Potential Hormetic Effects of Cimetidine on Aerobic Composting of Human Feces from Rural China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Composting Reactor
2.2. Human Feces Composting
2.3. Compost Physicochemical Properties
2.4. Characterization of Dissolved Organic Matters
2.5. Microbial Community Analysis
3. Results and Discussion
3.1. Effect of Cimetidine on Treatment Performance of Human Feces Composting
3.1.1. Physicochemical Properties of the Bulk Substrate
3.1.2. Transformation of Carbon-Containing Materials
3.1.3. Transformation of Nitrogen-Containing Materials
3.2. DOMs Analysis Using Fluorescence and FITR Spectroscopy
3.2.1. Fluorescence Spectroscopy
3.2.2. FTIR Spectroscopy
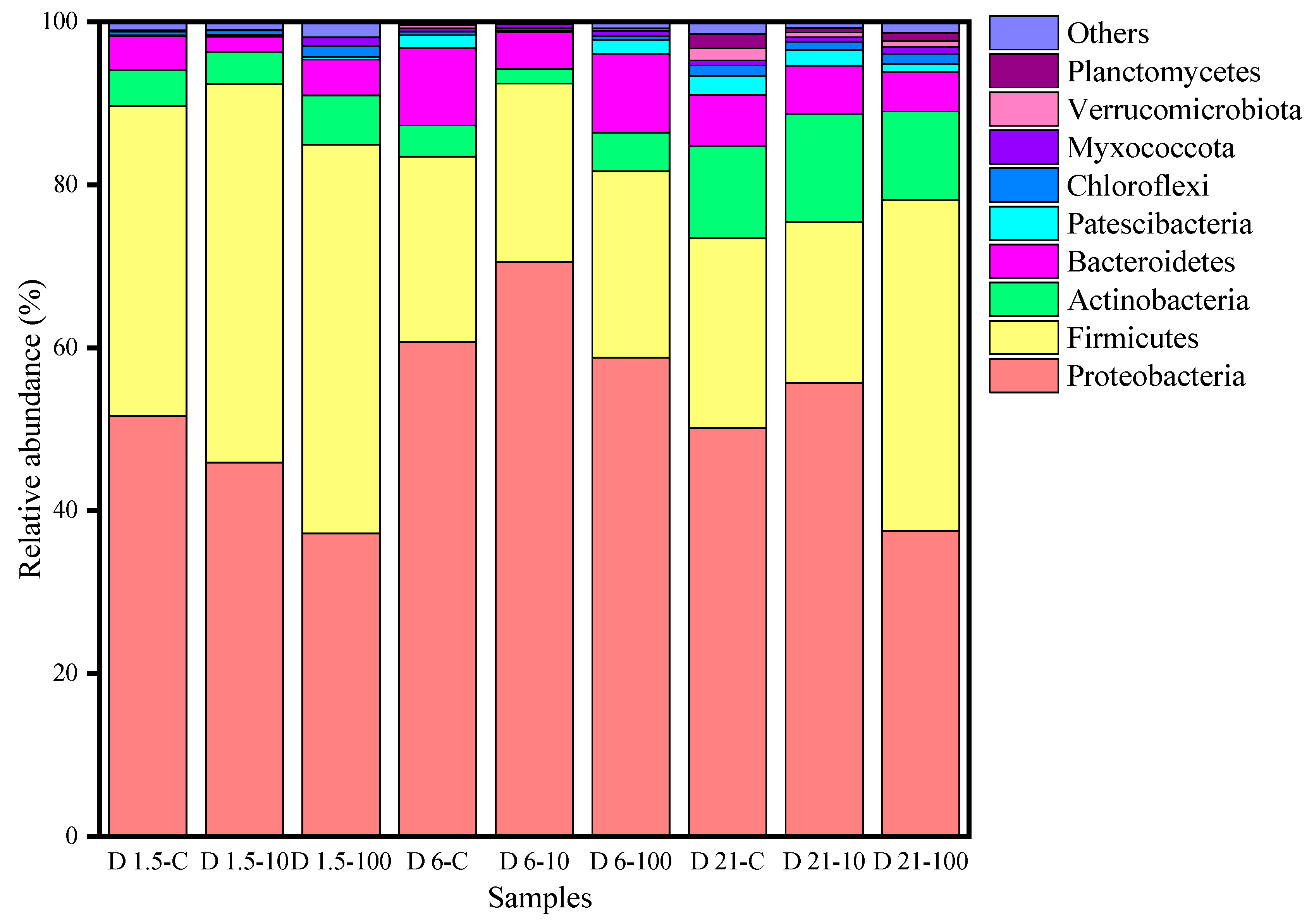
3.3. Effect of Cimetidine on Microbial Community Succession during Human Feces Composting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, T.; Yu, S.; Chen, W. Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China. Chemosphere 2016, 152, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Toor, G.S.; Wilson, P.C.; Williams, C.F. Micropollutants in groundwater from septic systems: Transformations, transport mechanisms, and human health risk assessment. Water Res. 2017, 123, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Gupta, V. Utilizing drug repurposing against COVID-19-Efficacy, limitations, and challenges. Life Sci. 2020, 259, 118275. [Google Scholar] [CrossRef] [PubMed]
- MedTech, E. World Preview 2018, Outlook to 2024. 2018. Available online: https://info.evaluategroup.com/rs/607-YGS-364/images/WPMT2018.pdf (accessed on 21 August 2022).
