In Vitro Multiplication of Lophostemon suaveolens (Sol.ex Gaertn.) Peter G.Wilson & J.T. Waterh): Peatland Tree Species for Rehabilitation
Abstract
:1. Introduction
2. Materials and Methods
3. Multiplication of L. suaveolens
Rooting and Acclimatization of L. suaveolens
4. Results
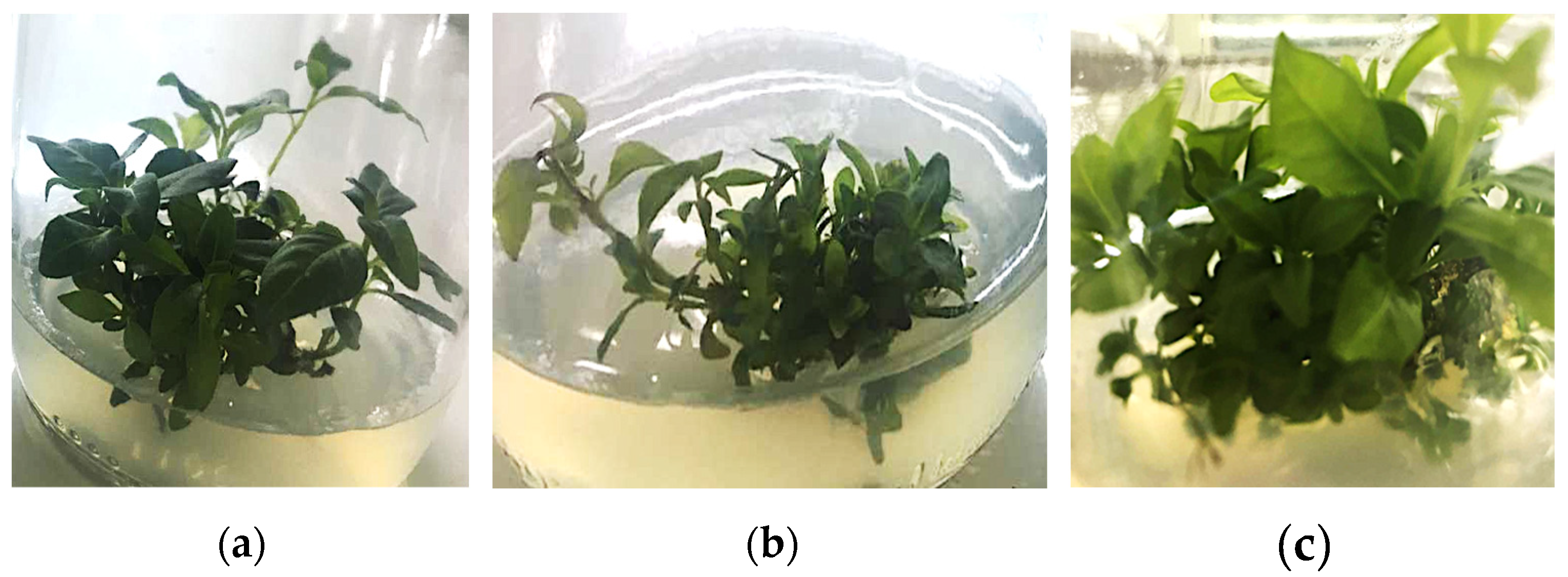
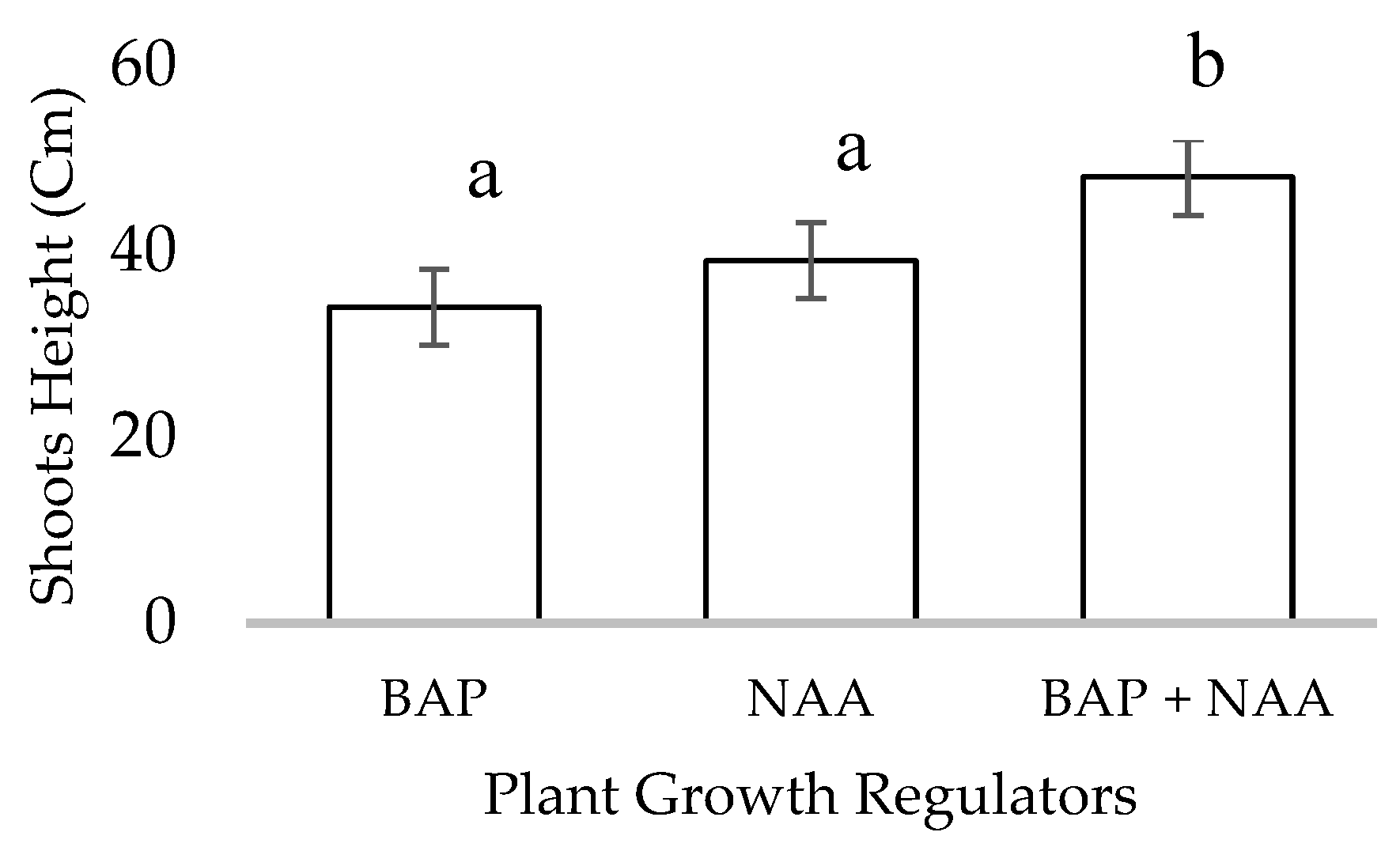
4.1. Multiplication of L. suaveolens
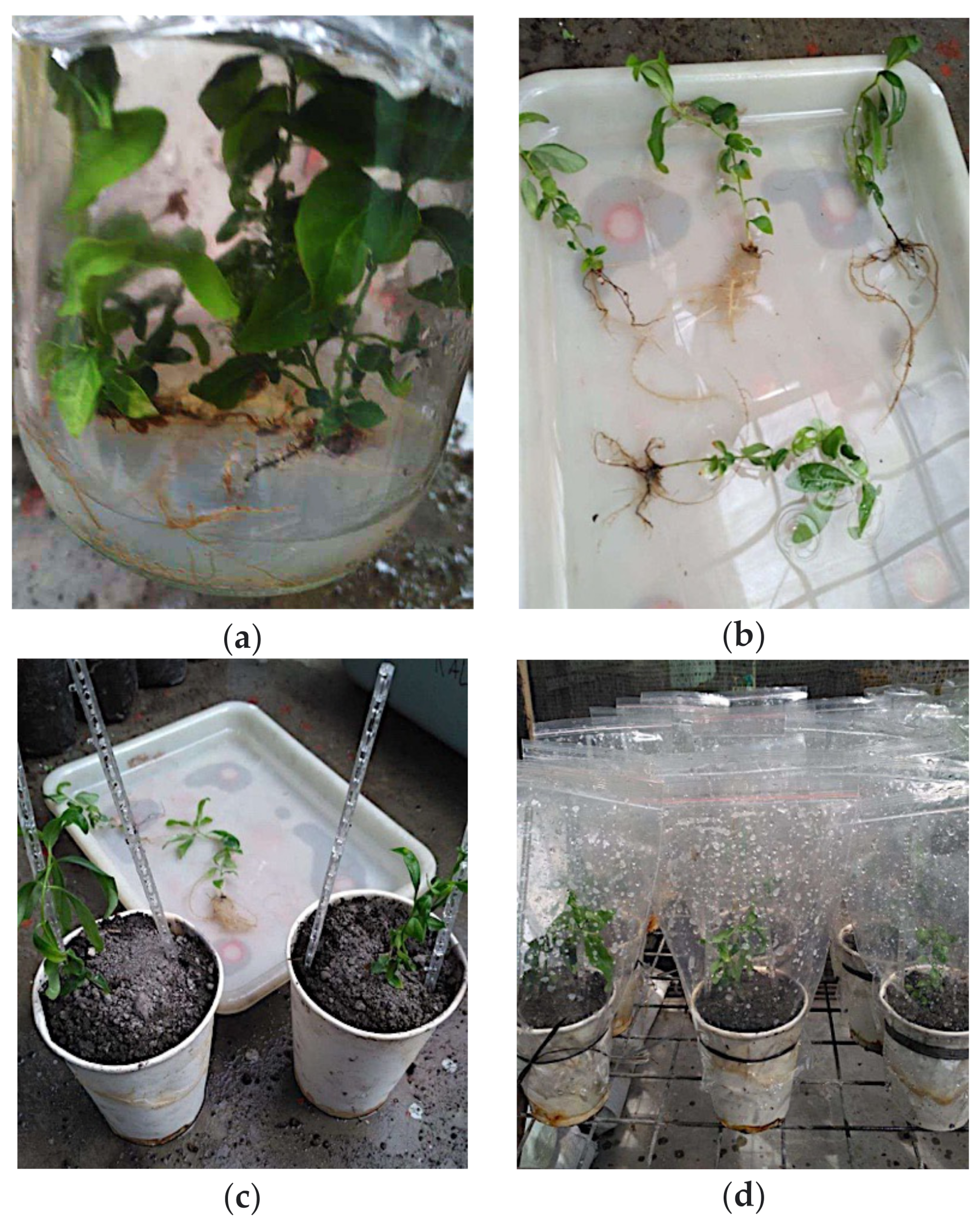
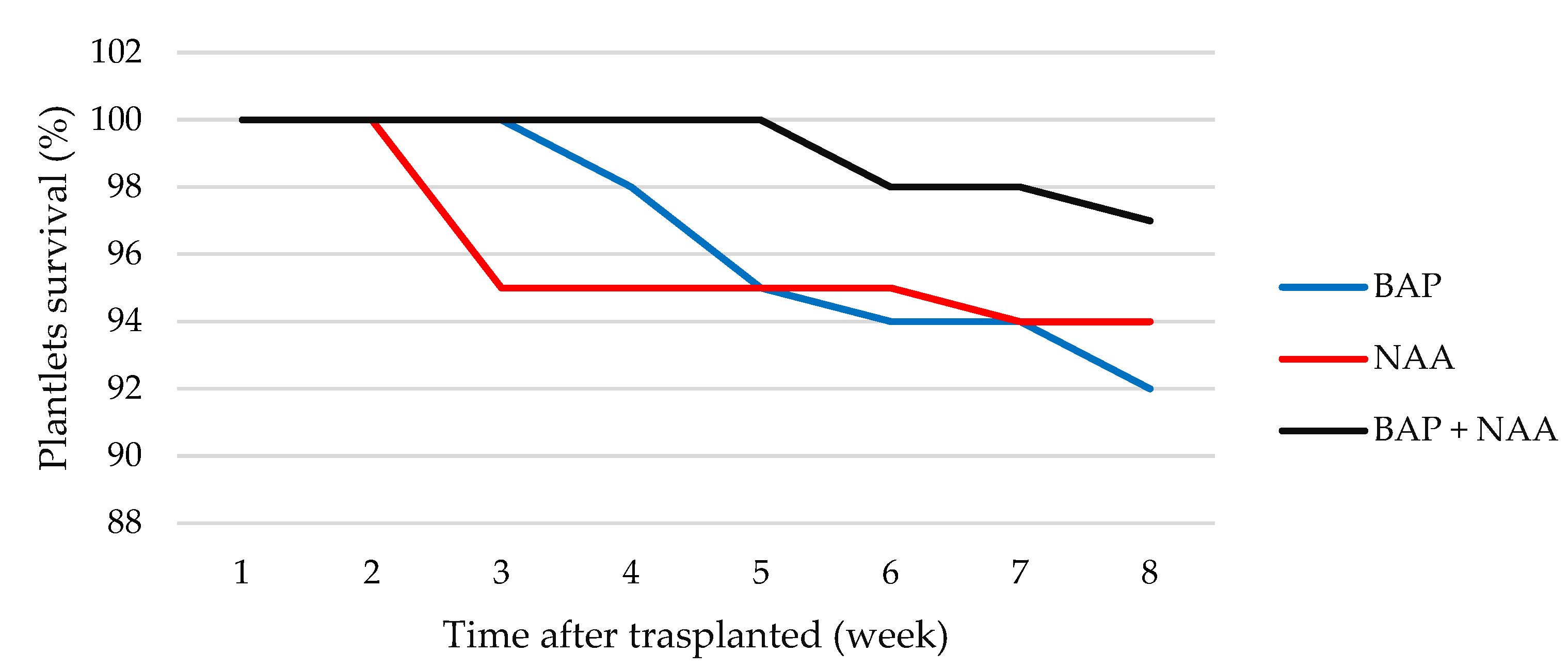
4.2. Rooting and Acclimatization of L. suaveolens
5. Discussion
5.1. Disinfection Culture Condition
5.2. Multiplication of L. suaveolens
5.3. Rooting and Acclimatization of L. suaveolens
6. Implication
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agus, F.; Anda, M.; Jamil, A. Lahan Gambut Indonesia: Pembentukan, Karakteristik, dan Potensi Mendukung Ketahanan Pangan, Edisi Revisi Cetakan I ed.; IAARD Press: Jakarta, Indonesia, 2016. [Google Scholar]
- Cochrane, M.A. Fire in the Tropics. In Tropical Fire Ecology; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Page, J.; Siegert, S.; Rieley, F. The Amount of Carbon Released from Peat and Forest Fires in Indonesia During 1997. Nature 2002, 420, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, W.; Song, Q.; Qu, K.; Wang, G.; Wang, Y.; Li, X.; Li, H.; Liy, J.; Li, G. Remotely sensing the ecological influences of ditches in Zoige Peatland, eastern Tibetan Plateau. Int. J. Remote Sens. 2014, 35, 5186–5197. [Google Scholar] [CrossRef]
- Valjarević, A.; Djekić, T.; Stevanović, V.; Ivanović, R.; Jandziković, B. GIS numerical and remote sensing analyses of forest changes in the Toplica region for the period of 1953–2013. Appl. Geogr. 2018, 92, 131–139. [Google Scholar] [CrossRef]
