Responses of Soil Phosphorus Fractions to Land-Use Change in Colombian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Areas
2.2. Soil Sampling and Analysis
2.3. Statistical Analysis
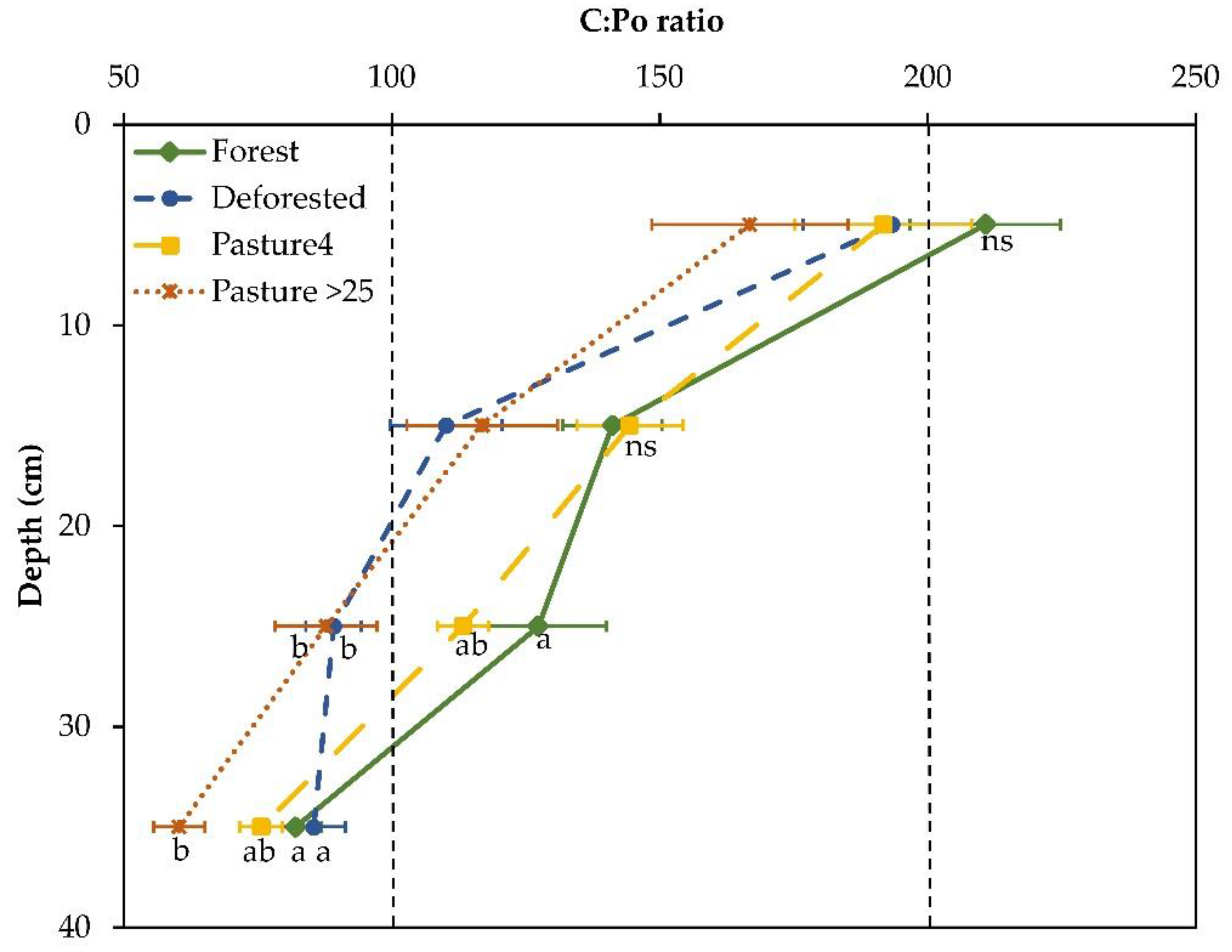
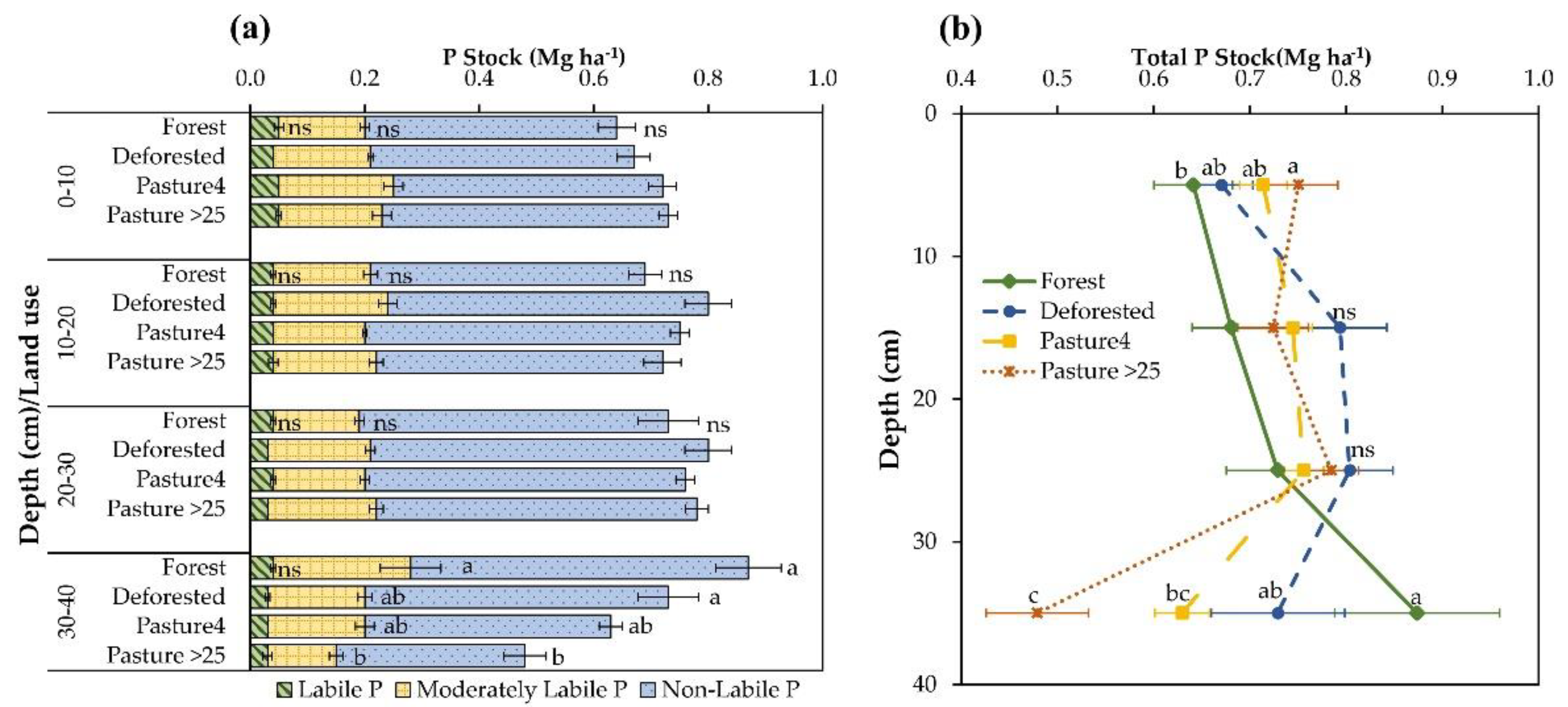
3. Results
3.1. Effect of Land-Use Change in the Soil P Fractions
3.2. Responses of C:Po Ratio, P Stocks, and Phosphatase Activity to Land-Use Change
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherubin, M.R.; Chavarro-Bermeo, J.P.; Silva-Olaya, A.M. Agroforestry systems improve soil physical quality in northwestern Colombian Amazon. Agrofor. Syst. 2019, 93, 1741–1753. [Google Scholar] [CrossRef]
- Marengo, J.A.; Souza, C.M.; Thonicke, K.; Burton, C.; Halladay, K.; Betts, R.A.; Alves, L.M.; Soares, W.R. Changes in Climate and Land Use Over the Amazon Region: Current and Future Variability and Trends. Front. Earth Sci. 2018, 6, 228. [Google Scholar] [CrossRef]
- Finer, M. Deforestación en la Amazonía 2020 (Final). MAAP: 136. 2020. Available online: https://maaproject.org/2021/amazon-2020/ (accessed on 20 September 2021).
- Finer, M. Deforestación en la Amazonía Colombiana—2020. MAAP #120. 2020. Available online: https://maaproject.org/2020/colombia-2020/#:~:text=En%20nuestro%20primer%20vistazo%20al,desde%20el%20acuerdo%20de%20paz (accessed on 21 September 2021).