- Jechalke, S.; Heuer, H.; Siemens, J.; Amelung, W.; Smalla, K. Fate and effects of veterinary antibiotics in soil. Trends Microbiol. 2014, 22, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhang, J.; Liu, R.; Gan, J.; Liu, J.; Liu, W.P. Endocrine-Disrupting Effects of Pesticides through Interference with Human Glucocorticoid Receptor. Environ. Sci. Technol. 2016, 50, 435–443. [Google Scholar] [CrossRef]
- Ji, C.Y.; Song, Q.; Chen, Y.C.; Zhou, Z.Q.; Wang, P.; Liu, J.; Sun, Z.; Zhao, M.R. The potential endocrine disruption of pesticide transformation products (TPs): The blind spot of pesticide risk assessment. Environ. Int. 2020, 137, 105490. [Google Scholar] [CrossRef]
- Payne, N.A.; Gerber, J.G. The effect of beta andrenoceptor stimulation of canine stomach on pentagastrin-stimulated histamine release. J. Pharmacol. Exp. Ther. 1996, 276, 984–988. [Google Scholar]
- Duan, L.; Zhang, Y.; Wang, B.; Yu, G.; Gao, J.; Cagnetta, G.; Huang, C.; Zhai, N. Wastewater surveillance for 168 pharmaceuticals and metabolites in a WWTP: Occurrence, temporal variations and feasibility of metabolic biomarkers for intake estimation. Water Res. 2022, 216, 118321. [Google Scholar] [CrossRef]
- Anderson, P.D.; D’Aco, V.J.; Shanahan, P.; Chapra, S.C.; Buzby, M.E.; Cunningham, V.L.; Duplessie, B.M.; Hayes, E.P.; Mastrocco, F.J.; Parke, N.J.; et al. Screening analysis of human pharmaceutical compounds in US surface waters. Environ. Sci. Technol. 2004, 38, 838–849. [Google Scholar] [CrossRef]
- Choi, M.; Furlong, E.T.; Werner, S.L.; Pait, A.S.; Lee, I.-S.; Choi, H.-G. Cimetidine, acetaminophen, and 1, 7-dimethylxanthine, as indicators of wastewater pollution in marine sediments from Masan Bay, Korea. Ocean. Sci. J. 2014, 49, 231–240. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Banks, E.; Grover, D.; Jiang, J. Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. J. Hazard. Mater. 2009, 166, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Carrera, E.; Hansen, M.; León, V.M.; Björklund, E.; Krogh, K.A.; Halling-Sørensen, B.; González-Mazo, E. Multiresidue method for the determination of 32 human and veterinary pharmaceuticals in soil and sediment by pressurized-liquid extraction and LC-MS/MS. Anal. Bioanal. Chem. 2010, 398, 1173–1184. [Google Scholar] [CrossRef]
- Prosser, R.S.; Trapp, S.; Sibley, P.K. Modeling Uptake of Selected Pharmaceuticals and Personal Care Products into Food Crops from Biosolids-Amended Soil. Environ. Sci. Technol. 2014, 48, 11397–11404. [Google Scholar] [CrossRef]
- Lee, S.; Jung, D.; Kho, Y.; Ji, K.; Kim, P.; Ahn, B.; Choi, K. Ecotoxicological assessment of cimetidine and determination of its potential for endocrine disruption using three test organisms: Daphnia magna, Moina macrocopa, and Danio rerio. Chemosphere 2015, 135, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, P.D.; Rosi-Marshall, E.J.; Bechtold, H.A. The antihistamine cimetidine alters invertebrate growth and population dynamics in artificial streams. Freshw. Sci. 2012, 31, 379–388. [Google Scholar] [CrossRef]
- Manzato, M.C.; de Santi, F.; da Silva, A.A.S.; Beltrame, F.L.; Cerri, P.S.; Sasso-Cerri, E. Cimetidine-induced androgenic failure causes cell death and changes in actin, EGF and V-ATPase immunoexpression in rat submandibular glands. J. Anat. 2021, 239, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Kiszkiel-Taudul, I. Determination of antihistaminic pharmaceuticals in surface water samples by SPE-LC-MS/MS method. Microchem. J. 2021, 162, 105874. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, L.; Liu, F.; Li, Q.; Wei, X.C.; Liu, L.Y.; Li, H.Y.; Zheng, X.Q.; Xu, Y. Optimization of the proportion of multi-component rural solid wastes in mixed composting using a simplex centroid design. Bioresour. Technol. 2021, 341, 125746. [Google Scholar] [CrossRef]