- Clark, J.S.; Bell, D.M.; Hersh, M.H.; Kwit, M.C.; Moran, E.; Salk, C.; Stine, A.; Valle, D.; Zhu, K. Individual-scale variation, species-scale differences: Inference needed to understand diversity. Ecol. Lett. 2011, 14, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Anzaldua, H.; Gerdes, G. Restoring Peatlands and Applying Concepts for Sustainable Management in Belarus—An Analysis of Project Implementation and Cost-Effectiveness; Gerardo Anzaldua and Holger Gerdes Ecologic Institute: Berlin, Germany, 2011. [Google Scholar]
- de Souza, R.A.; Dantas, P.V.; de Freitas Cavalcante, P.; Tenório, R.R.; Houllou, L.M. Cavalcante Basic procedure for the in vitro propagation of Brazilian trees for reforestation purposes. J. Environ. Anal. Prog. 2017, 2, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Jauhiainen, J.; Silvennoinen, H.; Könönen, M.; Limin, S.; Vasander, H. Management driven changes in carbon mineralization dynamics of tropical peat. Biogeochemistry 2016, 129, 115–132. [Google Scholar] [CrossRef]
- Jaenicke, J.; Englhart, S.; Siegert, F. Monitoring the effect of restoration measures in Indonesian peatlands by radar satellite imagery. J. Environ. Manag. 2010, 92, 630–638. [Google Scholar] [CrossRef]
- Wilson, P.G.; Heslewood, M.M. Phylogenetic position of Meteoromyrtus (Myrtaceae). Telopea J. Plant Syst. 2016, 19, 45–55. [Google Scholar]
- Mangera, Y. Analisis vegetasi jenis pohon di kawasan hutan kampung wasur pada taman nasional wasur distrik merauke kabupaten merauke. J. Agric. 2008, 1, 18–36. [Google Scholar]
