Influence of Seed Soaking and Foliar Application Using Ozonated Water on Two Sweet Pepper Hybrids under Cold Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seed Soaking in Ozonated Water
2.3. Foliar Application Using Ozonated Water
2.4. Germination and Seedling Parameters
2.5. Physiological Parameters
2.6. Data Analysis
3. Results
3.1. Germination Parameters
3.2. Fresh and Dry Weight
3.3. Seedling Measurements
3.4. Physiological Parameters
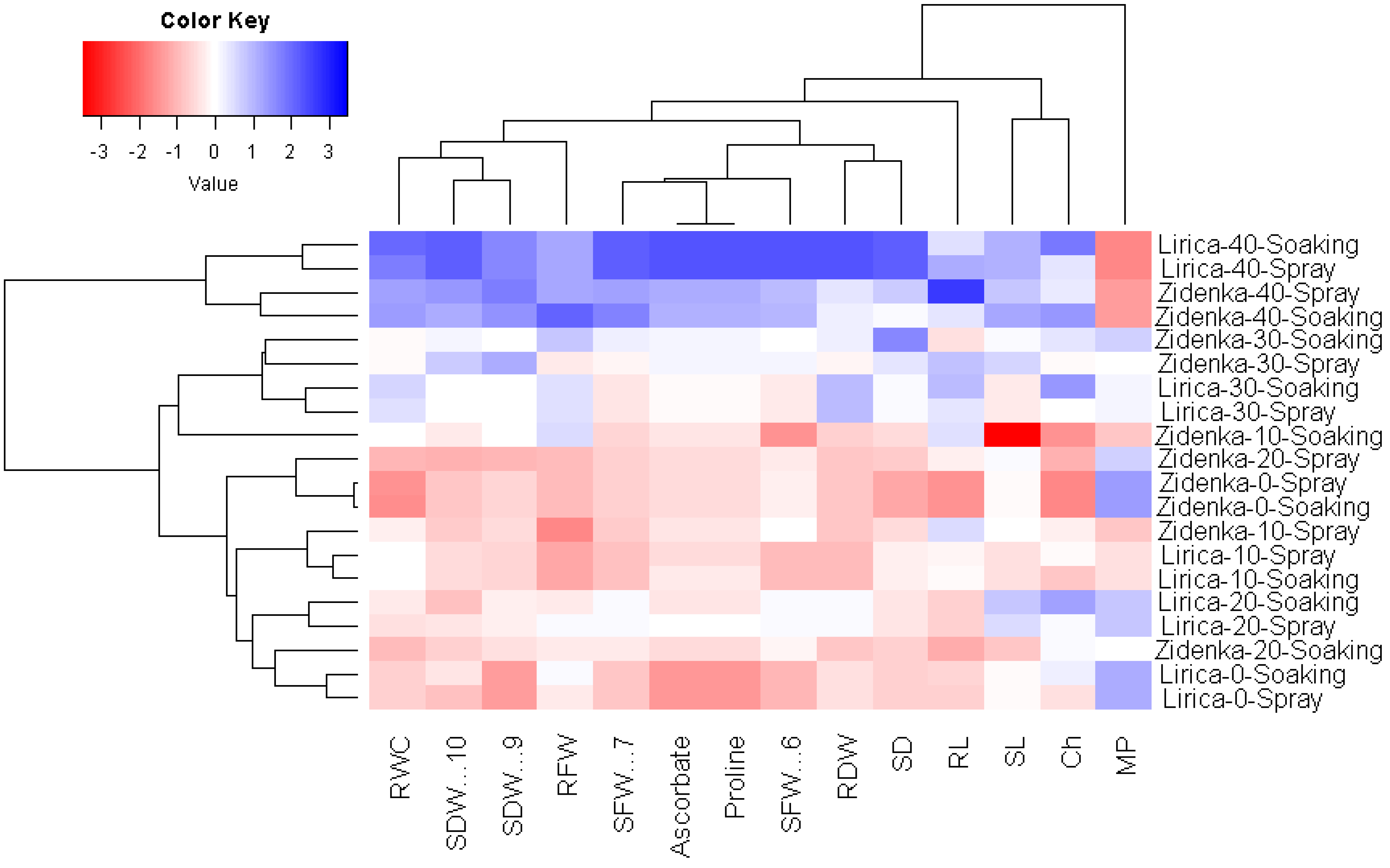
3.5. Interrelationship among the Evaluated Treatments and Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Zou, C.; Zhang, Y.; Shi, X.; Liu, J.; Fan, S.; Liu, Y.; Du, Y.; Zhao, Q.; Tan, Y.; et al. Environmental impacts of pepper (Capsicum annuum L) production affected by nutrient management: A case study in southwest China. J. Clean. Prod. 2018, 171, 934–943. [Google Scholar] [CrossRef]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. Food Sci. Technol. 2007, 40, 121–129. [Google Scholar] [CrossRef]
- Lahbib, K.; Dabbou, S.; Bnejdi, F.; Pandino, G.; Lombardo, S.; El Gazzah, M.; El Bok, S. Agro-morphological, biochemical and antioxidant characterization of a tunisian chili pepper germplasm collection. Agriculture 2021, 11, 1236. [Google Scholar] [CrossRef]
- Gracia, M.P.; Mansour, E.; Casas, A.M.; Lasa, J.M.; Medina, B.; Molina-Cano, J.L.; Moralejo, M.A.; López, A.; López-Fuster, P.; Escribano, J.; et al. Progress in the Spanish national barley breeding program. Span. J. Agric. Res. 2012, 10, 741. [Google Scholar] [CrossRef] [Green Version]
- Kamara, M.M.; Ghazy, N.A.; Mansour, E.; Elsharkawy, M.M.; Kheir, A.M.S.; Ibrahim, K.M. Molecular genetic diversity and line × tester analysis for resistance to late wilt disease and grain yield in maize. Agronomy 2021, 11, 898. [Google Scholar] [CrossRef]
- Moustafa, E.; El-Sobky, E.-S.; Farag, H.; Yasin, M.; Attia, A.; Rady, M.; Awad, M.; Mansour, E. Sowing date and genotype influence on yield and quality of dual-purpose barley in a salt-affected arid region. Agronomy 2021, 11, 717. [Google Scholar] [CrossRef]
- Moustafa, E.S.A.; Ali, M.M.A.; Kamara, M.M.; Awad, M.F.; Hassanin, A.A.; Mansour, E. Field screening of wheat advanced lines for salinity tolerance. Agronomy 2021, 11, 281. [Google Scholar] [CrossRef]
- Kamara, M.M.; Ibrahim, K.M.; Mansour, E.; Kheir, A.M.S.; Germoush, M.O.; Abd El-Moneim, D.; Motawei, M.I.; Alhusays, A.Y.; Farid, M.A.; Rehan, M. Combining ability and gene action controlling grain yield and its related traits in bread wheat under heat stress and normal conditions. Agronomy 2021, 11, 1450. [Google Scholar] [CrossRef]
- Mansour, E.; Moustafa, E.S.A.; Qabil, N.; Abdelsalam, A.; Wafa, H.A.; Kenawy, A.E.; Casas, A.M.; Igartua, E. Assessing different barley growth habits under Egyptian conditions for enhancing resilience to climate change. Field Crops Res. 2018, 224, 67–75. [Google Scholar] [CrossRef]
- Omar, M.; Rabie, H.A.; Mowafi, S.A.; Othman, H.T.; El-Moneim, D.A.; Alharbi, K.; Mansour, E.; Ali, M.M.A. Multivariate analysis of agronomic traits in newly developed maize hybrids grown under different agro-environments. Plants 2022, 11, 1187. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; Elrys, A.S.; Mansour, E.; Eid, R.S.M.; Selem, E.; Rady, M.M.; Ali, E.F.; Mersal, G.A.M.; Semida, W.M. Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci. Hortic. 2021, 288, 110340. [Google Scholar] [CrossRef]
- El-Mageed, T.A.; Belal, E.; Rady, M.; El-Mageed, S.A.; Mansour, E.; Awad, M.; Semida, W. Acidified biochar as a soil amendment to drought stressed (Vicia faba L.) plants: Influences on growth and productivity, nutrient status, and water use efficiency. Agronomy 2021, 11, 1290. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Bouw, W. Pepper seed treatment for low-temperature germination. Can. J. Plant Sci. Can. J. Plant Sci. 1984, 64, 387–393. [Google Scholar] [CrossRef]
- Yang, S.; Tang, X.-F.; Ma, N.-N.; Wang, L.-Y.; Meng, Q.-W. Heterology expression of the sweet pepper CBF3 gene confers elevated tolerance to chilling stress in transgenic tobacco. J. Plant Physiol. 2011, 168, 1804–1812. [Google Scholar] [CrossRef]
