Experimental Study on the Hydroponics of Wetland Plants for the Treatment of Acid Mine Drainage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic AMD Composition
2.2. Wetland Plants
2.3. Experimental Operation
2.4. Water Sample Analysis
2.5. Plant and Water Sediment Analysis
2.6. Statistical Analysis
3. Results and Discussion
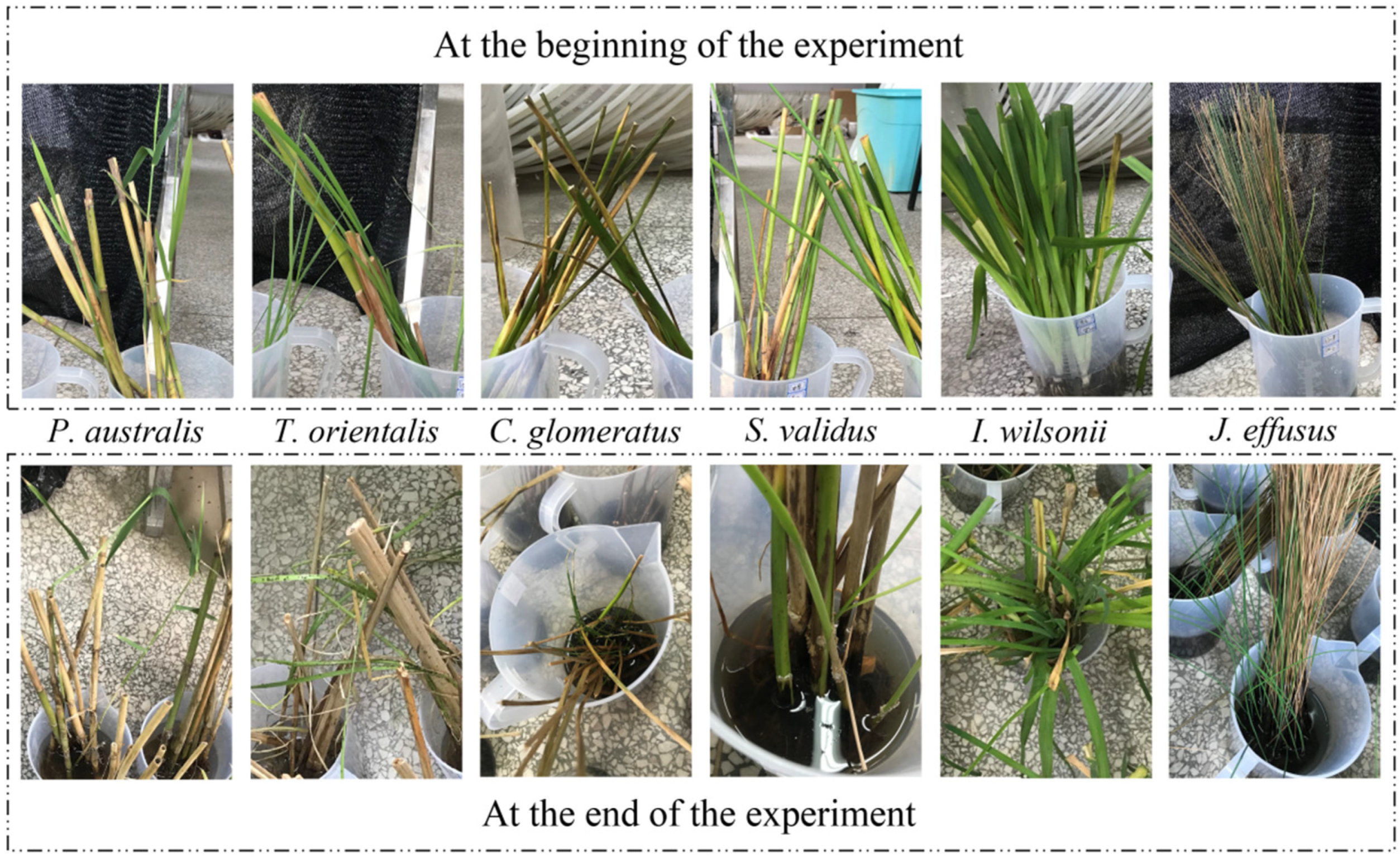
3.1. The Growth State of Plants
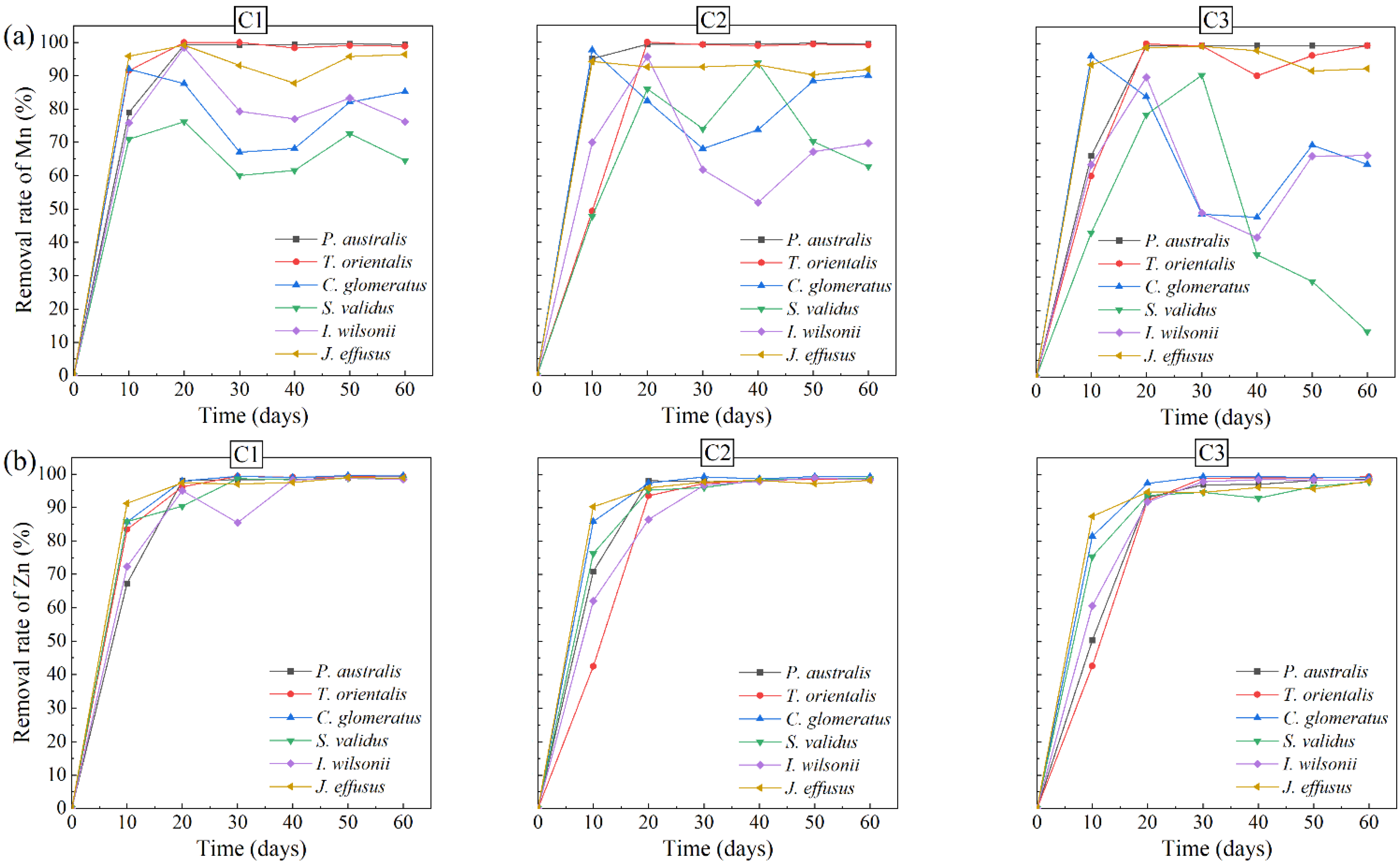
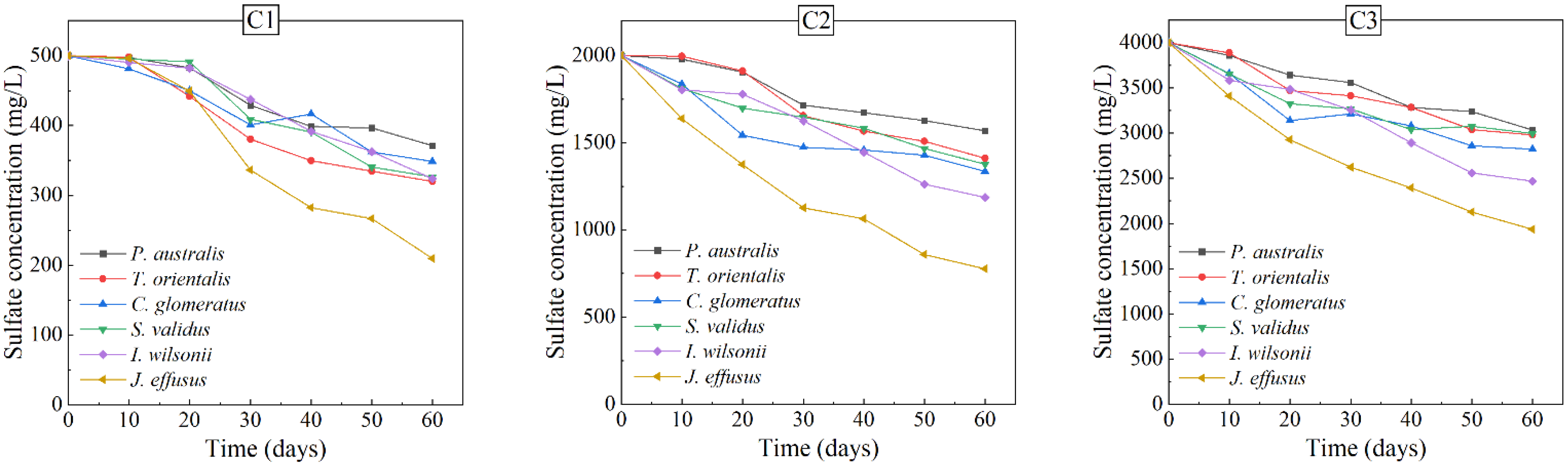
3.2. Removal of Contaminants in AMD
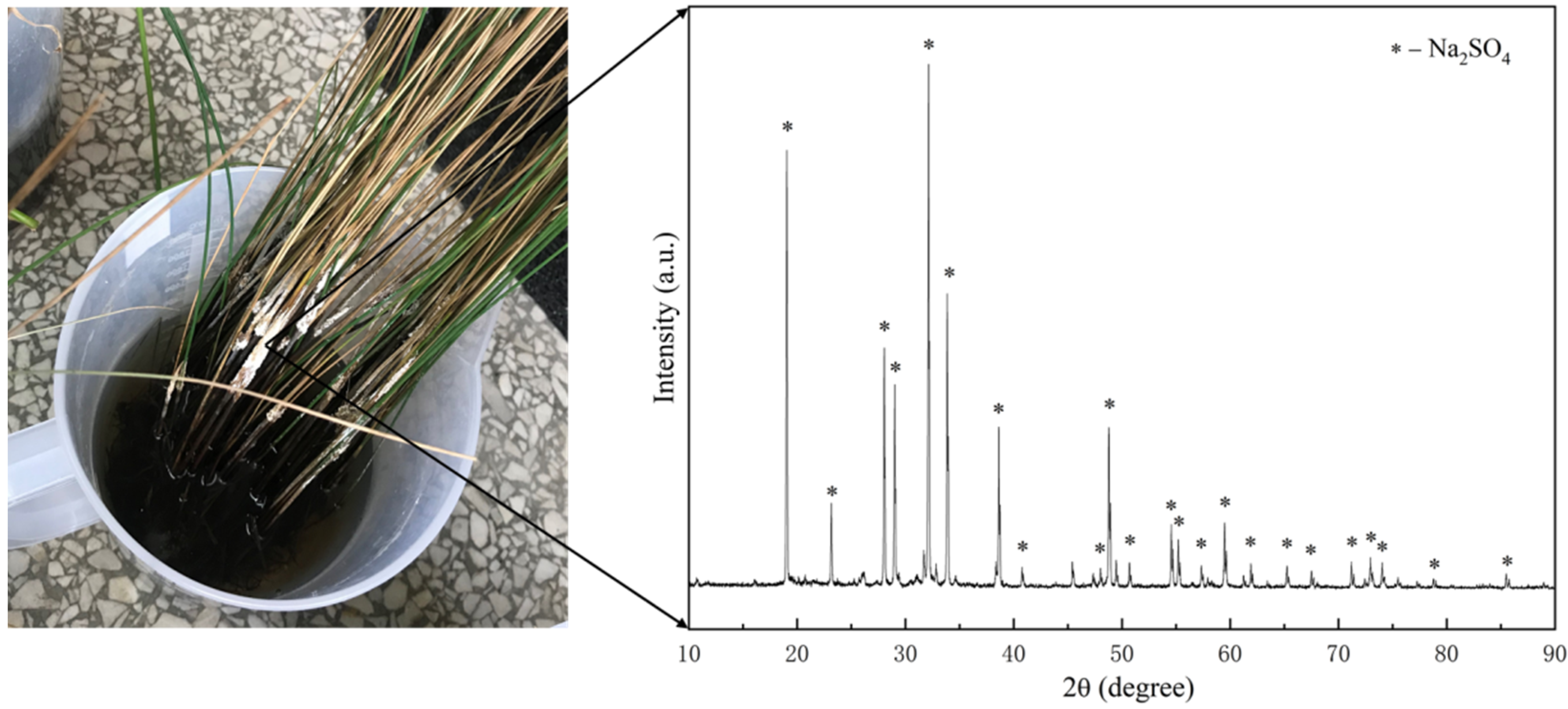
3.3. Removal Mechanism of Pollutants in AMD
4. Conclusions
- (1)
- There was no significant difference in the removal rates of Zn and Cd in AMD among the six plants, while the removal rates of SO42– and Mn in AMD varied greatly. Therefore, the six wetland plants were screened in terms of their growth status and the removal effects of the plants on pollutants in AMD, and Juncus effusus, Iris wilsonii and Phragmites australis were preferably finally selected as the dominant plants for the treatment of AMD.
- (2)
- The analysis of the uptake of pollutants in plants and the precipitates in AMD showed that the removal pathway of pollutants in AMD consisted of two aspects: one part was absorbed by the plants, and the other part was removed by means of hydrolysis, precipitation, etc. It was noteworthy that the plants first absorbed sodium sulfate into their bodies and then excreted the part that could not be absorbed and utilized by their own tissues, which precipitated as white crystals on the plant surface; hence, sulfate could be removed by harvesting.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernando, W.A.M.; Ilankoon, I.M.S.K.; Syed, T.H.; Yellishetty, M. Challenges and opportunities in the removal of sulphate ions in contaminated mine water: A review. Miner. Eng. 2018, 117, 74–90. