Investigating the Effect of Spherical Aluminum Particles on the Photothermal Performance of a Solar Air Collector
Abstract
:1. Introduction
2. Related Work
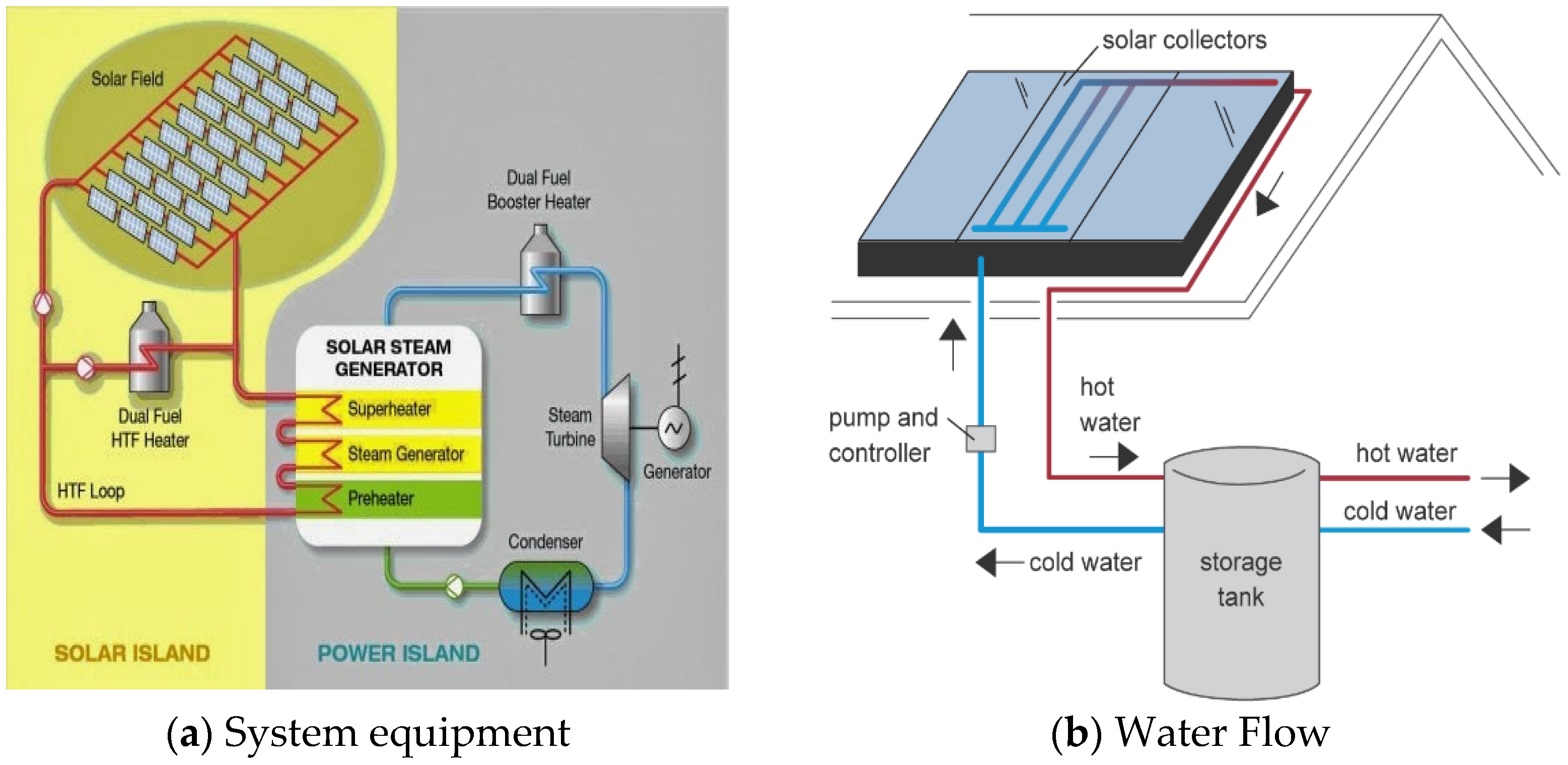
2.1. Thermal Energy Storage System
2.2. Structural of Phase Change Material Microcapsules
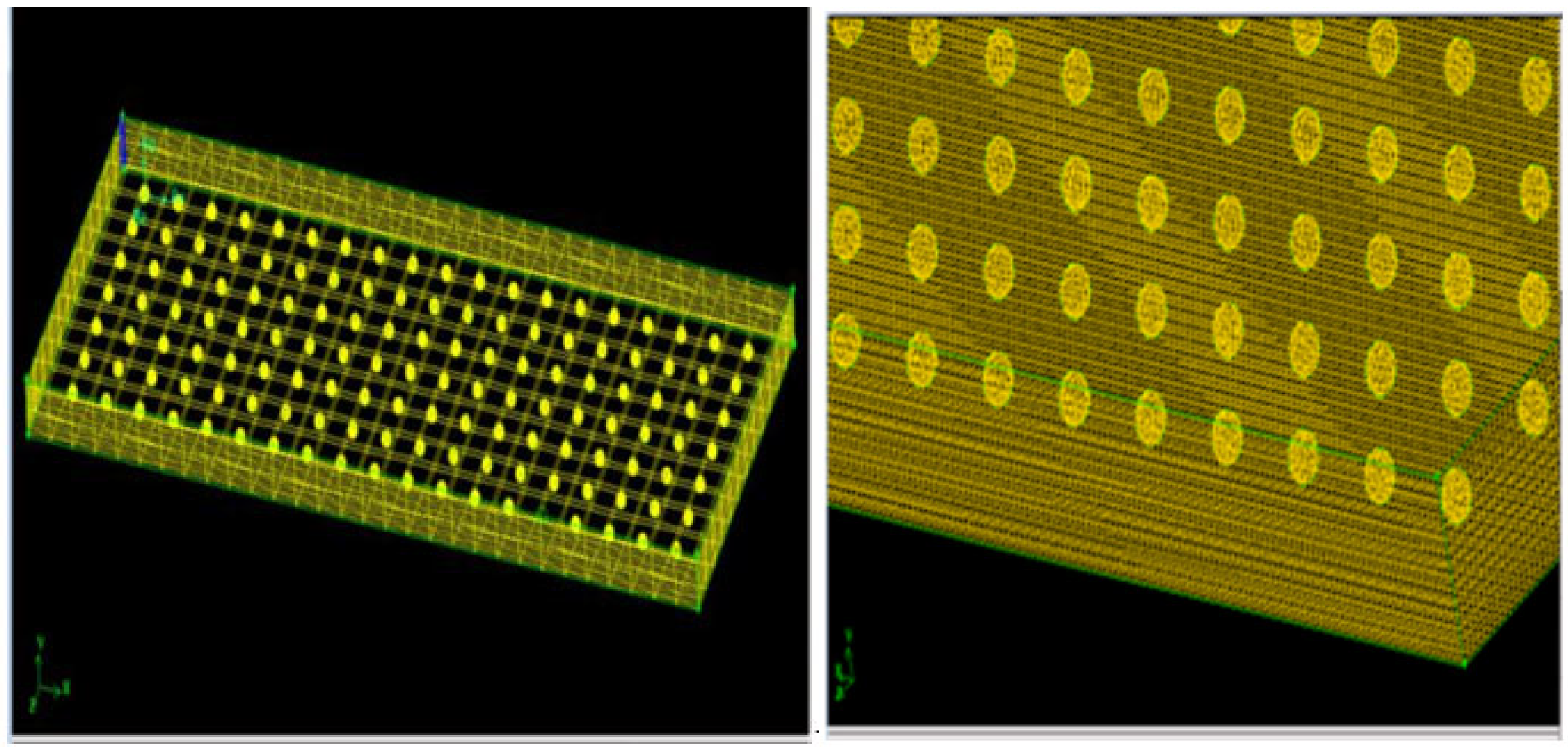
3. Solar Collector System Mathematical Model
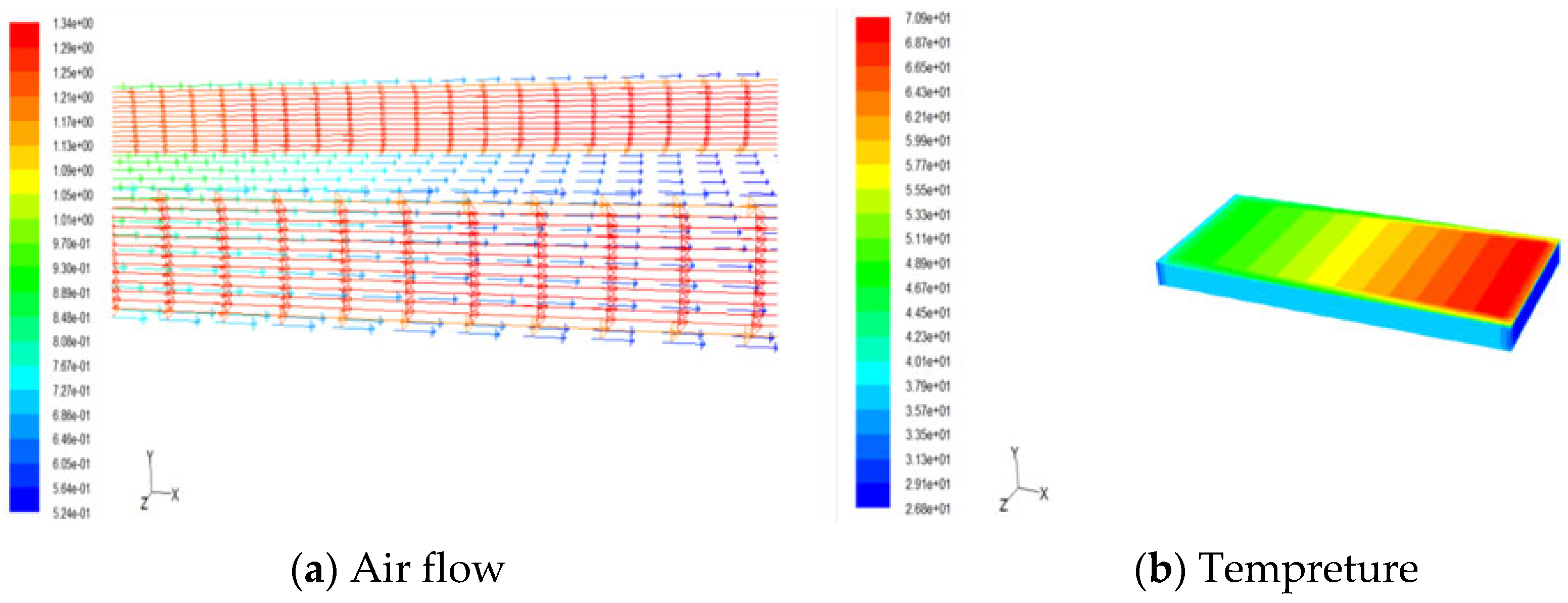
4. Result and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheikholeslami, M. Influence of magnetic field on Al2O3-H2O nanofluid forced convection heat transfer in a porous lid driven cavity with hot sphere obstacle by means of LBM. J. Mol. Liq. 2018, 263, 472–488. [Google Scholar] [CrossRef]
- Yun, J.H.; Kim, J.H.; Jeong, S.Y.; Yang, Y.S.; Kim, S.H.; Song, D.Y. An Experimental Study on the Freezing Protection Valve Using Phase Change Material (PCM) for the Heat Exchanger. J. Korean Sol. Energy Soc. 2012, 32, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Alkan, C.; Günther, E.; Hiebler, S.; Ensari, Ö.F.; Kahraman, D. Polyethylene glycol-sugar composites as shape stabilized phase change materials for thermal energy storage. Polym. Compos. 2012, 33, 1728–1736. [Google Scholar] [CrossRef]
- Ode, M.; Kim, S.G.; Suzuki, T. Recent advances in the phase-field model for solidification. ISIJ Int. 2001, 41, 1076–1082. [Google Scholar] [CrossRef]