- El-Aidy, F.; Sharaf-Eldin, M. Modifying microclimatic conditions in plastic walk-in tunnels through solar energy system for improving yield and quality of four sweet pepper hybrids. Plasticulture 2015, 134, 6–22. [Google Scholar]
- Zhang, L.; Hao, X.; Li, Y.; Jiang, G. Response of greenhouse tomato to varied low pre-night temperatures at the same daily integrated temperature. HortScience 2010, 45, 1654–1661. [Google Scholar] [CrossRef] [Green Version]
- Ensminger, I.; Busch, F.; Huner, N.P. Photostasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- El-Sanatawy, A.; Ash-Shormillesy, S.; Qabil, N.; Awad, M.; Mansour, E. Seed halo-priming improves seedling vigor, grain yield, and water use efficiency of maize under varying irrigation regimes. Water 2021, 13, 2115. [Google Scholar] [CrossRef]
- Raj, A.B.; Raj, S.K. Seed priming: An approach towards agricultural sustainability. J. Appl. Nat. Sci. 2019, 11, 227–234. [Google Scholar] [CrossRef]
- El-Sanatawy, A.; El-Kholy, A.; Ali, M.; Awad, M.; Mansour, E. Maize seedling establishment, grain yield and crop water productivity response to seed priming and irrigation management in a Mediterranean arid environment. Agronomy 2021, 11, 756. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M. Pre-sowing seed treatment—A shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- Desoky, E.-S.M.; Mansour, E.; Ali, M.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; Rady, M.M.; Ali, E.F. Exogenously used 24-epibrassinolide promotes drought tolerance in maize hybrids by improving plant and water productivity in an arid environment. Plants 2021, 10, 354. [Google Scholar] [CrossRef]
- ElShamey, E.A.Z.; Hamad, H.S.; Alshallash, K.S.; Alghuthaymi, M.A.; Ghazy, M.I.; Sakran, R.M.; Selim, M.E.; ElSayed, M.A.A.; Abdelmegeed, T.M.; Okasha, S.A.; et al. Growth regulators improve outcrossing rate of diverse rice cytoplasmic male sterile lines through affecting floral traits. Plants 2022, 11, 1291. [Google Scholar] [CrossRef]
- Desoky, E.-S.; Merwad, A.-R.; El-Maati, M.A.; Mansour, E.; Arnaout, S.; Awad, M.; Ramadan, M.; Ibrahim, S. Physiological and biochemical mechanisms of exogenously applied selenium for alleviating destructive impacts induced by salinity stress in bread wheat. Agronomy 2021, 11, 926. [Google Scholar] [CrossRef]
- Swailam, M.A.; Mowafy, S.A.E.; El-Naggar, N.Z.A.; Mansour, E. Agronomic responses of diverse bread wheat genotypes to phosphorus levels and nitrogen forms in a semiarid environment. Sabrao J. Breed. Genet. 2021, 53, 592–608. [Google Scholar] [CrossRef]
- El-Hady, A.; Mohamed, A.; Abd-Elkrem, Y.M.; Rady, M.O.; Mansour, E.; El-Tarabily, K.A.; AbuQamar, S.F.; El-Temsah, M.E. Impact on plant productivity under low fertility sandy soil in arid environment by revitalization of lentil roots. Front. Plant Sci. 2022, 13, 937073. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Mohamed, A.H.; Rafudeen, M.S.; Omar, A.A.; Awad, M.F.; Mansour, E. Polyamines mitigate the destructive impacts of salinity stress by enhancing photosynthetic capacity, antioxidant defense system and upregulation of Calvin cycle-related genes in rapeseed (Brassica napus L.). Saudi J. Biol. Sci. 2022, 29, 3675–3686. [Google Scholar] [CrossRef]
- Kelly, F.J.; Mudway, I.; Krishna, M.; Holgate, S. The free radical basis of air pollution: Focus on ozone. Respir. Med. 1995, 89, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Flowers, M.D.; Fiscus, E.L.; Burkey, K.O.; Booker, F.L.; Dubois, J.-J.B. Photosynthesis, chlorophyll fluorescence, and yield of snap bean (Phaseolus vulgaris L.) genotypes differing in sensitivity to ozone. Environ. Exp. Bot. 2007, 61, 190–198. [Google Scholar] [CrossRef]
- Dizengremel, P. Effects of ozone on the carbon metabolism of forest trees. Plant Physiol. Biochem. 2001, 39, 729–742. [Google Scholar] [CrossRef]
- Nagendra-Prasad, D.; Sudhakar, N.; Murugesan, K.; Mohan, N. Pre-exposure of calli to ozone promotes tolerance of regenerated Lycopersicon esculentum cv. PKM1 plantlets against acute ozone stress. J. Plant Physiol. 2008, 165, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, I.; Elvira, S.; Calatayud, V.; Calvo, E.; Aparicio, P.; Sánchez, M.; Alonso, R.; Bermejo, V.B. Ozone effects on the physiology and marketable biomass of leafy vegetables under Mediterranean conditions: Spinach (Spinacia oleracea L.) and Swiss chard (Beta vulgaris L. Var. cycla). Agric. Ecosyst. Environ. 2016, 235, 215–228. [Google Scholar] [CrossRef]
