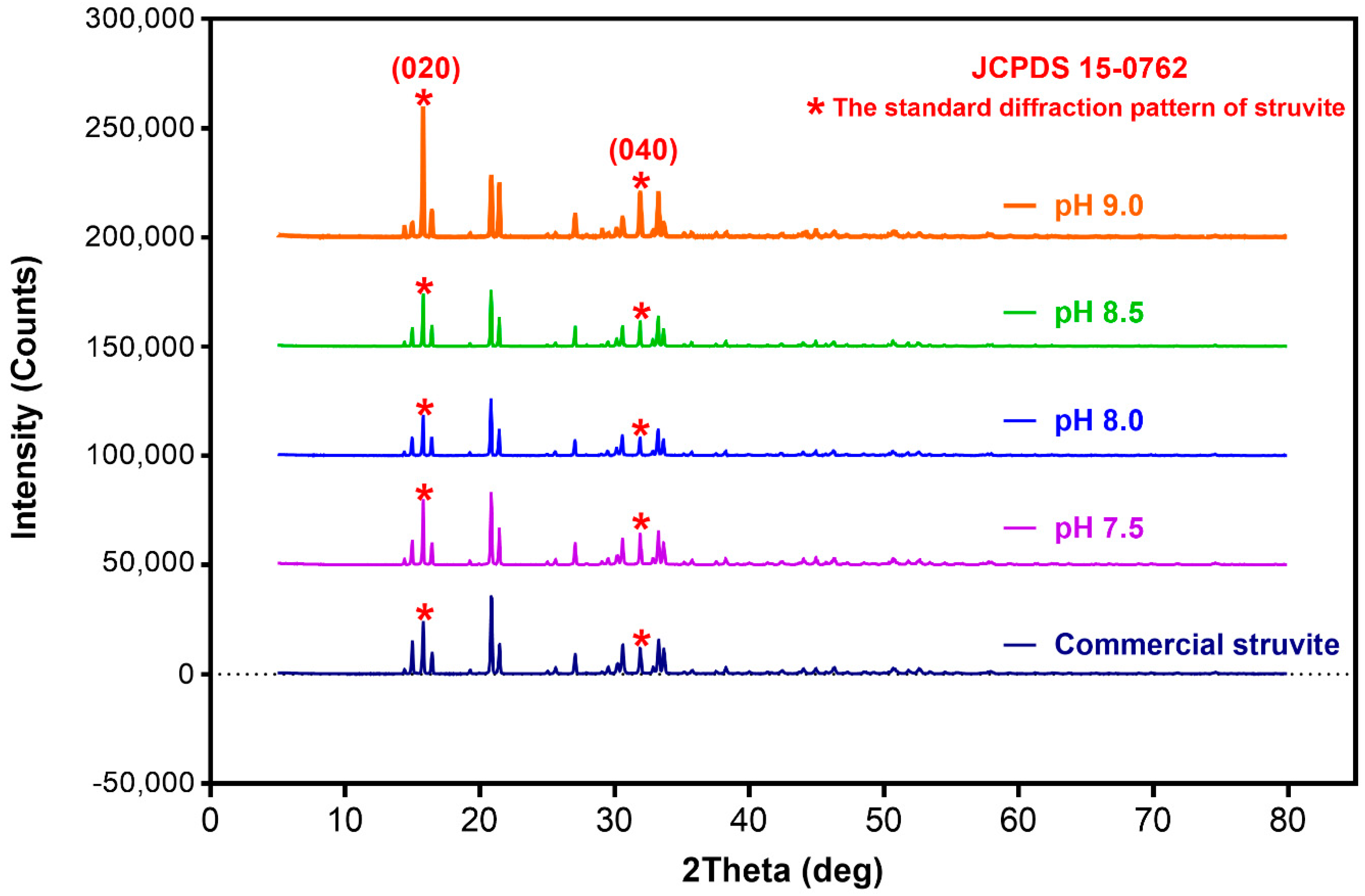
3.4. Struvite Precipitation from Swine Wastewater and Its Characterization
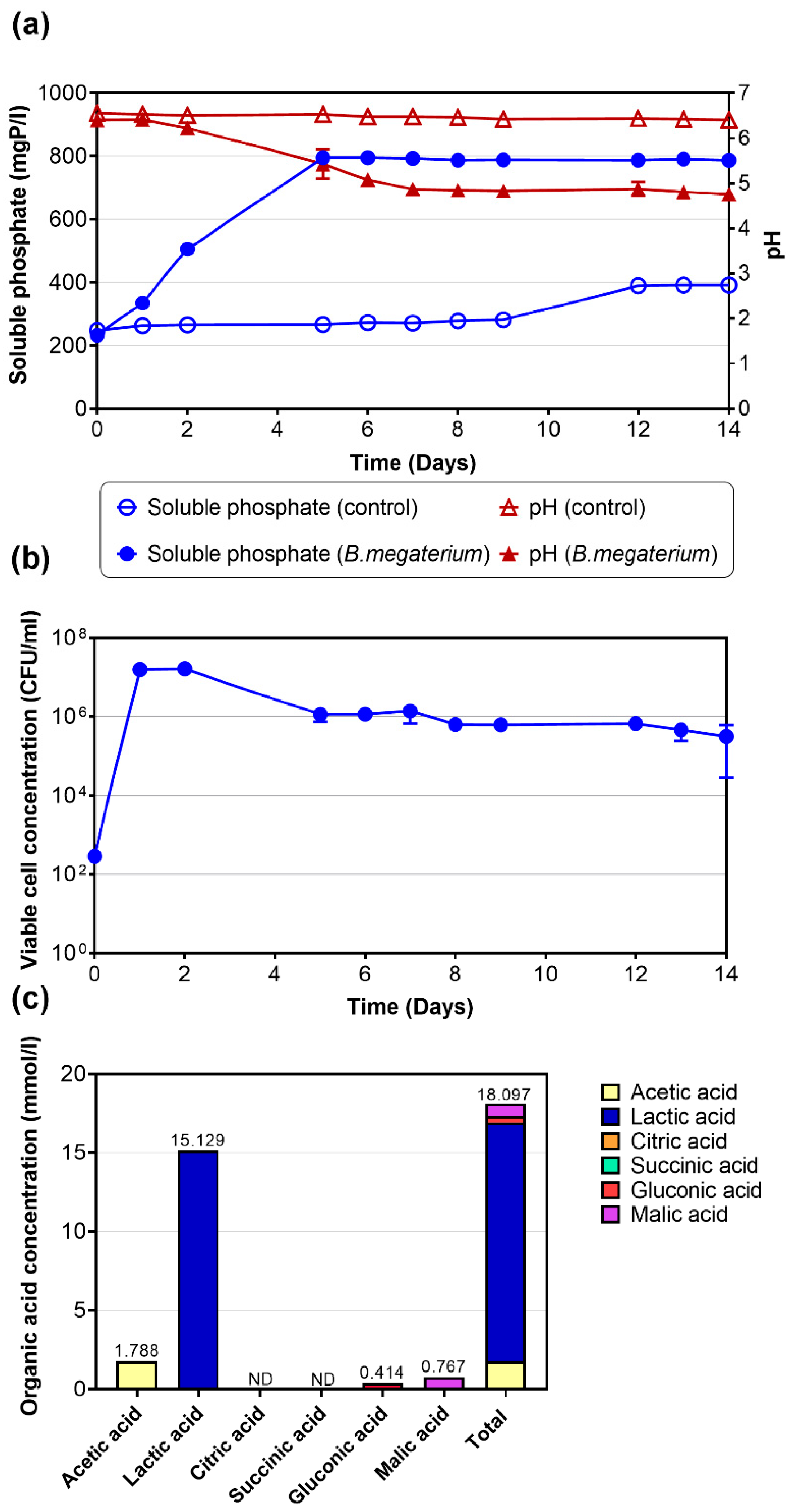
3.5. Dissolution of Swine Wastewater-Derived Struvite Using B. megaterium (TBRC 1396)
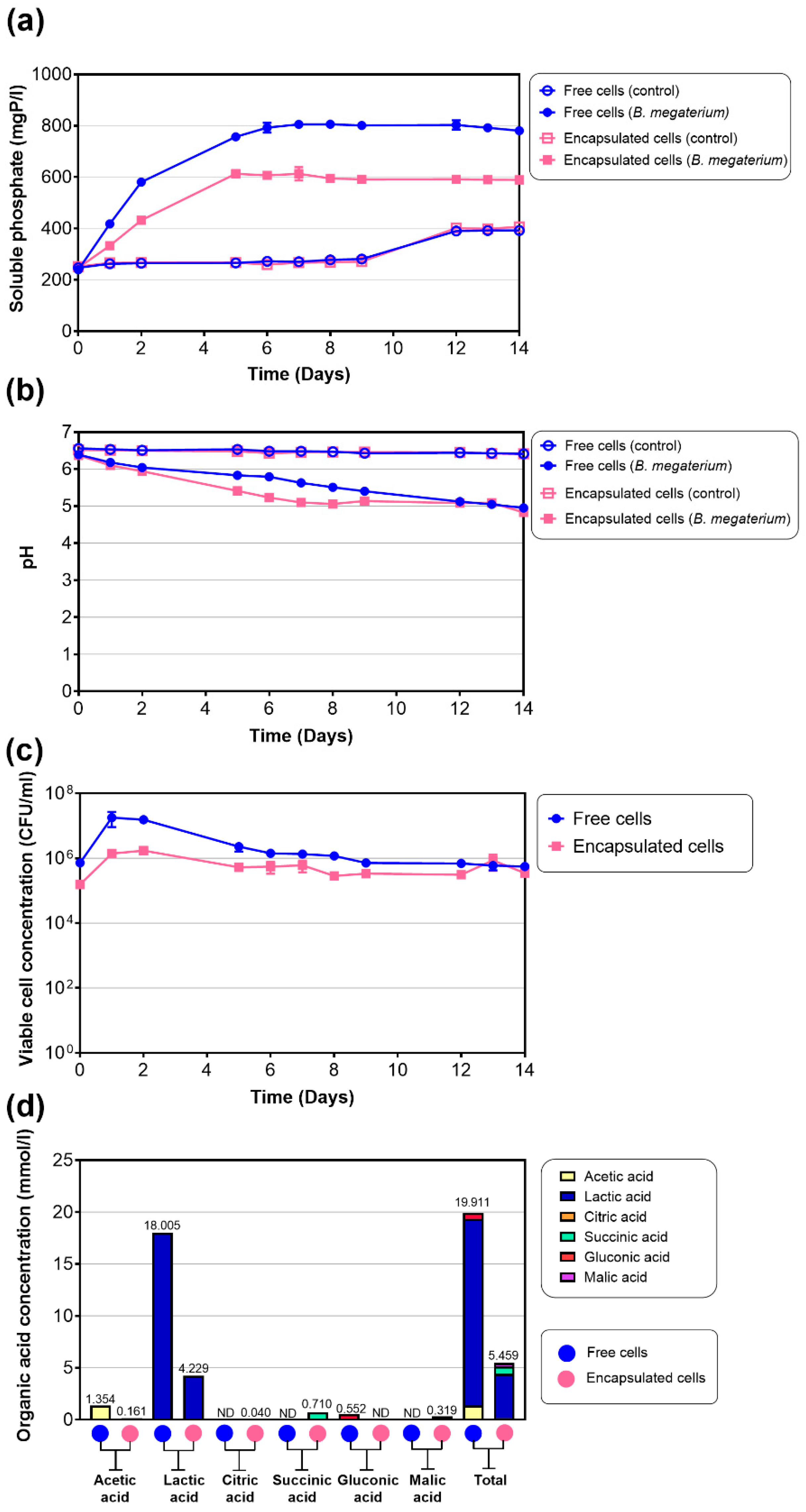
3.6. Applications of Free Cells and Encapsulated Cells for the Dissolution of Swine Wastewater-Derived Struvite
3.7. Implications and Future Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Tan, X.; Zhu, H.; Sun, T.; Wang, X. Effects of commonly occurring metal ions on hydroxyapatite crystallization for phosphorus recovery from wastewater. Water 2018, 10, 1619. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Boiarkina, I.; Young, B.; Yu, W.; Singhal, N. Prediction of future phosphate rock: A demand-based model. J. Environ. Inform. 2018, 31, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Perera, M.K.; Englehardt, J.D.; Dvorak, A.C. Technologies for recovering nutrients from wastewater: A critical review. Environ. Eng. Sci. 2019, 36, 511–529. [Google Scholar] [CrossRef]
- Cichy, B.; Kuzdzal, E.; Krzton, H. Phosphorus recovery from acidic wastewater by hydroxyapatite precipitation. J. Environ. Manage. 2019, 232, 421–427. [Google Scholar] [CrossRef]
- Dai, H.; Lu, X.; Peng, Y.; Zou, H.; Shi, J. An efficient approach for phosphorus recovery from wastewater using series-coupled air-agitated crystallization reactors. Chemosphere 2016, 165, 211–220. [Google Scholar] [CrossRef]
- Degryse, F.; Baird, R.; da Silva, R.C.; McLaughlin, M.J. Dissolution rate and agronomic effectiveness of struvite fertilizers—Effect of soil pH, granulation and base excess. Plant Soil. 2017, 410, 139–152. [Google Scholar] [CrossRef]
- Uysal, A.; Yilmazel, Y.D.; Demirer, G.N. The determination of fertilizer quality of the formed struvite from effluent of a sewage sludge anaerobic digester. J. Hazard. Mater. 2010, 181, 248–254. [Google Scholar] [CrossRef]