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, S. Performance of organic substrate amended constructed wetland treating acid mine drainage (AMD) of North-Eastern India. J. Hazard. Mater. 2020, 397, 122719. [Google Scholar] [CrossRef]
- Guo, L.; Cutright, T.J. Remediation of AMD Contaminated Soil by Two Types of Reeds. Int. J. Phytore Mediat. 2015, 17, 391–403. [Google Scholar] [CrossRef]
- Mang, K.C.; Ntushelo, K. Phytoextraction and phytostabilisation approaches of heavy metal remediation in acid mine drainage with case studies: A review. Appl. Ecol. Environ. Res. 2019, 17, 6129–6149. [Google Scholar] [CrossRef]
- Cutright, T.J.; Senko, J.; Sivaram, S.; York, M. Evaluation of the Phytoextraction Potential at an Acid-Mine-Drainage-Impacted Site. Soil Sediment Contam. 2012, 21, 970–984. [Google Scholar] [CrossRef]
- Karaca, O.; Cameselle, C.; Reddy, K.R. Mine tailing disposal sites: Contamination problems, remedial options and phytocaps for sustainable remediation. Rev. Environ. Sci. Bio-Technol. 2018, 17, 205–228. [Google Scholar] [CrossRef]
- Nagy, A.; Magyar, T.; Juhasz, C.; Tamas, J. Phytoremediation of acid mine drainage using by-product of lysine fermentation. Water Sci. Technol. 2020, 81, 1507–1517. [Google Scholar] [CrossRef]
- Younger, P.L. The longevity of minewater pollution: A basis for decision-making. Sci. Total Environ. 1997, 194–195, 457–466. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Zhang, Z.; Li, H.; Wu, A.; Shi, H. Experimental research on various slags as a potential adsorbent for the removal of sulfate from acid mine drainage. J. Environ. Manag. 2020, 270, 110880. [Google Scholar] [CrossRef]