- Sharps, K.; Hayes, F.; Harmens, H.; Mills, G. Ozone-induced effects on leaves in African crop species. Environ. Pollut. 2021, 268, 115789. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sun, W.; Zhu, Y.-G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Hafez, M.; Popov, A.I.; Rashad, M. Integrated use of bio-organic fertilizers for enhancing soil fertility–plant nutrition, germination status and initial growth of corn (Zea mays L.). Environ. Technol. Innov. 2021, 21, 101329. [Google Scholar] [CrossRef]
- Edwards, R.; Sundstrom, F. Afterripening and harvesting effects on Tabasco pepper seed germination performance. HortScience 1987, 22, 473–475. [Google Scholar] [CrossRef]
- Coste, S.; Baraloto, C.; Leroy, C.; Marcon, É.; Renaud, A.; Richardson, A.D.; Roggy, J.-C.; Schimann, H.; Uddling, J.; Hérault, B. Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: A calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann. For. Sci. 2010, 67, 607. [Google Scholar] [CrossRef] [Green Version]
- Valentovic, P.; Luxova, M.; Kolarovic, L.; Gasparikova, O. Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant Soil Environ. 2006, 52, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.A.; Farooq, M.; Xie, X.-Y.; Liu, X.-J.; Ijaz, M.F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hortic. 2012, 140, 66–73. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- López-Marín, J.; Gálvez, A.; del Amor, F.M.; Albacete, A.; Fernández, J.A.; Egea-Gilabert, C.; Pérez-Alfocea, F. Selecting vegetative/generative/dwarfing rootstocks for improving fruit yield and quality in water stressed sweet peppers. Sci. Hortic. 2017, 214, 9–17. [Google Scholar] [CrossRef]
- Motamedi, M.; Haghighi, M.; Goli, A. Physiological changes of sweet and hot peppers in vegetative and reproductive growth stages treated by Ca and H2O2 under unforeseen heat stresses. Sci. Hortic. 2019, 249, 306–313. [Google Scholar] [CrossRef]
- Korkmaz, A.; Korkmaz, Y.; Demirkıran, A.R. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 2010, 67, 495–501. [Google Scholar] [CrossRef]
- Penella, C.; Nebauer, S.G.; López-Galarza, S.; San Bautista, A.; Rodríguez-Burruezo, A.; Calatayud, Á. Evaluation of some pepper genotypes as rootstocks in water stress conditions. Hortic. Sci. 2014, 41, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Alam, A.; Ullah, H.; Cha-um, S.; Tisarum, R.; Datta, A. Effect of seed priming with potassium nitrate on growth, fruit yield, quality and water productivity of cantaloupe under water-deficit stress. Sci. Hortic. 2021, 288, 110354. [Google Scholar] [CrossRef]
- Kulak, M.; Jorrín-Novo, J.V.; Romero-Rodriguez, M.C.; Yildirim, E.D.; Gul, F.; Karaman, S. Seed priming with salicylic acid on plant growth and essential oil composition in basil (Ocimum basilicum L.) plants grown under water stress conditions. Ind. Crops Prod. 2021, 161, 113235. [Google Scholar] [CrossRef]
- Mansour, E.; Mahgoub, H.A.M.; Mahgoub, S.A.; El-Sobky, E.-S.E.A.; Abdul-Hamid, M.I.; Kamara, M.M.; AbuQamar, S.F.; El-Tarabily, K.A.; Desoky, E.-S.M. Enhancement of drought tolerance in diverse Vicia faba cultivars by inoculation with plant growth-promoting rhizobacteria under newly reclaimed soil conditions. Sci. Rep. 2021, 11, 24142. [Google Scholar] [CrossRef]
- Selem, E.; Hassan, A.A.S.A.; Awad, M.F.; Mansour, E.; Desoky, E.-S.M. Impact of exogenously sprayed antioxidants on physio-biochemical, agronomic, and quality parameters of potato in salt-affected soil. Plants 2022, 11, 210. [Google Scholar] [CrossRef]
- Mannan, M.A.; Tithi, M.A.; Islam, M.R.; Al Mamun, M.A.; Mia, S.; Rahman, M.Z.; Awad, M.F.; ElSayed, A.I.; Mansour, E.; Hossain, M.S. Soil and foliar applications of zinc sulfate and iron sulfate alleviate the destructive impacts of drought stress in wheat. Cereal Res. Commun. 2022, 1–11. [Google Scholar] [CrossRef]