- Cabeza, R.; Steingrobe, B.; Römer, W.; Claassen, N. Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr. Cycl. Agroecosyst. 2011, 91, 173–184. [Google Scholar] [CrossRef]
- Muys, M.; Phukan, R.; Brader, G.; Samad, A.; Moretti, M.; Haiden, B.; Pluchon, S.; Roest, K.; Vlaeminck, S.E.; Spiller, M. A systematic comparison of commercially produced struvite: Quantities, qualities and soil-maize phosphorus availability. Sci. Total Environ. 2021, 756, 143726. [Google Scholar] [CrossRef]
- Chen, Y.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil. Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Latif, A.; Jilani, G.; Hayat, R.; Khan, A.A.; Azeem, M.; Ehsan, M.; Mubarak, M.U. Isolation, characterization of PSB stains from rock phosphate and their potential as biofertilizer. Int. J. Biosci. 2017, 10, 72–80. [Google Scholar]
- Rodriguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Jha, A.; Saxena, J.; Sharma, V. Investigation on phosphate solubilization potential of agricultural soil bacteria as affected by different phosphorus sources, temperature, salt, and pH. Commun. Soil Sci. Plant. Anal. 2013, 44, 2443–2458. [Google Scholar] [CrossRef]
- Yu., X.; Liu, X.; Zhu, T.H.; Liu, G.H.; Mao, C. Isolation and characterization of phosphate-solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization. Biol. Fertil. Soils. 2011, 47, 437–446. [Google Scholar]
- Nosrati, R.; Owlia, P.; Saderi, H.; Rasooli, I.; Ali Malboobi, M. Phosphate solubilization characteristics of efficient nitrogen fixing soil Azotobacter strains. Iran. J. Microbiol. 2014, 6, 285–295. [Google Scholar]
- Schoebitz, M.; López, M.D.; Antonio Roldán, A. Bioencapsulation of microbial inoculants for better soil–plant fertilization. A review. Agron. Sustain. Dev. 2013, 33, 751–765. [Google Scholar]