- Tong, L.; Fan, R.; Yang, S.; Li, C. Development and Status of the Treatment Technology for Acid Mine Drainage. Min. Metall. Explor. 2021, 38, 315–327. [Google Scholar] [CrossRef]
- Dutta, M.; Islam, N.; Rabha, S.; Narzary, B.; Bordoloi, M.; Saikia, D.; Silva, L.F.O.; Saikia, B.K. Acid mine drainage in an Indian high-sulfur coal mining area: Cytotoxicity assay and remediation study. J. Hazard. Mater. 2019, 389, 121851. [Google Scholar] [CrossRef]
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of Passive Systems for Acid Mine Drainage Treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Blanco, I.; Sapsford, D.J.; Trumm, D.; Pope, J.; Kruse, N.; Cheong, Y.-W.; McLauchlan, H.; Sinclair, E.; Weber, P.; Olds, W. International Trials of Vertical Flow Reactors for Coal Mine Water Treatment. Mine Water Environ. 2018, 37, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Pat-Espadas, A.M.; Loredo Portales, R.; Amabilis-Sosa, L.E.; Gomez, G.; Vidal, G. Review of Constructed Wetlands for Acid Mine Drainage Treatment. Water 2018, 10, 1685. [Google Scholar] [CrossRef] [Green Version]
- Palihakkara, C.R.; Dassanayake, S.; Jayawardena, C.; Senanayake, I.P. Floating Wetland Treatment of Acid Mine Drainage using Eichhornia crassipes (Water Hyacinth). J. Health Pollut. 2018, 8, 14–19. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Tech. 2008, 407, 3923–3930. [Google Scholar] [CrossRef]
- Oyuela Leguizamo, M.A.; Fernandez Gomez, W.D.; Sarmiento, M.C.G. Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands—A review. Chemosphere 2017, 168, 1230–1247. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Yang, Y.; Yuan, H.; Zhao, J.; Gu, J.; Huang, S. Pb uptake and toxicity to Iris halophila tested on Pb mine tailing materials. Environ. Pollut. 2016, 214, 510–516. [Google Scholar] [CrossRef]