- Rehman, R.N.U.; Malik, A.U.; Khan, A.S.; Hasan, M.U.; Anwar, R.; Ali, S.; Haider, M.W. Combined application of hot water treatment and methyl salicylate mitigates chilling injury in sweet pepper (Capsicum annuum L.) fruits. Sci. Hortic. 2021, 283, 110113. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Y.; Fang, Z. Ozone pollution: A major health hazard worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, I.J.; Rathore, D. Assessment of dose–response relationship between ozone dose and groundnut (Arachis hypogaea L) cultivars using Open Top Chamber (OTC) and Ethylenediurea (EDU). Environ. Technol. Innov. 2021, 22, 101494. [Google Scholar] [CrossRef]
- Yadav, A.; Bhatia, A.; Yadav, S.; Kumar, V.; Singh, B. The effects of elevated CO2 and elevated O3 exposure on plant growth, yield and quality of grains of two wheat cultivars grown in north India. Heliyon 2019, 5, e02317. [Google Scholar] [CrossRef] [Green Version]
- Msayleb, N.; Ibrahim, S. Treatment of nematodes with ozone gas: A sustainable alternative to nematicides. Phys. Procedia 2011, 21, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhang, H.; Dong, C.; Ji, H.; Zhang, X.; Li, L.; Ban, Z.; Zhang, N.; Xue, W. Effect of ozone treatment on the phenylpropanoid biosynthesis of postharvest strawberries. RSC Adv. 2019, 9, 25429–25438. [Google Scholar] [CrossRef] [Green Version]
- Marchica, A.; Ascrizzi, R.; Flamini, G.; Cotrozzi, L.; Tonelli, M.; Lorenzini, G.; Nali, C.; Pellegrini, E. Ozone as eustress for enhancing secondary metabolites and bioactive properties in Salvia officinalis. Ind. Crops Prod. 2021, 170, 113730. [Google Scholar] [CrossRef]
- Calzone, A.; Podda, A.; Lorenzini, G.; Maserti, B.E.; Carrari, E.; Deleanu, E.; Hoshika, Y.; Haworth, M.; Nali, C.; Badea, O. Cross-talk between physiological and biochemical adjustments by Punica granatum cv. Dente di Cavallo mitigates the effects of salinity and ozone stress. Sci. Total Environ. 2019, 656, 589–597. [Google Scholar] [CrossRef]
- Pellegrini, E.; Cotrozzi, L.; Neri, L.; Baraldi, R.; Carrari, E.; Nali, C.; Lorenzini, G.; Paoletti, E.; Hoshika, Y. Stress markers and physiochemical responses of the Mediterranean shrub Phillyrea angustifolia under current and future drought and ozone scenarios. Environ. Res. 2021, 201, 111615. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Zhang, H.; Chen, C.; Li, L.; Dong, C.; Cheng, Y. Comparative transcriptomic analysis of cantaloupe melon under cold storage with ozone treatment. Int. Food Res. J. 2021, 140, 109993. [Google Scholar] [CrossRef]
- Sudhakar, N.; Nagendra-Prasad, D.; Mohan, N.; Murugesan, K. A preliminary study on the effects of ozone exposure on growth of the tomato seedlings. Aust. J. Crop Sci. 2008, 2, 33–39. [Google Scholar]
- Kleiber, T.; Borowiak, K.; Schroeter-Zakrzewska, A.; Budka, A.; Osiecki, S. Effect of ozone treatment and light colour on photosynthesis and yield of lettuce. Sci. Hortic. 2017, 217, 130–136. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transduction in plants. Curr. Sci. 2005, 89, 1113–1121. [Google Scholar]
- Desoky, E.-S.M.; Mansour, E.; El-Sobky, E.-S.E.A.; Abdul-Hamid, M.I.; Taha, T.F.; Elakkad, H.A.; Arnaout, S.M.A.I.; Eid, R.S.M.; El-Tarabily, K.A.; Yasin, M.A.T. Physio-biochemical and agronomic responses of faba beans to exogenously applied nano-silicon under drought stress conditions. Front. Plant Sci. 2021, 12, 637783. [Google Scholar] [CrossRef]
- Habibullah, M.; Sarkar, S.; Islam, M.M.; Ahmed, K.U.; Rahman, M.Z.; Awad, M.F.; ElSayed, A.I.; Mansour, E.; Hossain, M.S. Assessing the response of diverse sesame genotypes to waterlogging durations at different plant growth stages. Plants 2021, 10, 2294. [Google Scholar] [CrossRef]
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol. Plant. 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signaling transduction. Annu. Rev. Plant Biol. 2004, 55, 373. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaal, M.; Ouerghi, Z.; Navari-Izzo, F. Antioxidative responses of Ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol. Biochem. 2010, 48, 772–777. [Google Scholar] [CrossRef]
- Abeli, T.; Guasconi, D.B.; Mondoni, A.; Dondi, D.; Bentivoglio, A.; Buttafava, A.; Cristofanelli, P.; Bonasoni, P.; Rossi, G.; Orsenigo, S. Acute and chronic ozone exposure temporarily affects seed germination in alpine plants. Plant Biosyst. 2017, 151, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Avdeeva, V.; Zorina, E.; Bezgina, J.; Kolosova, O. Influence of ozone on germination and germinating energy of winter wheat seeds. Eng. Rural Dev. 2018, 23, 543–546. [Google Scholar]
