Synergistic Activity of Dimethyl Disulfide Mixtures with Two Chemical Compounds against Meloidogyne incognita
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nematode Source
2.2. Test Compounds
2.3. Determination of the Toxicity of DMDS, CuSO4, and NH4HCO3 on M. incognita J2
2.4. Determination of Toxicity of DMDS with CuSO4 or NH4HCO3 on M. incognita J2
2.5. Statistical Analysis of Data
3. Results and Discussion
3.1. Lethal Effect of DMDS, CuSO4, and NH4HCO3 on M. incognita J2
3.2. Lethal Effect of DMDS with CuSO4 or NH4HCO3 on M. incognita J2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qinge, L.I.U. Research Progress in the Control of Plant Nematode. J. Anhui Agric. Sci. 2006, 34, 4644–4645, 4664. [Google Scholar]
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasser, J.N. Plant-Parasitic Nematodes: The Farmer’s Hidden Enemy; Excel India Publishers: New Delhi, India, 1989. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-Lopez, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Peshin, R.; Dhawan, A.K. Integrated Pest Management: Innovation-Development Process; Springer: Dordrecht, The Netherlands, 2009; p. 688. [Google Scholar]
- Ntalli, N.G.; Caboni, P. Botanical Nematicides: A Review. J. Agric. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef]
- Chen, J.X.; Li, Q.X.; Song, B.A. Chemical Nematicides: Recent Research Progress and Outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef]
- Hajihassani, A. Chemical Nematicides for Control of Plant-Parasitic Nematodes in Georgia Vegetable Crops; University of Georgia: Athens, GA, USA, 2018. [Google Scholar]
- Desaeger, J.; Wram, C.; Zasada, I. New reduced-risk agricultural nematicides—Rationale and review. J. Nematol. 2020, 52, e2020-91. [Google Scholar] [CrossRef]
- MBTOC. Methyl Bromide Technical Options Committee 2018 Assessment Report; United Nations Environment Programme: Nairobi, Kenya, 2019. [Google Scholar]
- Wang, Q.; Yan, D.; Wang, X.; Xiang, L.P.; Li, X.; Cao, A. Research advances in soil fumigants. J. Plant Prot. 2017, 44, 529–543. [Google Scholar]
- EPA. Aldicarb; Cancellation Order for Amendments to Terminate Usesfed. Regist; EPA: Washington, DC, USA, 2012. [Google Scholar]
- EPA. Carbofuran; Product Cancellation Orderfed. Regist; EPA: Washington, DC, USA, 2009. [Google Scholar]
- Hemmati, S.; Saeedizadeh, A. Root-knot nematode, Meloidogyne javanica, in response to soil fertilization. Braz. J. Biol. 2020, 80, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Kesba, H.H.; Al-Shalaby, M.E.M. Survival and reproduction of Meloidogyne incognita on tomato as affected by humic acid. Nematology 2008, 10, 243–249. [Google Scholar]