- von Arnold, S.; Eriksson, T. In vitro studies of adventitious shoot formation in Pinus contorta. Can. J. Bot. 1981, 59, 870–874. [Google Scholar] [CrossRef]
- Khan, T.; Khan, M.A.; Karam, K.; Ullah, N.; Mashwani, Z.U.; Nadhman, A. Plant in vitro Culture Technologies; A Promise Into Factories of Secondary Metabolites Against COVID-19. Front. Plant Sci. 2021, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Brockman, H.G.; Brennan, R.F.; van Burgel, A. The impact of phytohormone concentration in Moringa oleifera leaf extract on wheat yield and components of yield. J. Plant Nutr. 2020, 43, 396–406. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2012, 107, 1203–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, M.; Robert, S.; Kleine-Vehn, J. Auxin: Simply complicated. J. Exp. Bot. 2013, 64, 2565–2577. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin–Cytokinin Interaction Regulates Meristem Development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chang, J.C. Development of an Improved Micropropagation Protocol for Red-Fleshed Pitaya ‘Da Hong’ with and without Activated Charcoal and Plant Growth Regulator Combinations. Horticulturae 2022, 8, 104. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Micropropagation. In Plant Tissue Culture: An Introductory Text; Bhojwani, S.S., Dantu, P.K., Eds.; Springer: New Delhi, India, 2013; pp. 245–274. [Google Scholar]