- Rodrigues, V.O.; Penido, A.C.; Pereira, D.D.S.; Oliveira, A.M.S.; Mendes, A.E.S.; Oliveira, J.A. Sanitary and physiological quality of soybean seeds treated with ozone. J. Agric. Sci. 2019, 11, 183–196. [Google Scholar] [CrossRef]
- Vandermeiren, K.; Black, C.; Pleijel, H.; De Temmerman, L. Impact of rising tropospheric ozone on potato: Effects on photosynthesis, growth, productivity and yield quality. Plant Cell Environ. 2005, 28, 982–996. [Google Scholar] [CrossRef]
- Tetteh, R.; Yamaguchi, M.; Wada, Y.; Funada, R.; Izuta, T. Effects of ozone on growth, net photosynthesis and yield of two African varieties of Vigna unguiculata. Environ. Pollut. 2015, 196, 230–238. [Google Scholar] [CrossRef]
- Wilkinson, S.; Mills, G.; Illidge, R.; Davies, W.J. How is ozone pollution reducing our food supply? J. Exp. Bot. 2012, 63, 527–536. [Google Scholar] [CrossRef]
- Mansour, E.; Desoky, E.-S.M.; Ali, M.M.A.; Abdul-Hamid, M.I.; Ullah, H.; Attia, A.; Datta, A. Identifying drought-tolerant genotypes of faba bean and their agro-physiological responses to different water regimes in an arid Mediterranean environment. Agric. Water Manag. 2021, 247, 106754. [Google Scholar] [CrossRef]
- Wright, M. The effect of chilling on ethylene production, membrane permeability and water loss of leaves of Phaseolus vulgaris. Planta 1974, 120, 63–69. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Igarashi, Y.; Sanada, Y.; Yamaguchi-Shinozaki, K.; Wada, K.; Shinozaki, K. Characterization of the gene for Δ1-pyrroline-5-carboxylate synthetase and correlation between the expression of the gene and salt tolerance in Oryza sativa L. Plant Mol. Biol. 1997, 33, 857–865. [Google Scholar] [CrossRef]
- ElShamey, E.A.Z.; Sakran, R.M.; ElSayed, M.A.A.; Aloufi, S.; Alharthi, B.; Alqurashi, M.; Mansour, E.; Abd El-Moneim, D. Heterosis and combining ability for floral and yield characters in rice using cytoplasmic male sterility system. Saudi J. Biol. Sci. 2022, 29, 3727–3738. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Zhou, W.; Takeuchi, Y.; Yoneyama, K. Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regul. 2006, 49, 27–34. [Google Scholar]
- Desoky, E.-S.M.; Mansour, E.; Yasin, M.A.T.; El-Sobky, E.-S.E.A.; Rady, M.M. Improvement of drought tolerance in five different cultivars of Vicia faba with foliar application of ascorbic acid or silicon. Span. J. Agric. Res. 2020, 18, e0802. [Google Scholar] [CrossRef]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiol. Plant. 2008, 132, 220–235. [Google Scholar] [CrossRef]
- Kishor, P.K.; Sangam, S.; Amrutha, R.; Laxmi, P.S.; Naidu, K.; Rao, K.S.; Rao, S.; Reddy, K.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Xu, P.-L.; Guo, Y.K.; Bai, J.G.; Shang, L.; Wang, X.J. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol. Plant. 2008, 132, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Sakran, R.M.; Ghazy, M.I.; Rehan, M.; Alsohim, A.S.; Mansour, E. Molecular genetic diversity and combining ability for some physiological and agronomic traits in rice under well-watered and water-deficit conditions. Plants 2022, 11, 702. [Google Scholar] [CrossRef]
- Arbona, V.; Hossain, Z.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant. 2008, 132, 452–466. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Rogers, A.; Ainsworth, E.A. Growth at elevated ozone or elevated carbon dioxide concentration alters antioxidant capacity and response to acute oxidative stress in soybean (Glycine max). J. Exp. Bot. 2011, 62, 2667–2678. [Google Scholar] [CrossRef]
- Umponstira, C.; Pimpa, W.; Nanegrungsun, S. Physiological and biochemical responses of cowpea (Vigna unguiculata (L.) Walp) to ozone. Songklanakarin J. Sci. Technol. 2006, 28, 861–869. [Google Scholar]
- Yan, K.; Chen, W.; He, X.; Zhang, G.; Xu, S.; Wang, L. Responses of photosynthesis, lipid peroxidation and antioxidant system in leaves of Quercus mongolica to elevated O3. Environ. Exp. Bot. 2010, 69, 198–204. [Google Scholar] [CrossRef]
| Studied Factor | GP (%) | MGT (Day) | GI (Seed/Day) | CV (%) | |
|---|---|---|---|---|---|