- Wang, Z.; Shi, Y.; Yuan, H.; Yang, W.; Li, H. The effects of nutrient elements on the hatching of cyst and vitality of secondary stage juvenile of cereal cyst nematode. Acta Phytopathol. Sin. 2013, 43, 333–336. [Google Scholar]
- Zhang, K.; Jia, Z. Method of Dyeing Perineal-striae of Root-knot Nematiodes. North. Hortic. 2008, 3, 207–208. [Google Scholar]
- Feng, Z. Plant Nematology; China Agriculture Press: Beijing, China, 2001; pp. 135–154. [Google Scholar]
- Feng, G.; Dong, L.; Chen, Y.; Shang, H.; Liu, Y.; Li, J.; Yang, J.; Cui, X.; Yang, P. PCR detection of nematode isolated from Panax notoginseng. Southwest China J. Agric. Sci. 2008, 21, 100–102. [Google Scholar]
- Zhang, F.; Yang, M.; Sun, J.; Hong, B.; Zhang, S. Specific Molecular Assay for the Detection of Greenhouse Vegetables Meloidogyne spp. in Shaanxi. Chin. Agric. Sci. Bull. 2014, 30, 136–140. [Google Scholar]
- Meng, Q.; Long, H.; Xu, J. PCR assays for rapid and sensitive identification of three major root-knot nematodes, Meloidogyne incognita, M. javanica and M. arenaria. Acta Phytopathol. Sin. 2004, 34, 204–210. [Google Scholar]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Mao, L.G.; Wang, Q.X.; Yan, D.D.; Xie, H.W.; Li, Y.; Guo, M.X.; Cao, A.C. Evaluation of the combination of 1,3-dichloropropene and dazomet as an efficient alternative to methyl bromide for cucumber production in China. Pest Manag. Sci. 2012, 68, 602–609. [Google Scholar] [CrossRef]
- Colby, S.R. Calculating synergistic and antagonistic responses of herbicide combinations. Weeds 1967, 15, 20–22. [Google Scholar] [CrossRef]
- Yan, D.; Cao, A.; Wang, Q.; Li, Y.; Canbin, O.; Guo, M.; Guo, X. Dimethyl disulfide (DMDS) as an effective soil fumigant against nematodes in China. PLoS ONE 2019, 14, e0224456. [Google Scholar] [CrossRef]
- Charles, P. DMDS: A New Alternative for Soil Disinfestation; MBAO: San Diego, CA, USA, 2003; pp. 1–4. [Google Scholar]
- Cabrera, J.A.; Wang, D.; Gerik, J.S.; Gan, J. Spot drip application of dimethyl disulfide as a post-plant treatment for the control of plant parasitic nematodes and soilborne pathogens in grape production. Pest Manag. Sci. 2014, 70, 1151–1157. [Google Scholar] [CrossRef]
- Charles, P. Biogenic Emission, Biological Origin, and Mode of Action of DMDS, a Natural Ubiquitous Fumigant; MBAO: San Diego, CA, USA, 2003; p. 138/1. [Google Scholar]
- Dugravot, E.; Grolleau, F.; Macherel, D.; Rochetaing, A.; Hue, B.; Stankiewicz, M.; Huignard, J.; Lapied, B. Dimethyl disulfide exerts insecticidal neurotoxicity through mitochondrial dysfunction and activation of insect K-ATP channels. J. Neurophysiol. 2003, 90, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouzounidou, G. Copper-induced changes in growth, metal content and photosynthetic function of Alyssum montanum L. plants. Environ. Exp. Bot. 1994, 34, 165–172. [Google Scholar] [CrossRef]