- Bhrawy, A.; Zaky, M.A. A method based on the Jacobi tau approximation for solving multi-term time–space fractional partial differential equations. J. Comput. Phys. 2015, 281, 876–895. [Google Scholar] [CrossRef]
- Kürklü, A.; Özmerzi, A.; Bilgin, S. Thermal performance of a water-phase change material solar collector. Renew. Energy 2002, 26, 391–399. [Google Scholar] [CrossRef]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2019, 25, 251–295. [Google Scholar] [CrossRef]
- Barreneche, C.; Solé, A.; Miró, L.; Martorell, I.; Fernández, A.I.; Cabeza, L.F. Study on differential scanning calorimetry analysis with two operation modes and organic and inorganic phase change material (PCM). Thermochim. Acta 2013, 553, 23–26. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Kao, H.; Tan, J. Experimental investigation of preparation and thermal performances of paraffin/bentonite composite phase change material. Energy Convers. Manag. 2011, 52, 3275–3281. [Google Scholar] [CrossRef]
- Abuşka, M.; Şevik, S.; Kayapunar, A. A comparative investigation of the effect of honeycomb core on the latent heat storage with PCM in solar air heater. Appl. Therm. Eng. 2019, 148, 684–693. [Google Scholar] [CrossRef]
- Mirković, D.; Gröbner, J.; Schmid-Fetzer, R. Solidification paths of multicomponent monotectic aluminum alloys. Acta Mater. 2008, 56, 5214–5222. [Google Scholar] [CrossRef]