| Pepper hybrid (H) | |||||
| Zidenka | 92.26 | 7.31 a | 6.35 b | 13.72 b | |
| Lirica | 92.00 | 6.79 b | 7.22 a | 14.91 a | |
| Seed soaking (S) | |||||
| 0 ppm O3 | 92.00 | 7.29 b | 5.73 d | 13.71 d | |
| 10 ppm O3 | 92.00 | 8.06 a | 6.76 e | 12.41 e | |
| 20 ppm O3 | 91.33 | 7.02 c | 6.71 c | 14.26 c | |
| 30 ppm O3 | 92.66 | 6.55 d | 8.04 b | 15.32 b | |
| 40 ppm O3 | 92.66 | 6.34 e | 8.68 a | 15.86 a | |
| Interaction | |||||
| Zidenka | 0 ppm O3 | 92.00 | 7.29 b | 5.73 c | 13.72 e |
| 10 ppm O3 | 92.00 | 8.06 a | 4.77 d | 12.41 f | |
| 20 ppm O3 | 90.67 | 7.02 c | 6.17 c | 13.57 e | |
| 30 ppm O3 | 92.00 | 6.56 d | 7.22 b | 14.23 d | |
| 40 ppm O3 | 93.33 | 6.34 e | 7.87 b | 14.68 c | |
| Lirica | 0 ppm O3 | 92.00 | 7.29 b | 5.73 c | 13.72 e |
| 10 ppm O3 | 92.00 | 8.06 a | 4.77 d | 12.41 f | |
| 20 ppm O3 | 92.00 | 6.68 d | 7.25 b | 14.97 c | |
| 30 ppm O3 | 93.33 | 6.09 e | 8.87 a | 16.43 b | |
| 40 ppm O3 | 93.33 | 5.87 f | 9.48 a | 17.04 a | |
| ANOVA | df | ||||
| H | 1 | 0.539 NS | <0.001 | <0.001 | <0.001 |
| S | 4 | 0.284 NS | <0.001 | <0.001 | <0.001 |
| H × S | 4 | 0.237 NS | <0.001 | <0.001 | <0.001 |
| Studied Factor | Fresh Weight (g/Plant) | Dry Weight (g/Plant) | |||||
|---|---|---|---|---|---|---|---|
| Roots | Shoots | Root/Shoot Ratio | Roots | Shoots | Root/Shoot Ratio | ||
| Pepper hybrids | |||||||
| Zidenka | 0.141 b | 0.211 b | 0.668 a | 0.009 | 0.016 | 0.563 | |
| Lirica | 0.150 a | 0.228 a | 0.658 b | 0.014 | 0.015 | 0.933 | |
| Seed soaking | |||||||
| 0 ppm O3 | 0.141 c | 0.205 e | 0.688 b | 0.021 | 0.015 b | 1.400 | |
| 10 ppm O3 | 0.147 b | 0.195 d | 0.754 a | 0.009 | 0.016 b | 0.563 | |
| 20 ppm O3 | 0.136 d | 0.219 c | 0.621 d | 0.007 | 0.014 c | 0.500 | |
| 30 ppm O3 | 0.147 a | 0.232 b | 0.634 c | 0.008 | 0.015 b | 0.53 | |
| 40 ppm O3 | 0.155 b | 0.245 a | 0.633 c | 0.012 | 0.017 a | 0.706 | |
| Interaction | |||||||
| Zidenka | 0 ppm O3 | 0.133 g | 0.192 i | 0.693 b | 0.009 | 0.013 c | 0.692 |
| 10 ppm O3 | 0.159 b | 0.193 i | 0.824 a | 0.010 | 0.018 a | 0.556 | |
| 20 ppm O3 | 0.132 g | 0.211 g | 0.626 e | 0.009 | 0.015 b | 0.600 | |
| 30 ppm O3 | 0.140 e | 0.224 e | 0.625 e | 0.009 | 0.016 b | 0.563 | |
| 40 ppm O3 | 0.142 e | 0.236 c | 0.602 f | 0.012 | 0.018 a | 0.667 | |
| Lirica | 0 ppm O3 | 0.151 d | 0.219 f | 0.689 b | 0.033 | 0.018 a | 1.833 |
| 10 ppm O3 | 0.135 f | 0.198 h | 0.682 b | 0.009 | 0.013 c | 0.692 | |
| 20 ppm O3 | 0.141 e | 0.227 d | 0.621 e | 0.007 | 0.013 c | 0.538 | |
| 30 ppm O3 | 0.155 c | 0.241 b | 0.643 d | 0.008 | 0.014 c | 0.57 | |
| 40 ppm O3 | 0.169 a | 0.255 a | 0.663 c | 0.008 | 0.014 c | 0.571 | |
| ANOVA | df | p value | |||||
| H | 1 | <0.001 | <0.001 | <0.001 | 0.387 NS | <0.001 | 0.375 NS |
| S | 4 | <0.001 | <0.001 | <0.001 | 0.423 NS | <0.001 | 0.388 NS |
| H × S | 4 | <0.001 | <0.001 | <0.001 | 0.411 NS | <0.001 | 0.557 NS |
| Studied Factor | Stem Diameter (mm) | Root Length (cm) | Stem Length (cm) | ||||
|---|---|---|---|---|---|---|---|
| Soaking | Spray | Soaking | Spray | Soaking | Spray | ||
| Pepper hybrid (H) | |||||||
| Zidenka | 2.66 b | 2.65 b | 8.17 a | 8.25 a | 11.70 b | 12.11 | |
| Lirica | 2.71 a | 2.71 a | 7.90 b | 7.80 b | 12.21 a | 12.21 | |
| Seed treatment (T) | |||||||
| 0 ppm O3 | 2.55 e | 2.55 e | 7.26 e | 7.26 d | 10.90 e | 12.18 bc | |
| 10 ppm O3 | 2.77 b | 2.69 b | 8.26 b | 7.62 c | 12.30 b | 12.30 b | |
| 20 ppm O3 | 2.61 d | 2.60 d | 7.62 d | 8.00 b | 11.80 d | 12.06 cd | |
| 30 ppm O3 | 2.65 c | 2.65 c | 8.00 c | 9.03 a | 11.96 c | 11.93 d | |
| 40 ppm O3 | 2.83 a | 2.87 a | 9.03 a | 7.98 b | 12.81 a | 12.60 a | |
| Mean (T) | 2.67 | 2.66 | 7.68 B | 8.02 A | 11.88 B | 12.09 A | |
| Interaction | |||||||
| Zidenka | 0 ppm O3 | 2.520 f | 2.520 f | 7.000 f | 7.00 i | 12.000 cd | 12.000 cd |
| 10 ppm O3 | 2.900 b | 2.733 c | 8.167 b | 8.40 c | 9.900 f | 12.467 b | |
| 20 ppm O3 | 2.593 g | 2.587 e | 7.233 e | 7.75 g | 11.600 e | 12.133 c | |
| 30 ppm O3 | 2.617 ef | 2.613 de | 7.633 d | 8.20 d | 12.133 c | 12.063 cd | |
| 40 ppm O3 | 2.700 c | 2.780 b | 8.133 b | 9.50 a | 12.867 a | 12.600 ab | |
| Lirica | 0 ppm O3 | 2.597 f | 2.597 e | 7.533 d | 7.53 h | 12.600 b | 12.433 b |
| 10 ppm O3 | 2.650 d | 2.650 d | 8.467 a | 8.13 e | 11.900 cd | 11.900 cd | |