- Hesami, M.; Naderi, R.; Tohidfar, M. Modeling and Optimizing in vitro Sterilization of Chrysanthemum via Multilayer Perceptron-Non-dominated Sorting Genetic Algorithm-II (MLP-NSGAII). Front. Plant Sci. 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Permanasari, A.E.; Rambli, D.R.A.; Dominic, P.D.D. Forecasting method selection using ANOVA and Duncan multiple range tests on time series dataset. In Proceedings of the 2010 International Symposium on Information Technology—Engineering Technology, Kuala Lumpur, Malaysia, 15–17 June 2010; Volume 2, pp. 941–945. [Google Scholar]
- Kartikaningtyas, D.; Setiyaji, T.; Surip, N.F.N. The Effectivity of Planting Media and Fertilizer in Sprouting Ability of Kess (Lophostemon suaveolens (Sol.ex Gaertn.) Peter G.Wilson & J.T. Waterh) Stool Plant. J. Perbenihan Tanam. Hutan 2019, 7, 67–76. [Google Scholar]
- Williams, T.M. The Mechanism of Action of Isothiazolone Biocide. In Proceedings of the CORROSION 2006, San Diego, CA, USA, 12–16 March 2006; p. NACE-06090. [Google Scholar]
- Altan, F.; Burun, B.; Sahin, N. Fungal contaminants observed during micropropagation of Lilium candidum L. and the effect of chemotherapeutic substances applied after sterilization. African J. Biotechnol. 2010, 7, 991–995. [Google Scholar]
- Valente, T.A.M.; Silva, D.M.; Gomes, P.S.; Fernandes, M.H.; Santos, J.D.; Sencadas, V. Effect of Sterilization Methods on Electrospun Poly(lactic acid) (PLA) Fiber Alignment for Biomedical Applications. ACS Appl. Mater. Interfaces 2014, 8, 3241–3249. [Google Scholar] [CrossRef] [Green Version]
- Hesami, M.; Naderi, R.; Yoosefzadeh, N.M. Optimizing sterilization conditions and growth regulator effects on in vitro shoot regeneration through direct organogenesis in Chenopodium quinoa. J. Biotechnol. Comput. Biol. Bionanotechnol. 2018, 99, 49–57. [Google Scholar] [CrossRef]