- Miyamoto, J.; Sassa, S.; Sekiguchi, H. Progressive solidification of a liquefied sand layer during continued wave loading. Geotechnique 2004, 54, 617–629. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Singh, R.; Lazarus, I.J.; Souliotis, M. Recent developments in integrated collector storage (ICS) solar water heaters: A review. Renew. Sustain. Energy Rev. 2016, 54, 270–298. [Google Scholar] [CrossRef]
- Cui, X.; Chen, K.; Xing, H.; Yang, Q.; Krishna, R.; Bao, Z.; Han, Y. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 2016, 353, 141–144. [Google Scholar] [CrossRef]
- Glasser, L. Thermodynamics of inorganic hydration and of humidity control, with an extensive database of salt hydrate pairs. J. Chem. Eng. Data 2014, 59, 526–530. [Google Scholar] [CrossRef]
- Mohammadjavad, K.; Sheikholeslami, M. Heat transfer efficiency and electrical performance evaluation of photovoltaic unit under influence of NEPCM. Int. J. Heat Mass Transf. 2022, 183, 122232. [Google Scholar]
- Javad, M.; Ann, L.; Victoria, T.; Robert, T. Nano-Enhanced Phase Change Materials for Thermal Energy Storage: A Bibliometric Analysis. Energies 2022, 15, 3426. [Google Scholar]
- Amin, A.D.; Sajjad, K.; Fuli, H. Direct Numerical Simulation of pulsating flow effect on the distribution of non-circular particles with increased levels of complexity: IB-LBM. Comput. Math. Appl. 2022, 121, 115–130. [Google Scholar]