- Bai, C.; Duan, Y.; Chen, L.; Liu, Y.; Yu, X. The modes of action of inorganic compounds to Meloidogyne incognita. Plant Prot. 2011, 37, 74–78. [Google Scholar]
- Long, D.; Wu, Y.; Li, W.; Wang, Q.; Qin, Q. Toxicities of eight chemicals on Bursaphelenchus xylophilus. J. Zhejiang For. Sci. Technol. 2006, 26, 39–42. [Google Scholar]
- Zuo, Q.; Wu, F.; Zhang, S.; Xing, L.; Li, J.; Xiao, Q.; Liu, J. Effect of different nitrogen on root-knot nematodes and its soil microorganisms. Plant Prot. 2022, 48, 329–336 + 376. [Google Scholar]
- Treskova, V.S. Microelements and nematicides in the control of root-knot nematodes. Zashchita Rastenii Vred. Bolezn. 1959, 4, 26–27. [Google Scholar]
- Oka, Y.; Pivonia, S. Use of ammonia-releasing compounds for control of the root-knot nematode Meloidogyne javanica. Nematology 2002, 4, 65–71. [Google Scholar] [CrossRef]
- Su, L.; Ruan, Y.; Yang, X.; Wang, K.; Li, R.; Shen, Q. Suppression on plant-parasitic nematodes using a soil fumigation strategy based on ammonium bicarbonate and its effects on the nematode community. Sci. Rep. 2015, 5, 17597. [Google Scholar] [CrossRef] [Green Version]
- Oka, Y.; Tkachi, N.; Shuker, S.; Rosenberg, R.; Suriano, S.; Roded, L.; Fine, P. Field studies on the enhancement of nematicidal activity of ammonia-releasing fertilisers by alkaline amendments. Nematology 2006, 8, 881–893. [Google Scholar] [CrossRef]
- Cao, A.; Guo, M.; Wang, Q.; Li, Y.; Yan, D. Progress of Soil Disinfection Technology in the World. China Veg. 2010, 21, 17–22. [Google Scholar]
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; do Espirito Santo Pereira, A.; de Freitas Proença, P.L.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, Y.; Tkachi, N.; Shuker, S.; Yerumiyahu, U. Enhanced Nematicidal Activity of Organic and Inorganic Ammonia-Releasing Amendments by Azadirachta indica Extracts. J. Nematol. 2007, 39, 9–16. [Google Scholar] [PubMed]
- Zhao, M.; Zhou, H.; Hao, L.; Chen, H.; Zhou, X. A high-efficient nano pesticide-fertilizer combination fabricated by amino acid-modified cellulose based carriers. Pest Manag. Sci. 2022, 78, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.L.; Schrimsher, D.W.; Lawrence, K.S. Additional fertilizer and nematicide combinations on upland cotton to manage Rotylenchulus reniformis and Meloidogyne incognita in Alabama. J. Nematol. 2022, 54, 20220003. [Google Scholar]