- Blin, T.; Purohit, V.; Leprince, J.; Jouenne, T.; Glinel, K. Bactericidal Microparticles Decorated by an Antimicrobial Peptide for the Easy Disinfection of Sensitive Aqueous Solutions. Biomacromolecules 2011, 12, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Kane, M.E.; Chen, J. Effects of genotype, explant source, and plant growth regulators on indirect shoot organogenesis in Dieffenbachia cultivars. Vitr. Celluler Dev. Biol. Plant 2008, 44, 282–288. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; Klerk, G.J. Plant Growth Regulators I: Introduction; Auxins, their Analogues and Inhibitors. In Plant Propagation by Tissue Culture; Springer: Dordrecht, The Netherlands, 2008; pp. 175–204. [Google Scholar]
- George, E.F.; Hall, M.A.; Klerk, G.J. Plant Growth Regulators II: Cytokinins, their Analogues and Antagonists. In Plant Propagation by Tissue Culture; Springer: Dordrecht, The Netherlands, 2008; pp. 205–226. [Google Scholar]
- Herman, E.B. Recent Advances in Plant Tissue Culture; Agritech Consultants, Inc.: Shrub Oak, NY, USA, 2006. [Google Scholar]
- Thorpe, T.A. History of Plant Tissue Culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Hamill, J.D. Ultrastructural Studies of Somatic Embryos of Eucalyptus nitens and Comparisons with Zygotic Embryos Found in Mature Seeds. Ann. Bot. 2000, 86, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Carmen, S.J.; Jose, C.M.; Corredoira, E. Histology of the regeneration of Paulownia tomentosa (Paulowniaceae) by organogenesis. Rev. Biol. Trop. 2014, 62, 809–818. [Google Scholar]