- Behrooz, A.; Amin, A.D.; Mostafavi, M.; Ali, T. Fluid-structure interaction for the flexible filament’s propulsion hanging in the free stream. J. Mol. Liq. 2021, 323, 114941. [Google Scholar]
- Ali, J.; Amin, A.D.; Mojtaba, K.; Golmohamadi, A.M.; Sajjad, K. Mesoscopic Simulation of Forced Convective Heat Transfer of Carreau-Yasuda Fluid Flow over an Inclined Square. Temp.-Depend. Viscosity 2020, 6, 307–319. [Google Scholar]
- Hassan, H.M.A.; Amjad, M.; Qamar, A.; Noor, F.; Hu, Y.; Yaqub, T.B. Performance analysis of nanofluid-based water desalination system using integrated solar still, flat plate and parabolic trough collectors. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 427. [Google Scholar] [CrossRef]
- Amjad, M.; Haruna, M.A.; Gardy, J. Chapter two–Nanomaterials for solar energy capture and steam generation. In Emerging Nanotechnologies for Renewable Energy, Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 37–48. [Google Scholar]
- Khalil, A.; Amjad, M.; Noor, F.; Hussain, A.; Nawaz, S.; Du, X. Performance analysis of direct absorption-based parabolic trough solar collector using hybrid nanofluids. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 573. [Google Scholar] [CrossRef]
- Sattar, A.; Farooq, M.; Amjad, M.; Saeed, M.A.; Nawaz, S.; Mujtaba, M.A.; Anwar, S.; El-Sherbeeny, A.M.; Soudagar, M.E.M.; Bandarra Filho, E.P.; et al. Performance Evaluation of a Direct Absorption Collector for Solar Thermal Energy Conversion. Energies 2020, 13, 4956. [Google Scholar] [CrossRef]