- Zheng, Q. The Study on Mechanism of the Treatment of Acid Mine Drainage Using PRB with Loess-Steel Slag. Ph.D. Thesis, Taiyuan University of Technology, Taiyuan, China, 2020. [Google Scholar] [CrossRef]
- Chinese Academy of Sciences, Editorial Committee of the Flora of China. Flora of China; Science Press: Beijing, China, 1993. [Google Scholar]
- Park, J.H. Contrasting effects of Cr(III) and Cr(VI) on lettuce grown in hydroponics and soil: Chromium and manganese speciation. Environ. Pollut. 2020, 266, 115073. [Google Scholar] [CrossRef] [PubMed]
- Dan, A.; Oka, M.; Fujii, Y.; Soda, S.; Ishigaki, T.; Machimura, T.; Ike, M. Removal of heavy metals from synthetic landfill leachate in lab-scale vertical flow constructed wetlands. Sci. Total Environ. 2017, 584, 742–750. [Google Scholar] [CrossRef]
- Maser, P.; Eckelman, B.; Vaidyanathan, R.; Horie, T.; Fairbairn, D.J.; Kubo, M.; Yamagami, M.; Yamaguchi, K.; Nishimura, M.; Uozumi, N.; et al. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett. 2002, 531, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Wu, H. Plant salt tolerance and Na+ sensing and transport. Crop. J. 2018, 6, 215–225. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response Mechanisms of Plants Under Saline-Alkali Stress. Front. Plant Sci. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of heavy metals in a horizontal sub-surface flow constructed wetland. J. Environ. Sci. Health 2005, 40, 1369–1379. [Google Scholar] [CrossRef]
- Lesage, E. Behaviour of Heavy Metals in Constructed Treatment Wetlands; Ghent University: Ghent, Belgium, 2006. [Google Scholar]
- Wu, S.; Kuschk, P.; Wiessner, A.; Mueller, J.; Saad, R.A.B.; Dong, R. Sulphur transformations in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2013, 52, 278–289. [Google Scholar] [CrossRef]