- Anggarwal, G.; Gaur, A.; Srivastava, D.K. Establishment of high frequency shoot regeneration system in Himalayan poplar (Populus ciliata Wall. ex Royle) from petiole explants using Thidiazuron cytokinin as plant growth regulator. J. For. Res. 2015, 26, 651–656. [Google Scholar] [CrossRef]
- Moteuuis, O.; Galiana, A.; Goh, D. In Vitro Propagation of Acacia mangium and A. mangium × A. auriculiformis. Methods Mol. Biol. 2013, 11013, 199–211. [Google Scholar]
- Ibanez, C.M.; Camus, P.A.; Rocha, F.J. Diversity and distribution of cephalopod species off the coast of Chile. Mar. Biol. Res. 2019, 5, 374. [Google Scholar] [CrossRef]
- Darby, D.; Bai, S.H.; Wallace, H.M.; Truemen, S.J. Micropropagation of the therapeutic-honey plants Leptospermum polygalifolium and L. scoparium (Myrtaceae). Aust. J. Bot. 2021, 69, 310–317. [Google Scholar] [CrossRef]
- Lebedev, V.; Schestibratov, K. Effect of natural and synthetic growth stimulators on in vitro rooting and acclimatization of common ash (Fraxinus excelsior L.) microplants. Nat. Sci. 2013, 5, 1095. [Google Scholar]
- Mathur, A.; Mathur, A.K.; Verma, P.; Yadav, S.; Gupta, M.L.; Darokar, M.P. Biological hardening and genetic fidelity testing of micro-cloned progeny of Chlorophytum borivilianum Sant. et Fernand. African J. Biotechnol. 2008, 7, 1046–1053. [Google Scholar]
- Indonesia Water Portal, Sustainability Times. A Peatland Restoration Project in Indonesia Could set a Global Example. Sustainability Times, 3 December 2021. Available online: https://www.indonesiawaterportal.com/news/a-peatland-restoration-project-in-indonesia-could-set-a-global-example.html (accessed on 17 September 2022).