- Javad, M.; Fatemeh, S.; Ann, L. Performance of nano encapsulated phase change material slurry heat transfer in a microchannel heat sink with dual-circular synthetic jets. Int. J. Heat Mass Transf. 2022, 184, 122265. [Google Scholar]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Gürel, B. A numerical investigation of the melting heat transfer characteristics of phase change materials in different plate heat exchanger (latent heat thermal energy storage) systems. Int. J. Heat Mass Transf. 2020, 148, 119117. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ghasemi, A.; Li, Z.; Shafee, A.; Saleem, S. Influence of CuO nanoparticles on heat transfer behavior of PCM in solidification process considering radiative source term. Int. J. Heat Mass Transf. 2018, 126, 1252–1264. [Google Scholar] [CrossRef]
- Pablo, D.; Javier, M.; Ana, L.; José, M.M.; Belén, Z. Experimental validation of a theoretical model: Uncertainty propagation analysis to a PCM-air thermal energy storage unit. Energy Build. 2012, 45, 124–131. [Google Scholar]
- Wang, Y.; Weidner, D.J.; Liebermann, R.C.; Liu, X.; Ko, J.; Vaughan, M.T.; Pacalo, R.E. Phase transition and thermal expansion of MgSiO3 perovskite. Science 1991, 251, 410–413. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2011, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Hu, F.X.; Bao, L.F.; Wang, J.; Wu, H.; Huang, Q.Z.; Kuang, H. Giant negative thermal expansion in bonded MnCoGe-based compounds with Ni2In-type hexagonal structure. J. Am. Chem. Soc. 2015, 137, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, K. A Study on Solar Water Heater Based On Phase Change Material. Int. J. Eng. Manag. Res. 2016, 6, 504–508. [Google Scholar]
- Solomon, L.; Elmozughi, A.F.; Oztekin, A.; Neti, S. Effect of internal void placement on the heat transfer performance–Encapsulated phase change material for energy storage. Renew. Energy 2015, 78, 438–447. [Google Scholar] [CrossRef]



| Collector Information | Value (cm) |
|---|---|
| Collector Height | 15 |
| Collector Width | 109.6 |
| Collector Length | 234.9 |
| Effective glazing area | 194.9*107 |
| Glass thickness | 0.4 cm |
| Property | Value |
|---|---|
| Liquid density | 770 kg/m3 |
| Specific heat | 2.5 kJ/Kg·K |
| Thermal conductivity | 0.2 W/m·K |
| Melting temperature | 51–54 °C |
| Latent heat of fusion | 189 kJ/kg |
| Capsule surface absorbance | 0.97 |
| Kinematic viscosity | 3.3–3.6 mm2/s at 373 K |
| Spherical Capsule | Value |
|---|---|
| Diameter | 7.7 cm |
| thickness | 0.15 cm |
| Weight of capsules | 0.05 kg |
| Material | PVC, polyethylene or Cupper (Any of this) |
| Number of capsules | 152 |