- Aoyagi, T.; Hamai, T.; Hori, T.; Sato, Y.; Kobayashi, M.; Sato, Y.; Inaba, T.; Ogata, A.; Habe, H.; Sakata, T. Hydraulic retention time and pH affect the performance and microbial communities of passive bioreactors for treatment of acid mine drainage. AMB Express 2017, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Andrea, I.; Luis Sanz, J.; Bijmans, M.F.M.; Stams, A.J.M. Sulfate reduction at low pH to remediate acid mine drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef]
- Mulenga, C.; Clarke, C.; Meincken, M. Bioaccumulation of Cu, Fe, Mn and Zn in native Brachystegia longifolia naturally growing in a copper mining environment of Mufulira, Zambia. Environ. Monit. Assess. 2022, 194, 13. [Google Scholar] [CrossRef]
- Cruzado-Tafur, E.; Bierla, K.; Torro, L.; Szpunar, J. Accumulation of As, Ag, Cd, Cu, Pb, and Zn by Native Plants Growing in Soils Contaminated by Mining Environmental Liabilities in the Peruvian Andes. Plants 2021, 10, 241. [Google Scholar] [CrossRef] [PubMed]





| Theoretical Concentration (mg/L) | Reagent Used | Amount (mg) Per L Water | |
|---|---|---|---|
| SO42− | 500 (C1) | Na2SO4 | 739.5833 |
| 2000 (C2) | 2958.3333 | ||
| 4000 (C3) | 5916.6667 | ||
| Mn | 18 | MnCl2 | 41.1853 |
| Zn | 10 | Zn (NO3)2·6H2O | 45.7677 |
| Cd | 0.5 | Cd (NO3)2·4H2O | 1.3721 |
| pH | 4 | HCl |
| The Plant | Ecological Habits | Economic Value |
|---|---|---|
| P. australis | The perennial aquatic herb that grows along irrigation ditches, riverbank marshes, etc. It is found throughout the world and often forms contiguous reed colonies due to its rapidly expanding reproductive capacity. | It can be used for making medicine, paper, weaving, and construction, and has ornamental value. |