- Yang, L.; Zhou, Q.; Wang, D.; Guo, R.; Fu, H.; Zhang, W.; Ge, B.; Li, S. Management of tomato diseases by a combined technique of soil fumigation with fertigation and biological agents in greenhouse. Chin. J. Biol. Control. 2022, 38, 890–895. [Google Scholar]
| Compound | Regression Equation | LC50 (mg/L) | 95% Confidence Interval | Correlation Coefficient R2 |
|---|---|---|---|---|
| DMDS | y = 2.22x − 2.77 | 19.28 | 17.25–21.61 | 0.93 |
| CuSO4 | y = 1.24x − 2.78 | 187.42 | 155.03–234.84 | 0.85 |
| NH4HCO3 | y = 1.57x − 3.66 | 213.49 | 191.59–236.13 | 0.99 |
| Treatment | Corrected Mortality (%) | |||||
|---|---|---|---|---|---|---|
| No. | Compound | Concentration (mg/L) | Ea | E0b | E − E0 c | CE d |
| 1 | DMDS | 2.5 | 4.42 | \ | \ | \ |
| 2 | DMDS | 10 | 11.59 | \ | \ | \ |
| 3 | DMDS | 20 | 35.13 | \ | \ | \ |
| 4 | CuSO4 | 46.58 | 13.02 | \ | \ | \ |
| 5 | CuSO4 | 186.33 | 50.11 | \ | \ | \ |
| 6 | NH4HCO3 | 80.25 | 26.02 | \ | \ | \ |
| 7 | NH4HCO3 | 241 | 52.15 | \ | \ | \ |
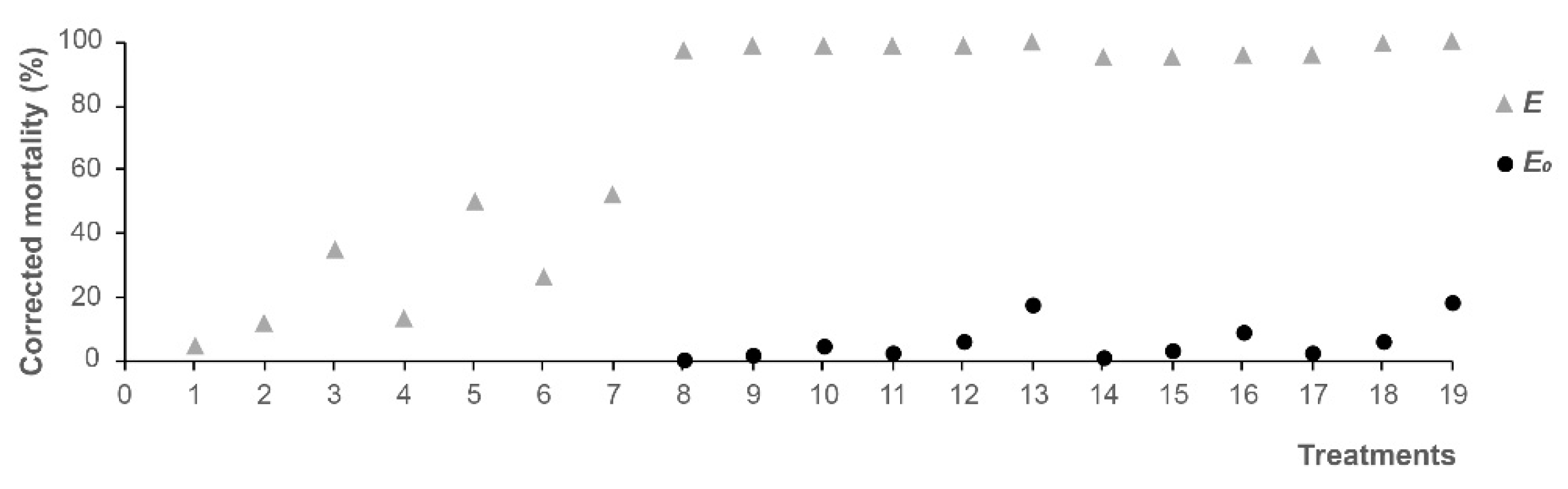
| 8 | DMDS + CuSO4 | 2.5 + 46.58 | 97.67 | 0.58 | 97.09 a | + |
| 9 | DMDS + CuSO4 | 10 + 46.58 | 98.46 | 1.51 | 96.95 a | + |
| 10 | DMDS + CuSO4 | 20 + 46.58 | 98.78 | 4.57 | 94.20 b | + |
| 11 | DMDS + CuSO4 | 2.5 + 186.33 | 98.99 | 2.21 | 96.78 a | + |
| 12 | DMDS + CuSO4 | 10 + 186.33 | 99.02 | 5.81 | 93.21 b | + |
| 13 | DMDS + CuSO4 | 20 + 186.33 | 100.00 | 17.60 | 82.40 c | + |
| 14 | DMDS + NH4HCO3 | 2.5 + 80.25 | 95.15 | 1.15 | 94.00 a | + |
| 15 | DMDS + NH4HCO3 | 10 + 80.25 | 95.50 | 3.02 | 92.48 a | + |
| 16 | DMDS + NH4HCO3 | 20 + 80.25 | 95.55 | 9.14 | 86.41 b | + |
| 17 | DMDS + NH4HCO3 | 2.5 + 241 | 96.06 | 2.30 | 93.76 a | + |
| 18 | DMDS + NH4HCO3 | 10 + 241 | 99.63 | 6.04 | 93.59 a | + |
| 19 | DMDS + NH4HCO3 | 20 + 241 | 100.00 | 18.32 | 81.68 c | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ren, L.; Zhang, D.; Song, Z.; Fang, W.; Li, Y.; Wang, Q.; Cao, A.; Yan, D. Synergistic Activity of Dimethyl Disulfide Mixtures with Two Chemical Compounds against Meloidogyne incognita. Sustainability 2022, 14, 16711. https://doi.org/10.3390/su142416711
Wang Q, Ren L, Zhang D, Song Z, Fang W, Li Y, Wang Q, Cao A, Yan D. Synergistic Activity of Dimethyl Disulfide Mixtures with Two Chemical Compounds against Meloidogyne incognita. Sustainability. 2022; 14(24):16711. https://doi.org/10.3390/su142416711
Chicago/Turabian StyleWang, Qing, Lirui Ren, Daqi Zhang, Zhaoxin Song, Wensheng Fang, Yuan Li, Qiuxia Wang, Aocheng Cao, and Dongdong Yan. 2022. "Synergistic Activity of Dimethyl Disulfide Mixtures with Two Chemical Compounds against Meloidogyne incognita" Sustainability 14, no. 24: 16711. https://doi.org/10.3390/su142416711