- Hernández Jiménez, J.E.; Nyiraneza, J.; Fraser, T.D.; Brown, H.C.P.; Lopez-Sanchez, I.J.; Botero-Botero, L.R. Enhancing phosphorus release from struvite with biostimulants. Can. J. Soil Sci. 2021, 101, 22–32. [Google Scholar] [CrossRef]
- Bashan, Y.; Kamnev, A.A.; de-Bashan, L.E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biol. Fertil. Soils. 2013, 49, 465–479. [Google Scholar]
- Reyes, I.; Valery, A.; Valduz, Z. Phosphate-solubilizing microorganisms isolated from rhizospheric and bulk soils of colonizer plants at an abandoned rock phosphate mine. Plant Soil. 2006, 287, 69–75. [Google Scholar] [CrossRef]
- Ben Farhat, M.; Farhat, A.; Bejar, W.; Kammoun, R.; Bouchaala, K.; Fourati, A.; Antoun, H.; Bejar, S.; Chouayekh, H. Characterization of the mineral phosphate solubilizing activity of Serratia marcescens CTM 50650 isolated from the phosphate mine of Gafsa. Arch. Microbiol. 2009, 191, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; McDonald, G.A.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils. 1997, 24, 347–352. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.-O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar]
- Gao, L.; Kong, F.; Feng, C.; Wang, J.; Gao, J.; Shen, G.; Zhang, C. Isolation, characterization, and growth promotion of phosphate-solubilizing bacteria associated with Nicotiana Tabacum (Tobacco). Pol. J. Environ. Stud. 2016, 25, 993–1003. [Google Scholar] [CrossRef] [Green Version]
- El-Brady, M.A.; Elbarbary, T.A.; Ibrahim, I.A.; Abdel-Fatah, Y.M. Azotobacter vinelandii evaluation and optimization of Abu Tartur Egyptian phosphate ore dissolution. Saudi J. Pathol. Microbiol. 2016, 1, 80–93. [Google Scholar]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Goteti, P.K.; Desai, S.; Emmanuel, L.D.A.; Taduri, M.; Sultana, U. Phosphate solubilization potential of fluorescent Pseudomonas spp. isolated from diverse agro-ecosystems of India. Int. J. Soil Sci. 2014, 9, 101–110. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Hashidoko, Y.; Santoso, E.; Tahara, S. Potent phosphate-solubilizing bacteria isolated from dipterocarps grown in peat swamp forest in Central Kalimantan and their possible utilization for biorehabilitation of degraded peatland. In Proceedings of the International Symposium and Workshop on Tropical Peatland, Yogyakarta, Indonesia, 27–29 August 2007; pp. 27–29. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Joe, M.M.; Deivaraj, S.; Benson, A.; Henry, A.J.; Narendrakumar, G. Soil extract calcium phosphate media for screening of phosphate-solubilizing bacteria. Agric. Nat. Resour. 2018, 52, 305–308. [Google Scholar] [CrossRef]
- Baird, R.B. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Water Environment Federation; American Public Health Association; American Water Works Association: Denver, CO, USA, 2017. [Google Scholar]
- Rahman, M.; Salleh, A.; Rashid, U.; Ahsan, A.; Hossain, M.; Ra, C. Production of slow-release crystal fertilizer from wastewaters through struvite crystallization—A Review. Arab. J. Chem. 2014, 7, 139–155. [Google Scholar]
- Bower, C.E.; Holm-Hansen, T. A salicylate-hypochlorite method for determining ammonia in seawater. Can. J. Fish. Aquat. Sci. 1980, 37, 794–798. [Google Scholar] [CrossRef]
- Afzaal, M.; Khan, A.U.; Saeed, F.; Ahmed, A.; Ahmad, M.H.; Maan, A.A.; Tufail, T.; Anjum, F.M.; Hussain, S. Functional exploration of free and encapsulated probiotic bacteria in yogurt and simulated gastrointestinal conditions. Food Sci. Nutr. 2019, 7, 3931–3940. [Google Scholar] [PubMed] [Green Version]