- Iranzo, M.; Gamon, M.; Boluda, R.; Mormeneo, S. Analysis of pharmaceutical biodegradation of WWTP sludge using composting and identification of certain microorganisms involved in the process. Sci. Total Environ. 2018, 640, 840–848. [Google Scholar] [CrossRef]
- Xu, X.; Ma, W.; Zhou, K.; An, B.; Huo, M.; Lin, X.; Wang, L.; Wang, H.; Liu, Z.; Cheng, G.; et al. Effects of composting on the fate of doxycycline, microbial community, and antibiotic resistance genes in swine manure and broiler manure. Sci. Total Environ. 2022, 832, 155039. [Google Scholar] [CrossRef]
- Guo, H.; Gu, J.; Wang, X.; Nasir, M.; Yu, J.; Lei, L.; Wang, J.; Zhao, W.; Dai, X. Beneficial effects of bacterial agent/bentonite on nitrogen transformation and microbial community dynamics during aerobic composting of pig manure. Bioresour. Technol. 2020, 298, 122384. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.-Z.; Zeng, Y.; Wang, S.-P.; Sun, Z.-Y.; Tang, Y.-Q.; Kida, K. Insight into the microbiology of nitrogen cycle in the dairy manure composting process revealed by combining high-throughput sequencing and quantitative PCR. Bioresour. Technol. 2020, 301, 122760. [Google Scholar] [CrossRef] [PubMed]
- Vieuble Gonod, L.; Dellouh, L.P.Y.; Andriamalala, A.; Dumeny, V.; Bergheaud, V.; Cambier, P. Fate of sulfamethoxazole in compost, manure and soil amended with previously stored organic wastes. Sci. Total. Environ. 2022, 803, 150023. [Google Scholar] [CrossRef]
- Liu, N.; Hou, T.; Yin, H.J.; Han, L.J.; Huang, G.Q. Effects of amoxicillin on nitrogen transformation and bacterial community succession during aerobic composting. J. Hazard. Mater. 2019, 362, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Qian, X.; Gu, J.; Wang, X.; Gao, H. Effects of oxytetracycline on the abundance and community structure of nitrogen-fixing bacteria during cattle manure composting. Bioresour. Technol. 2016, 216, 801–807. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.Z.; Cheng, W.T.; Zhao, Y.; Cui, H.Y.; Xie, X.Y.; Wu, J.Q.; Wei, Z.M.; Liu, Y. Oxytetracycline stress reconstruct the core microbial community related to nitrogen transformation during composting. Bioresour. Technol. 2021, 319. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Yin, Z.; Pei, F.; Ye, Z.; Song, G.; Ling, H.; Gao, D.; Jiang, X.; Zhang, C.; Ge, J. Aerobic composting of chicken manure with penicillin G: Community classification and quorum sensing mediating its contribution to humification. Bioresour. Technol. 2022, 352, 127097. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Zhang, J.; Kong, Z.; Wang, X.; Liu, D.; Shen, Q. Contributions of the biochemical factors and bacterial community to the humification process of in situ large-scale aerobic composting. Bioresour. Technol. 2021, 323, 124599. [Google Scholar] [CrossRef]
- Guo, B.B.; Wu, J.P.; Chen, J.W.; Zhang, H.; Li, J.J. Effects of Chinese medicine herbal residues on antibiotic resistance genes and the bacterial community in chicken manure composting. J. Antibiot. 2022, 75, 164–171. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Ma, X.; Idrees, F.; Xu, B.; Hao, X.; Lin, W. From rice bran to high energy density supercapacitors: A new route to control porous structure of 3D carbon. Sci. Rep. 2014, 4, 7260. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Li, G.X.; Jiang, T.; Schuchardt, F.; Chen, T.B.; Zhao, Y.Q.; Shen, Y.J. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.L.; Wang, X.C.C.; Li, Q.; Jiang, S.Q. Degradation of typical antibiotics during human feces aerobic composting under different temperatures. Environ. Sci. Pollut. Res. 2016, 23, 15076–15087. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huang, D.; Shao, M.; Sun, R.; Xu, Q. Use of activated carbon to reduce ammonia emissions and accelerate humification in composting digestate from food waste. Bioresour. Technol. 2022, 347, 126701. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Li, X.; Bi, F.; Yan, X.; Wang, H.; Wu, W. Accelerating Food Waste Composting Course with Biodrying and Maturity Process: A Pilot Study. ACS Sustain. Chem. Eng. 2020, 9, 224–235. [Google Scholar] [CrossRef]
- Zhu, P.; Pan, X.; Shen, Y.; Huang, X.; Yu, F.; Wu, D.; Feng, Q.; Zhou, J.; Li, X. Biodegradation and potential effect of ranitidine during aerobic composting of human feces. Chemosphere 2022, 296, 134062. [Google Scholar] [CrossRef]
- Li, X.; Xing, M.; Yang, J.; Zhao, L.; Dai, X. Organic matter humification in vermifiltration process for domestic sewage sludge treatment by excitation-emission matrix fluorescence and Fourier transform infrared spectroscopy. J. Hazard. Mater. 2013, 261, 491–499. [Google Scholar] [CrossRef]
- Maqbool, T.; Qin, Y.; Ly, Q.V.; Zhang, J.; Li, C.; Asif, M.B.; Zhang, Z. Exploring the relative changes in dissolved organic matter for assessing the water quality of full-scale drinking water treatment plants using a fluorescence ratio approach. Water Res. 2020, 183, 116125. [Google Scholar] [CrossRef]
- Li, X.W.; Dai, X.H.; Dai, L.L.; Liu, Z.G. Two-dimensional FTIR correlation spectroscopy reveals chemical changes in dissolved organic matter during the biodrying process of raw sludge and anaerobically digested sludge. RSC Adv. 2015, 5, 82087–82096. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Alburquerque, J.A.; Sanchez-Monedero, M.A.; Roig, A.; Cayuela, M.L. Biochar accelerates organic matter degradation and enhances N mineralisation during composting of poultry manure without a relevant impact on gas emissions. Bioresour. Technol. 2015, 192, 272–279. [Google Scholar] [CrossRef]
- Lang, L.; Zhang, Y.; Yang, A.; Dong, J.; Li, W.; Zhang, G. Macrophage polarization induced by quinolone antibiotics at environmental residue level. Int. Immunopharmacol. 2022, 106, 108596. [Google Scholar] [CrossRef]
- Sardar, M.F.; Zhu, C.; Geng, B.; Ahmad, H.R.; Song, T.; Li, H. The fate of antibiotic resistance genes in cow manure composting: Shaped by temperature-controlled composting stages. Bioresour. Technol. 2021, 320, 124403. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Y.; Zhang, X.; Feng, C.; Gao, M.; Shen, Q. Effects of composting process on the dissipation of extractable sulfonamides in swine manure. Bioresour. Technol. 2015, 175, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.; Ward, B.J.; Strande, L.; Maurer, M. Review of synthetic human faeces and faecal sludge for sanitation and wasteWater Res. Water Res. 2018, 132, 222–240. [Google Scholar] [CrossRef]
- Qi, H.S.; Zhao, Y.; Zhao, X.Y.; Yang, T.X.; Dang, Q.L.; Wu, J.Q.; Lv, P.; Wang, H.; Wei, Z.M. Effect of manganese dioxide on the formation of humin during different agricultural organic wastes compostable environments: It is meaningful carbon sequestration. Bioresour. Technol. 2020, 299, 122596. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ren, X.N.; Pan, J.T.; Zhang, Z.Q.; Tsui, T.H.; Luo, L.W.; Wang, Q. Effect of microplastics on greenhouse gas and ammonia emissions during aerobic composting. Sci. Total Environ. 2020, 737, 139856. [Google Scholar] [CrossRef]