| No. of rows | 10 |
| No. of spherical cap in each row | 7 |
| Gaps between each row | 2–4 cm |
| N | (kg/S) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.05 | 0.07 | 0.09 | |||||
| Exp (T °C) | Theo (T °C) | Exp (T °C) | Theo (T °C) | Exp (T °C) | Theo (T °C) | Exp (T °C) | Theo (T °C) | |
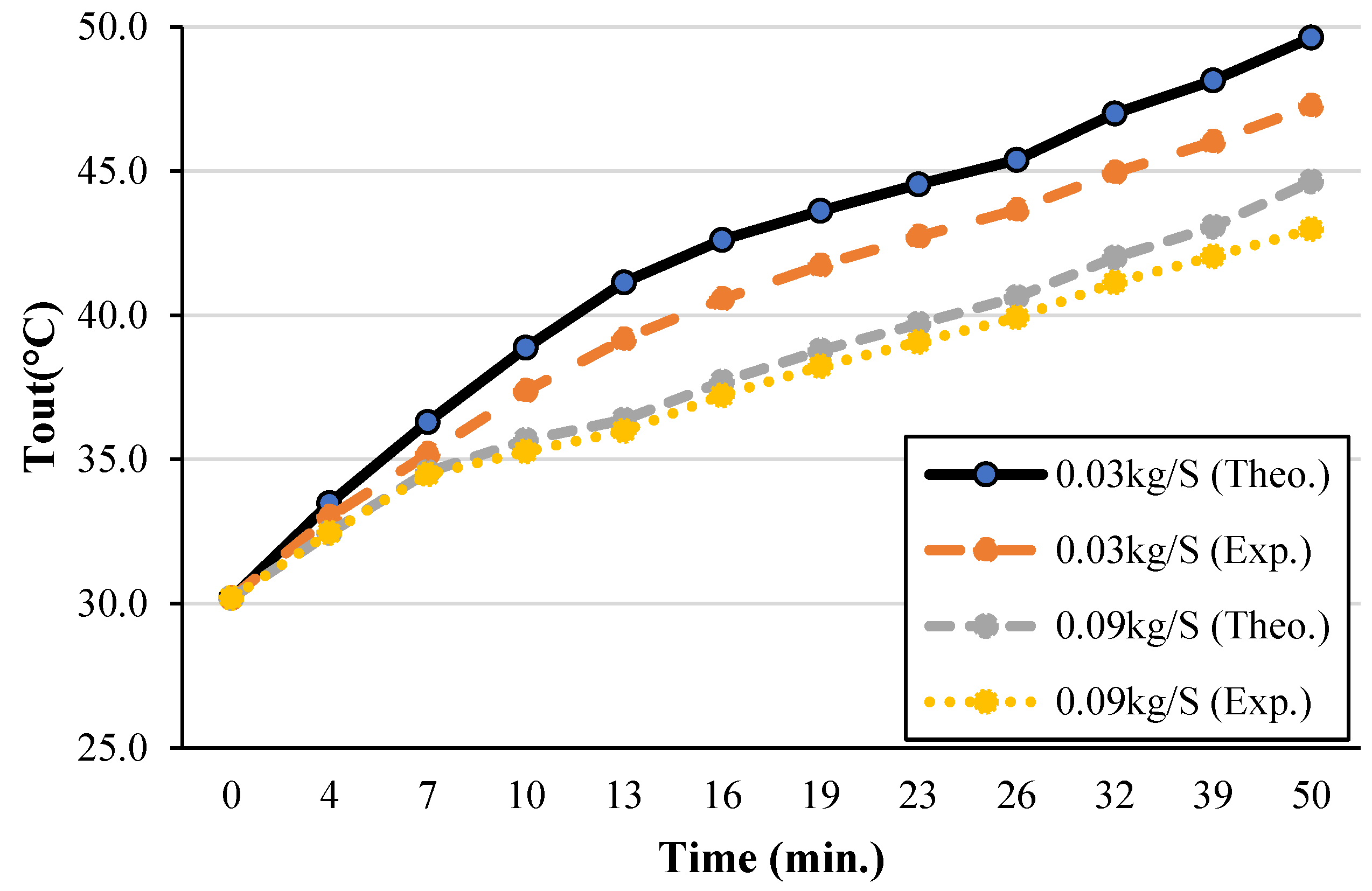
| T2 | 35.13 | 35 | 35.15 | 35 | 35.25 | 35 | 35.14 | 35 |
| T5 | 46.99 | 43.79 | 45.02 | 42.536 | 44.02 | 42.06 | 43.02 | 41.72 |
| T8 | 50.43 | 48.52 | 48.81 | 47.186 | 48.15 | 46.62 | 47.14 | 46.20 |
| T11 | 52.20 | 50.94 | 50.36 | 49.77 | 49.79 | 49.28 | 48.66 | 48.10 |
| T14 | 52.81 | 52.32 | 50.91 | 50.36 | 50.21 | 50.09 | 49.71 | 49.31 |
| (kg/S) | Tin = 30 °C | Tin= 35 °C | ||
|---|---|---|---|---|
| Exp(To − Tin) | The(To − Tin) | Exp(To − Tin) | The(To − Tin) | |
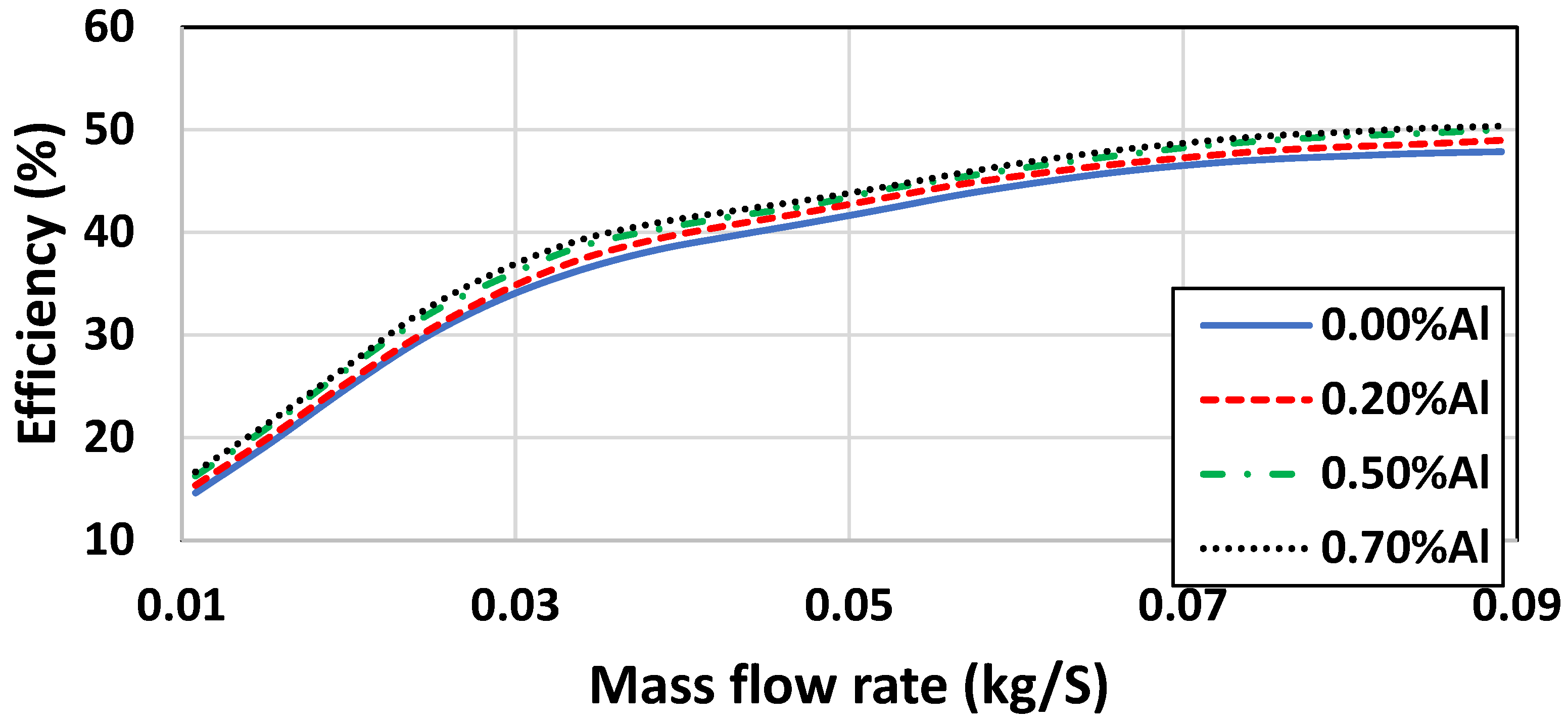
| 0.01 | 15.98 | 15.13 | 18.56 | 17.65 |
| 0.03 | 14.82 | 13.64 | 16.68 | 17.81 |
| 0.05 | 12.45 | 12.04 | 15.53 | 15.91 |
| 0.07 | 11.81 | 10.93 | 14.83 | 15.211 |
| 0.09 | 10.11 | 10.07 | 14.23 | 14. 31 |
| Mass Additive MAl (%) | Val. Additive Al (%) | Val. P.W (%) | Mass of Al (g) | Mass of P.W (g) | Mass of Compound (g) |
|---|---|---|---|---|---|
| 0.1 | 0.349 | 99.97 | 1.14 | 1138.86 | 1140 |
| 0.2 | 0.697 | 99.94 | 2.28 | 1137.72 | 1140 |
| 0.3 | 1.044 | 99.91 | 3.42 | 1136.58 | 1140 |
| 0.4 | 1.389 | 99.89 | 4.56 | 1135.44 | 1140 |