- Marra, L.M.; de Oliveira-Longatti, S.M.; Soares, C.R.F.S.; de Lima, J.M.; Olivares, F.L.; Moreira, F.M.S. Initial pH of medium affects organic acids production but do not affect phosphate solubilization. Braz. J. Microbiol. 2015, 46, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J. Struvite: A slow-release fertilizer for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [PubMed] [Green Version]
- Chen, W.; Yang, F.; Zhang, L.; Wang, J. Organic acid secretion and phosphate solubilizing efficiency of Pseudomonas sp. PSB12: Effects of phosphorus forms and carbon sources. Geomicrobiol. J. 2016, 33, 870–877. [Google Scholar] [CrossRef]
- Illmer, P.; Schinner, F. Solubilization of inorganic calcium phosphates—Solubilization mechanisms. Soil Biol. Biochem. 1995, 27, 257–263. [Google Scholar] [CrossRef]
- Prabhu, N.; Borkar, S.; Garg, S. Chapter 11—Phosphate solubilization by microorganisms: Overview, mechanisms, applications and advances. In Advances in Biological Science Research: A Practical Approach; Meena, S.N., Naik, M.M., Eds.; Academic Press: London, UK, 2019; pp. 161–176. [Google Scholar]
- Ali, M.I.; Schneider, P.A.; Hudson, N. Thermodynamics and solution chemistry of struvite. J. Indian Inst. Sci. 2005, 85, 141–149. [Google Scholar]
- Shim, S.; Won, S.; Reza, A.; Kim, S.; Ahmed, N.; Ra, C. Design and optimization of fluidized bed reactor operating conditions for struvite recovery process from Swine Wastewater. Processes 2020, 8, 422. [Google Scholar]
- Tarrago, E.; Puig, S.; Ruscalleda, M.; Balaguer, M.D.; Colprim, J. Controlling struvite particles’ size using the up-flow velocity. Chem. Eng. J. 2016, 302, 819–827. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-M.; Radhakrishnan, R.; You, Y.-H.; Joo, G.-J.; Lee, I.-J.; Lee, K.-E.; Kim, J.-H. Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J. Microbiol. 2014, 54, 427–433. [Google Scholar] [CrossRef]
- Yan, Z.; Zheng, X.-W.; Chen, J.-Y.; Han, J.-S.; Han, B.-Z. Effect of different Bacillus strains on the profile of organic acids in a liquid culture of Daqu. J. Inst. Brew. 2019, 119, 78–83. [Google Scholar] [CrossRef]