| T. orientalis | Perennial aquatic or marsh herb grows in lakes, ponds, ditches, rivers in slow-flowing shallow water, also seen in wetlands and swamps, can withstand low temperatures of −30 °C. | It is a weaving material, can be used for making medicine, paper, food, and has ornamental value |
| C. glomeratus | A perennial herb of the Cyperaceae family, growing mostly in wet places or swamps. | It can be used for weaving and making medicinal |
| S. validus | Perennial emergent aquatic herb, produced in many provinces in China, growing in lakesides or shallow ponds, and can tolerate low temperatures. | It can be used for weaving and has ornamental value. |
| I. wilsonii | Perennial herb, with fibers of old leaves remaining at the base of the plant, born on mountain slopes, forest margins, and wetlands along riverside ditches, light-loving, also more shade-tolerant, cold-hardy. | It has great ornamental value and can also be used to make medicine. |
| J. effusus | Perennial herbaceous aquatic plants, suitable for growing by rivers, ponds, ditches, rice fields, grasslands, marshes. | It can be used to weave utensils and make medicines, and the pith of the stem can be used to make lamp wicks and pillow wicks, etc. |
| Code | Experimental Water | pH | SO42− | Mn | Zn | Cd |
|---|---|---|---|---|---|---|
| mg/L | ||||||
| CK | Control group (distillate water) | 4 | 0 | 0 | 0 | 0 |
| C1 | Low sulfate concentration AMD | 500 | 18 | 10 | 0.5 | |
| C2 | Medium sulfate concentration AMD | 2000 | 18 | 10 | 0.5 | |
| C3 | High sulfate concentration AMD | 4000 | 18 | 10 | 0.5 | |
| The Plant | Large Number of New Shoots | Old Branches in Good Condition | No Root Rotted | No Pest Infestation | Aggregate |
|---|---|---|---|---|---|