| 20 ppm O3 | 2.630 de | 2.630 de | 7.500 d | 7.50 h | 12.000 cd | 12.000 cd | |
| 30 ppm O3 | 2.700 c | 2.700 c | 7.833 c | 7.80 f | 11.800 e | 11.800 d | |
| 40 ppm O3 | 2.973 a | 2.973 a | 8.167 b | 8.57 b | 12.767 ab | 12.767 a | |
| ANOVA | df | p value | |||||
| H | 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| S | 4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| H × S | 4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Studied Factor | Root Fresh Weight (g) | Shoot Fresh Weight (g) | Seedling Fresh Weight (g) | ||||
|---|---|---|---|---|---|---|---|
| Soaking | Spray | Soaking | Spray | Soaking | Spray | ||
| Pepper hybrid (H) | |||||||
| Zidenka | 0.797 a | 0.740 b | 1.76 b | 1.794 | 2.56 | 2.528 b | |
| Lirica | 0.774 b | 0.775 a | 1.78 a | 1.784 | 2.55 | 2.558 a | |
| Seed treatment (T) | |||||||
| 0 ppm O3 | 0.743 c | 0.743 c | 1.77 b | 1.773 b | 2.51 c | 2.51 b | |
| 10 ppm O3 | 0.756 c | 0.680 d | 1.71 d | 1.733 b | 2.46 e | 2.45 c | |
| 20 ppm O3 | 0.752 c | 0.733 c | 1.73 c | 1.728 d | 2.48 d | 2.46 c | |
| 30 ppm O3 | 0.801 b | 0.776 b | 1.74 c | 1.743 c | 2.54 b | 2.52 b | |
| 40 ppm O3 | 0.876 a | 0.850 a | 1.91 a | 1.916 a | 2.79 a | 2.76 a | |
| Mean (T) | 0.770 A | 0.752 B | 1.76 | 1.77 | 2.53 | 2.52 | |
| Interaction | |||||||
| Zidenka | 0 ppm O3 | 0.710 f | 0.710 e | 1.760 d | 1.760 d | 2.470 ef | 2.470 f |
| 10 ppm O3 | 0.820 c | 0.667 f | 1.660 f | 1.793 c | 2.480 e | 2.460 f | |
| 20 ppm O3 | 0.750 e | 0.713 e | 1.767 d | 1.757 d | 2.517 d | 2.470 f | |
| 30 ppm O3 | 0.803 c | 0.753 d | 1.783 c | 1.780 c | 2.587 c | 2.533 d | |
| 40 ppm O3 | 0.903 a | 0.850 a | 1.863 b | 1.860 b | 2.767 b | 2.710 b | |
| Lirica | 0 ppm O3 | 0.777 d | 0.777 a | 1.787 c | 1.787 c | 2.563 c | 2.563 c |
| 10 ppm O3 | 0.693 f | 0.693 c | 1.753 d | 1.753 d | 2.447 ef | 2.447 f | |
| 20 ppm O3 | 0.753 e | 0.753 e | 1.700 e | 1.700 e | 2.453 f | 2.453 f | |
| 30 ppm O3 | 0.800 c | 0.800 b | 1.707 e | 1.707 e | 2.507 d | 2.507 e | |
| 40 ppm O3 | 0.850 b | 0.850 a | 1.973 a | 1.973 a | 2.823 a | 2.823 a | |
| ANOVA | df | p value | |||||
| H | 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| S | 4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| H × S | 4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Studied Factor | Root Dry Weight (g) | Shoot Dry Weight (g) | Seedling Dry Weight (g) | ||||
|---|---|---|---|---|---|---|---|
| Soaking | Spray | Soaking | Spray | Soaking | Spray | ||
| Pepper hybrid (H) | |||||||
| Zidenka | 0.055 b | 0.055 b | 0.182 a | 0.183 a | 0.237 b | 0.237 b | |
| Lirica | 0.061 a | 0.061 a | 0.179 b | 0.179 b | 0.241 a | 0.240 a | |
| Seed treatment (T) | |||||||
| 0 ppm O3 | 0.053 d | 0.053 d | 0.175 d | 0.174 d | 0.226 d | 0.223 e | |
| 10 ppm O3 | 0.055 c | 0.053 d | 0.181 b | 0.188 b | 0.237 b | 0.241 b | |
| 20 ppm O3 | 0.058 b | 0.055 c | 0.170 e | 0.167 e | 0.229 c | 0.229 d | |
| 30 ppm O3 | 0.067 a | 0.058 b | 0.177 c | 0.175 c | 0.236 b | 0.233 c | |
| 40 ppm O3 | 0.052 e | 0.068 a | 0.200 a | 0.201 a | 0.267 a | 0.269 a | |
| Mean (T) | 0.057 | 0.057 | 0.180 | 0.181 | 0.239 | 0.239 | |
| Interaction | |||||||
| Zidenka | 0 ppm O3 | 0.052 e | 0.052 g | 0.174 f | 0.174 e | 0.226 f | 0.226 f |
| 10 ppm O3 | 0.060 c | 0.057 e | 0.182 c | 0.195 b | 0.242 c | 0.252 c | |
| 20 ppm O3 | 0.052 e | 0.052 g | 0.176 e | 0.169 f | 0.228 f | 0.221 g | |
| 30 ppm O3 | 0.053 e | 0.052 g | 0.181 c | 0.175 e | 0.234 e | 0.227 f | |
| 40 ppm O3 | 0.060 c | 0.061 c | 0.199 b | 0.202 a | 0.259 b | 0.263 b | |
| Lirica | 0 ppm O3 | 0.055 d | 0.055 f | 0.178 d | 0.178 d | 0.233 e | 0.233 e |
| 10 ppm O3 | 0.051 e | 0.051 g | 0.181 c | 0.181 c | 0.231 e | 0.231 e | |
| 20 ppm O3 | 0.059 c | 0.059 d | 0.165 g | 0.165 e | 0.225 f | 0.225 f | |
| 30 ppm O3 | 0.065 b | 0.065 b | 0.174 f | 0.174 g | 0.239 d | 0.239 d | |
| 40 ppm O3 | 0.075 a | 0.075 a | 0.201 a | 0.201 a | 0.276 a | 0.276 a | |
| ANOVA | df | p value | |||||
| H | 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| S | 4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| H × S | 4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Studied Factor | Chlorophyll (SPAD Value) | Membrane Permeability (MP %) | Relative Water Content (RWC %) | ||||