| 0.5 | 1.732 | 99.86 | 5.7 | 1134.30 | 1140 |
| Variable | Reference Value | Measured Value | Qaverage (W) | MAPE (%) |
|---|---|---|---|---|
| 1458 kg/m3 | 1389.5 | 345.5 | 4.698 | |
| 1518.5 | 370 | 4.149 | ||
| THTF;in | Equation (11) °C | Ref. − 1 | 345.6 | 4.8 |
| Ref. + 1 | 368.7 | 4.9 | ||
| Tmelt | 52.7 °C | 53.7 | 362.1 | 1.897 |
| 50.7 | 350.3 | 3.795 | ||
| kwall | 0.55 W/m·K | 0.575 | 356.8 | 4.545 |
| 0.482 | 356.7 | 3.636 | ||
| Air at THTF J/kg·K | Ref. + 5% | 357.9 | 2.3 | |
| Ref. − 5% | 347.6 | 2.4 | ||
| 0.06 (kg/S) | 0.0613 | 352.9 | 12.167 | |
| 0.059 | 352.6 | 1.667 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Dong, Y.; Fu, X.; Wang, Y.; Zhang, Q. Investigating the Effect of Spherical Aluminum Particles on the Photothermal Performance of a Solar Air Collector. Sustainability 2022, 14, 14107. https://doi.org/10.3390/su142114107
Li C, Dong Y, Fu X, Wang Y, Zhang Q. Investigating the Effect of Spherical Aluminum Particles on the Photothermal Performance of a Solar Air Collector. Sustainability. 2022; 14(21):14107. https://doi.org/10.3390/su142114107
Chicago/Turabian StyleLi, Chunbo, Yuwei Dong, Xuelong Fu, Yanzong Wang, and Qunyong Zhang. 2022. "Investigating the Effect of Spherical Aluminum Particles on the Photothermal Performance of a Solar Air Collector" Sustainability 14, no. 21: 14107. https://doi.org/10.3390/su142114107