- Yu, H.; Xie, B.; Khan, R.; Shen, G. The changes in carbon, nitrogen components and humic substances during organic-inorganic aerobic co-composting. Bioresour. Technol. 2019, 271, 228–235. [Google Scholar] [CrossRef]
- Zhang, D.F.; Luo, W.H.; Yuan, J.; Li, G.X.; Luo, Y. Effects of woody peat and superphosphate on compost maturity and gaseous emissions during pig manure composting. Waste Manag. 2017, 68, 56–63. [Google Scholar] [CrossRef]
- He, X.-S.; Yang, C.; You, S.-H.; Zhang, H.; Xi, B.-D.; Yu, M.-D.; Liu, S.-J. Redox properties of compost-derived organic matter and their association with polarity and molecular weight. Sci. Total Environ. 2019, 665, 920–928. [Google Scholar] [CrossRef]
- Guo, X.J.; He, X.S.; Li, C.W.; Li, N.X. The binding properties of copper and lead onto compost-derived DOM using Fourier-transform infrared, UV-vis and fluorescence spectra combined with two-dimensional correlation analysis. J. Hazard. Mater. 2019, 365, 457–466. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.Q. Advances in the characterization and monitoring of natural organic matter using spectroscopic approaches. Water Res. 2021, 190, 116759. [Google Scholar] [CrossRef] [PubMed]
- Carstea, E.M.; Bridgeman, J.; Baker, A.; Reynolds, D.M. Fluorescence spectroscopy for wastewater monitoring: A review. Water Res. 2016, 95, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, X.; Zhao, M.; Zhao, W.; Liu, J.; Tang, J.; Liao, H.; Chen, Z.; Zhou, S. Hyperthermophilic composting accelerates the humification process of sewage sludge: Molecular characterization of dissolved organic matter using EEM–PARAFAC and two-dimensional correlation spectroscopy. Bioresour. Technol. 2019, 274, 198–206. [Google Scholar] [CrossRef] [PubMed]
- He, X.S.; Xi, B.D.; Wei, Z.M.; Guo, X.J.; Li, M.X.; An, D.; Liu, H.L. Spectroscopic characterization of water extractable organic matter during composting of municipal solid waste. Chemosphere 2011, 82, 541–548. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.J.; Baudon, A.; Chow, A.T. Improved fluorescence excitation-emission matrix regional integration to quantify spectra for fluorescent dissolved organic matter. J. Environ. Qual. 2013, 42, 925–930. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Li, X.; Yang, J.; Huang, Z.; Lu, Y. Changes in the chemical characteristics of water-extracted organic matter from vermicomposting of sewage sludge and cow dung. J. Hazard. Mater. 2012, 205, 24–31. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Liu, J.; Evrendilek, F.; Xie, W.; He, Y.; Buyukada, M. Comparative (co-)pyrolytic performances and by-products of textile dyeing sludge and cattle manure: Deeper insights from Py-GC/MS, TG-FTIR, 2D-COS and PCA analyses. J. Hazard. Mater. 2021, 401, 123276. [Google Scholar] [CrossRef]
- Li, W.W.; Zhang, F.F.; Ye, Q.; Wu, D.; Wang, L.Y.; Yu, Y.H.; Deng, B.; Du, J.Z. Composition and copper binding properties of aquatic fulvic acids in eutrophic Taihu Lake, China. Chemosphere 2017, 172, 496–504. [Google Scholar] [CrossRef]
- Guo, A.; Gu, J.; Wang, X.; Zhang, R.; Yin, Y.; Sun, W.; Tuo, X.; Zhang, L. Effects of superabsorbent polymers on the abundances of antibiotic resistance genes, mobile genetic elements, and the bacterial community during swine manure composting. Bioresour. Technol. 2017, 244, 658–663. [Google Scholar] [CrossRef]
- Sitthi, S.; Hatamoto, M.; Watari, T.; Yamaguchi, T. Accelerating anaerobic propionate degradation and studying microbial community using modified polyvinyl alcohol beads during anaerobic digestion. Bioresour. Technol. Rep. 2022, 17, 100907. [Google Scholar] [CrossRef]