| P. australis | × | √ | √ | √ | 3√ 1× |
| T. orientalis | × | × | × | × | 0√ 4× |
| C. glomeratus | √ | × | × | √ | 2√ 2× |
| S. validus | × | √ | × | × | 1√ 3× |
| I. wilsonii | × | √ | × | √ | 2√ 2× |
| J. effusus | √ | × | √ | √ | 3√ 1× |
| Parameters | The Plant | Mn | Zn | Cd |
|---|---|---|---|---|
| Concentration(mg/kg) | P. australis | 171.75 ± 11.24 | 71.01 ± 5.68 | 5.67 ± 0.96 |
| T. orientalis | 210.55 ± 16.69 | 101.76 ± 10.57 | 5.54 ± 0.57 | |
| C. glomeratus | 406.12 ± 20.25 | 86.32 ± 8.98 | 6.03 ± 0.84 | |
| S. validus | 450.23 ± 15.55 | 111.83 ± 13.54 | 7.12 ± 1.11 | |
| I. wilsonii | 503.89 ± 23.57 | 171.00 ± 15.14 | 11.48 ± 1.21 | |
| J. effusus | 393.27 ± 8.89 | 121.38 ± 9.63 | 9.11 ± 0.98 | |
| BCF | P. australis | 9.54 | 7.10 | 11.35 |
| T. orientalis | 11.70 | 10.18 | 11.08 | |
| C. glomeratus | 22.56 | 8.63 | 12.06 | |
| S. validus | 25.01 | 11.18 | 14.24 | |
| I. wilsonii | 27.99 | 17.10 | 22.96 | |
| J. effusus | 21.85 | 12.14 | 18.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, A.; Zhang, Y.; Zhao, X.; Li, J.; Zhang, G.; Shi, H.; Guo, L.; Xu, S. Experimental Study on the Hydroponics of Wetland Plants for the Treatment of Acid Mine Drainage. Sustainability 2022, 14, 2148. https://doi.org/10.3390/su14042148
Wu A, Zhang Y, Zhao X, Li J, Zhang G, Shi H, Guo L, Xu S. Experimental Study on the Hydroponics of Wetland Plants for the Treatment of Acid Mine Drainage. Sustainability. 2022; 14(4):2148. https://doi.org/10.3390/su14042148
Chicago/Turabian StyleWu, Aijing, Yongbo Zhang, Xuehua Zhao, Jiamin Li, Guowei Zhang, Hong Shi, Lina Guo, and Shuyuan Xu. 2022. "Experimental Study on the Hydroponics of Wetland Plants for the Treatment of Acid Mine Drainage" Sustainability 14, no. 4: 2148. https://doi.org/10.3390/su14042148