|---|---|---|---|---|---|---|---|
| Soaking | Spray | Soaking | Spray | Soaking | Spray | ||
| Pepper hybrid (H) | |||||||
| Zidenka | 39.67 b | 38.48 b | 16.32 | 16.07 | 75.74 b | 76.25 b | |
| Lirica | 43.76 a | 40.73 a | 16.30 | 16.07 | 77.78 a | 78.21 a | |
| Seed treatment (T) | |||||||
| 0 ppm O3 | 43.20 d | 37.71 c | 18.08 a | 18.08 a | 72.75 e | 72.95 e | |
| 10 ppm O3 | 37.85 e | 37.68 c | 16.46 c | 17.31 b | 72.65 c | 74.06 d | |
| 20 ppm O3 | 43.43 c | 40.13 b | 17.31 b | 15.43 c | 74.40 d | 76.96 c | |
| 30 ppm O3 | 44.15 b | 41.93 a | 15.43 d | 14.26 e | 77.43 b | 81.95 a | |
| 40 ppm O3 | 46.95 a | 40.11 b | 14.26 e | 14.88 d | 82.60 a | 81.16 b | |
| Mean (T) | 41.72 A | 39.51 B | 16.31 A | 16.07 B | 77.01 B | 77.23 A | |
| Interaction | |||||||
| Zidenka | 0 ppm O3 | 34.20 i | 34.30 g | 18.233 a | 18.233 a | 71.20 h | 71.50 i |
| 10 ppm O3 | 34.80 h | 39.60 d | 16.367 c | 16.367 c | 76.60 d | 76.13 d | |
| 20 ppm O3 | 41.13 f | 36.47 f | 17.233 b | 17.233 b | 73.33 g | 73.07 h | |
| 30 ppm O3 | 42.10 d | 40.13 cd | 15.200 e | 15.200 e | 76.23 d | 75.70 e | |
| 40 ppm O3 | 46.13 c | 41.77 a | 14.467 f | 14.467 f | 81.37 b | 81.00 b | |
| Lirica | 0 ppm O3 | 41.50 e | 41.13 b | 17.933 a | 17.933 a | 74.30 f | 74.40 g |
| 10 ppm O3 | 37.60 g | 40.60 c | 16.567 c | 16.567 c | 76.70 d | 76.50 d | |
| 20 ppm O3 | 45.73 c | 38.90 e | 17.400 b | 17.400 b | 75.47 e | 75.07 f | |
| 30 ppm O3 | 46.20 b | 40.13 cd | 15.667 d | 15.667 d | 78.63 c | 78.23 c | |
| 40 ppm O3 | 47.77 a | 42.10 a | 14.067 g | 14.067 g | 83.83 a | 82.90 a | |
| ANOVA | df | p value | |||||
| H | 1 | <0.001 | <0.001 | 0.521 NS | 0.495 NS | <0.001 | <0.001 |
| S | 4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| H × S | 4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Studied Factor | Proline Content (μmol g−1) | Ascorbate Peroxides (μmol g−1 min−1) | |||
|---|---|---|---|---|---|
| Soaking | Spray | Soaking | Spray | ||
| Pepper hybrid (H) | |||||
| Zidenka | 0.959 b | 0.961 b | 0.399 b | 0.399 b | |
| Lirica | 0.964 a | 0.968 a | 0.400 a | 0.402 a | |
| Seed treatment (T) | |||||
| 0 ppm O3 | 0.917 d | 0.935 d | 0.381 d | 0.389 c | |
| 10 ppm O3 | 0.927 c | 0.918 c | 0.386 c | 0.382 d | |
| 20 ppm O3 | 0.864 e | 0.865 e | 0.359 e | 0.359 e | |
| 30 ppm O3 | 0.967 b | 0.967 b | 0.402 b | 0.402 b | |
| 40 ppm O3 | 1.132 a | 1.140 a | 0.471 a | 0.472 a | |
| Mean (T) | 0.961 | 0.965 | 0.400 | 0.401 | |
| Interaction | |||||
| Zidenka | 0 ppm O3 | 0.912 g | 0.913 f | 0.379 g | 0.379 f |
| 10 ppm O3 | 0.922 f | 0.923 e | 0.383 f | 0.384 e | |
| 20 ppm O3 | 0.912 g | 0.910 f | 0.379 g | 0.378 f | |
| 30 ppm O3 | 0.981 c | 0.979 c | 0.408 c | 0.407 c | |
| 40 ppm O3 | 1.070 b | 1.075 b | 0.445 b | 0.447 b | |
| Lirica | 0 ppm O3 | 0.922 f | 0.957 d | 0.383 f | 0.398 d |
| 10 ppm O3 | 0.933 e | 0.913 f | 0.388 e | 0.379 f | |
| 20 ppm O3 | 0.817 h | 0.819 g | 0.340 h | 0.341 g | |
| 30 ppm O3 | 0.953 d | 0.955 d | 0.396 d | 0.397 d | |
| 40 ppm O3 | 1.194 a | 1.197 a | 0.496 a | 0.497 a | |
| ANOVA | df | ||||
| H | 1 | <0.001 | <0.001 | <0.001 | <0.001 |
| S | 4 | <0.001 | <0.001 | <0.001 | <0.001 |
| H×S | 4 | <0.001 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharaf-Eldin, M.A.; Alshallash, K.S.; Alharbi, K.R.; Alqahtani, M.M.; Etman, A.A.; Yassin, A.M.; Azab, E.S.; El-Okkiah, S.A.F. Influence of Seed Soaking and Foliar Application Using Ozonated Water on Two Sweet Pepper Hybrids under Cold Stress. Sustainability 2022, 14, 13453. https://doi.org/10.3390/su142013453
Sharaf-Eldin MA, Alshallash KS, Alharbi KR, Alqahtani MM, Etman AA, Yassin AM, Azab ES, El-Okkiah SAF. Influence of Seed Soaking and Foliar Application Using Ozonated Water on Two Sweet Pepper Hybrids under Cold Stress. Sustainability. 2022; 14(20):13453. https://doi.org/10.3390/su142013453
Chicago/Turabian StyleSharaf-Eldin, Mohamed A., Khalid S. Alshallash, Khadiga R. Alharbi, Mesfer M. Alqahtani, Abdelwahab A. Etman, Ali M. Yassin, Enas S. Azab, and Samira A. F. El-Okkiah. 2022. "Influence of Seed Soaking and Foliar Application Using Ozonated Water on Two Sweet Pepper Hybrids under Cold Stress" Sustainability 14, no. 20: 13453. https://doi.org/10.3390/su142013453