| PGRs | Nodule Multiplication Coefficients (NMC) | Shoot Length (cm) | Root Length (cm) | Leave Number |
|---|---|---|---|---|
| BAP | 6.0 ± 0.07 b | 4.72 ± 0.13 b | 5.03 ± 0.11 a | 30 ± 0.01 a |
| NAA | 5.1 ± 0.09 a | 2.21 ± 0.09 a | 5.10 ± 0.09 a | 29 ± 0.12 a |
| BAP and NAA | 8.4 ± 0.13 c | 5.91 ± 0.17 c | 8.83 ± 0.69 b | 32 ± 0.07 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putri, A.I.; Kartikawati, N.K.; Nirsatmanto, A.; Sunarti, S.; Haryjanto, L.; Herawan, T.; Santosa, P.B.; Wahyuningtyas, R.S.; Lestari, F.; Rimbawanto, A. In Vitro Multiplication of Lophostemon suaveolens (Sol.ex Gaertn.) Peter G.Wilson & J.T. Waterh): Peatland Tree Species for Rehabilitation. Sustainability 2022, 14, 14720. https://doi.org/10.3390/su142214720
Putri AI, Kartikawati NK, Nirsatmanto A, Sunarti S, Haryjanto L, Herawan T, Santosa PB, Wahyuningtyas RS, Lestari F, Rimbawanto A. In Vitro Multiplication of Lophostemon suaveolens (Sol.ex Gaertn.) Peter G.Wilson & J.T. Waterh): Peatland Tree Species for Rehabilitation. Sustainability. 2022; 14(22):14720. https://doi.org/10.3390/su142214720
Chicago/Turabian StylePutri, Asri Insiana, Noor Khomsah Kartikawati, Arif Nirsatmanto, Sri Sunarti, Liliek Haryjanto, Toni Herawan, Purwanto Budi Santosa, Reni Setyo Wahyuningtyas, Fajar Lestari, and Anto Rimbawanto. 2022. "In Vitro Multiplication of Lophostemon suaveolens (Sol.ex Gaertn.) Peter G.Wilson & J.T. Waterh): Peatland Tree Species for Rehabilitation" Sustainability 14, no. 22: 14720. https://doi.org/10.3390/su142214720