- Bolan, N.S.; Naidu, R.; Mahimairaja, S.; Baskaran, S. Influence of low-molecular-weight organic acids on the solubilization of phosphates. Biol. Fertil. Soils. 1994, 18, 311–319. [Google Scholar] [CrossRef]
- Chien, M.; Nakahata, R.; Ono, T.; Miyauchi, K.; Endo, G. Mercury removal and recovery by immobilized Bacillus megaterium MB1. Front. Chem. Sci. Eng. 2012, 6, 192–197. [Google Scholar] [CrossRef]
- Wiwattanapatapee, R.; Chumthong, A.; Pengnoo, A.; Kanjanamaneesathian, M. Preparation and evaluation of Bacillus megaterium-alginate microcapsules for control of rice sheath blight disease. World J. Microbiol. Biotechnol. 2013, 8, 1487–1497. [Google Scholar]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jokkaew, S.; Jantharadej, K.; Pokhum, C.; Chawengkijwanich, C.; Suwannasilp, B.B. Free and Encapsulated Phosphate-Solubilizing Bacteria for the Enhanced Dissolution of Swine Wastewater-Derived Struvite—An Attractive Approach for Green Phosphorus Fertilizer. Sustainability 2022, 14, 12627. https://doi.org/10.3390/su141912627
Jokkaew S, Jantharadej K, Pokhum C, Chawengkijwanich C, Suwannasilp BB. Free and Encapsulated Phosphate-Solubilizing Bacteria for the Enhanced Dissolution of Swine Wastewater-Derived Struvite—An Attractive Approach for Green Phosphorus Fertilizer. Sustainability. 2022; 14(19):12627. https://doi.org/10.3390/su141912627
Chicago/Turabian StyleJokkaew, Suphatsorn, Krittayapong Jantharadej, Chonlada Pokhum, Chamorn Chawengkijwanich, and Benjaporn Boonchayaanant Suwannasilp. 2022. "Free and Encapsulated Phosphate-Solubilizing Bacteria for the Enhanced Dissolution of Swine Wastewater-Derived Struvite—An Attractive Approach for Green Phosphorus Fertilizer" Sustainability 14, no. 19: 12627. https://doi.org/10.3390/su141912627