- Zainudin, M.H.; Mustapha, N.A.; Maeda, T.; Ramli, N.; Sakai, K.; Hassan, M. Biochar enhanced the nitrifying and denitrifying bacterial communities during the composting of poultry manure and rice straw. Waste Manag. 2020, 106, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, M.; Bao, S.; Tang, W.; Fang, T. Nitrate removal from low carbon-to-nitrogen ratio wastewater by combining iron-based chemical reduction and autotrophic denitrification. Bioresour. Technol. 2020, 301, 122731. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Bai, X.; Liu, Y.; Huang, X. Simultaneous nitrification and aerobic denitrification by a novel isolated Ochrobactrum anthropi HND19. Bioresour. Technol. 2021, 340, 125582. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Pourcher, A.M.; Bouchez, T.; Gelhaye, E.; Peu, P. Occurrence of lignin degradation genotypes and phenotypes among prokaryotes. Appl. Microbiol. Biotechnol. 2014, 98, 9527–9544. [Google Scholar] [CrossRef]
- Zhao, H.P.; Van Ginkel, S.; Tang, Y.N.; Kang, D.W.; Rittmann, B.; Krajmalnik-Brown, R. Interactions between Perchlorate and Nitrate Reductions in the Biofilm of a Hydrogen-Based Membrane Biofilm Reactor. Environ. Sci. Technol. 2011, 45, 10155–10162. [Google Scholar] [CrossRef]
- Rosi-Marshall, E.J.; Kincaid, D.W.; Bechtold, H.A.; Royer, T.V.; Rojas, M.; Kelly, J.J. Pharmaceuticals suppress algal growth and microbial respiration and alter bacterial communities in stream biofilms. Ecol. Appl. 2013, 23, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Wei, Z.; Cao, Z.; Zhao, Y.; Zhao, X.; Lu, Q.; Wang, X.; Zhang, X. A regulating method for the distribution of phosphorus fractions based on environmental parameters related to the key phosphate-solubilizing bacteria during composting. Bioresour. Technol. 2016, 211, 610–617. [Google Scholar] [CrossRef]





| Samples | Peak 1 | Peak 2 | Peak 3 | Peak 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Ex/Em | SFI | Ex/Em | SFI | Ex/Em | SFI | Ex/Em | SFI | |
| 1.5 days | ||||||||
| Control | 225/320 | 110.35 | 280/325 | 174.81 | 335/425 | 178.93 | 285/420 | 167.71 |
| 10 mg/kg | 225/325 | 130.79 | 280/325 | 194.71 | 340/430 | 209.83 | 285/425 | 192.40 |
| 100 mg/kg | 225/325 | 115.59 | 280/325 | 169.41 | 340/430 | 191.33 | 285/420 | 182.51 |
| 6 days | ||||||||
| Control | 230/315 | 19.82 | 280/325 | 135.91 | 340/430 | 240.13 | 285/425 | 210.10 |
| 10 mg/kg | 230/325 | 27.73 | 280/325 | 128.11 | 340/430 | 232.83 | 285/425 | 193.90 |
| 100 mg/kg | 230/325 | 45.17 | 280/325 | 128.91 | 340/430 | 240.73 | 285/425 | 208.30 |
| 21 days | ||||||||
| Control | 230/325 | 9.00 | 280/325 | 96.85 | 335/425 | 311.01 | 285/425 | 247.55 |
| 10 mg/kg | 230/325 | 15.62 | 280/325 | 116.15 | 340/430 | 312.41 | 285/425 | 247.15 |
| 100 mg/kg | 230/325 | 10.57 | 280/325 | 90.05 | 340/430 | 296.81 | 285/425 | 241.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, X.; Pan, X.; Zhu, P.; Zhang, Q.; Huang, X.; Deng, X.; Wang, Z.; Ding, Y.; Liu, X.; et al. Potential Hormetic Effects of Cimetidine on Aerobic Composting of Human Feces from Rural China. Sustainability 2022, 14, 14454. https://doi.org/10.3390/su142114454
Li X, Wang X, Pan X, Zhu P, Zhang Q, Huang X, Deng X, Wang Z, Ding Y, Liu X, et al. Potential Hormetic Effects of Cimetidine on Aerobic Composting of Human Feces from Rural China. Sustainability. 2022; 14(21):14454. https://doi.org/10.3390/su142114454
Chicago/Turabian StyleLi, Xiaowei, Xuan Wang, Xusheng Pan, Ping Zhu, Qianzhi Zhang, Xiang Huang, Xiuquan Deng, Zhipu Wang, Yao Ding, Ximing Liu, and et al. 2022. "Potential Hormetic Effects of Cimetidine on Aerobic Composting of Human Feces from Rural China" Sustainability 14, no. 21: 14454. https://doi.org/10.3390/su142114454