- Garcia-Montiel, D.C.; Neill, C.; Melillo, J.; Thomas, S.; Steudler, P.A.; Cerri, C.C. Soil Phosphorus Transformations Following Forest Clearing for Pasture in the Brazilian Amazon. Soil Sci. Soc. Am. J. 2000, 64, 1792–1804. [Google Scholar] [CrossRef]
- Liu, J.; Cade-Menun, B.J.; Yang, J.; Hu, Y.; Liu, C.W.; Tremblay, J.; LaForge, K.; Schellenberg, M.; Hamel, C.; Bainard, L.D. Long-term land use affects phosphorus speciation and the composition of phosphorus cycling genes in agricultural soils. Front. Microbiol. 2018, 9, 1643. [Google Scholar] [CrossRef] [PubMed]
- Olaya-Montes, A.; Llanos-Cabrera, M.P.; Cherubin, M.R.; Herrera-Valencia, W.; Ortiz-Morea, F.A.; Silva-Olaya, A.M. Restoring soil carbon and chemical properties through silvopastoral adoption in the Colombian Amazon region. Land Degrad. Dev. 2020, 32, 3720–3730. [Google Scholar] [CrossRef]
- Silva-Olaya, A.M.; Mora-Motta, D.A.; Cherubin, M.R.; Grados, D.; Somenahally, A.; Ortiz-Morea, F.A. Soil enzyme responses to land use change in the tropical rainforest of the Colombian Amazon region. PLoS ONE 2021, 16, e0255669. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Hamer, U.; Potthast, K.; Burneo, J.I.; Makeschin, F. Nutrient stocks and phosphorus fractions in mountain soils of Southern Ecuador after conversion of forest to pasture. Biogeochemistry 2013, 112, 495–510. [Google Scholar] [CrossRef]
- Aguiar, A.D.C.F.; Cândido, C.S.; Carvalho, C.S.; Monroe, P.H.M.; De Moura, E.G. Organic matter fraction and pools of phosphorus as indicators of the impact of land use in the Amazonian periphery. Ecol. Indic. 2013, 30, 158–164. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Franco, A.L.C.; Cerri, C.E.P.; Karlen, D.L.; Pavinato, P.S.; Rodrigues, M.; Davies, C.A.; Cerri, C.C. Phosphorus pools responses to land-use change for sugarcane expansion in weathered Brazilian soils. Geoderma 2016, 265, 27–38. [Google Scholar] [CrossRef]
- Crews, T.E.; Brookes, P.C. Changes in soil phosphorus forms through time in perennial versus annual agroecosystems. Agric. Ecosyst. Environ. 2014, 184, 168–181. [Google Scholar] [CrossRef]
- Wright, A.L. Soil phosphorus stocks and distribution in chemical fractions for long-term sugarcane, pasture, turfgrass, and forest systems in Florida. Nutr. Cycl. Agroecosyst. 2009, 83, 223–231. [Google Scholar] [CrossRef]
- Gama-Rodrigues, A.C.; Sales, M.V.S.; Silva, P.S.D.; Comerford, N.B.; Cropper, W.P.; Gama-Rodrigues, E.F. An exploratory analysis of phosphorus transformations in tropical soils using structural equation modeling. Biogeochemistry 2014, 118, 453–469. [Google Scholar] [CrossRef]
- McGrath, D.A.; Duryea, M.L.; Cropper, W.P. Soil phosphorus availability and fine root proliferation in Amazonian agroforests 6 years following forest conversion. Agric. Ecosyst. Environ. 2001, 83, 271–284. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Dao, T.H.; Rosolem, C.A. Tillage and phosphorus management effects on enzyme-labile bioactive phosphorus availability in Cerrado Oxisols. Geoderma 2010, 156, 207–215. [Google Scholar] [CrossRef]
- Turner, B.L.; Engelbrecht, B.M.J. Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 2011, 103, 297–315. [Google Scholar] [CrossRef]
- Johnson, A.H.; Frizano, J.; Vann, D.R. Biogeochemical implications of labile phosphorus in forest soils determined by the Hedley fractionation procedure. Oecologia 2003, 135, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Jackson, M.L. Fractionation of soil phosphorus. Soil Sci. 1957, 84, 133–144. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. Soil Sampling and Methods of Analysis. Ed. MR Cart. P 1993, 7, 75–86. [Google Scholar]
- Gatiboni, L.C.; Condron, L.M. A rapid fractionation method for assessing key soil phosphorus parameters in agroecosystems. Geoderma 2021, 385, 114893. [Google Scholar] [CrossRef]
- Negassa, W.; Leinweber, P. How does the Hedley sequential phosphorus fractionation reflect impacts of land use and management on soil phosphorus: A review. J. Plant Nutr. Soil Sci. 2009, 172, 305–325. [Google Scholar] [CrossRef]
- Richter, D.D.; Allen, H.L.; Li, J.; Markewitz, D.; Raikes, J. Bioavailability of slowly cycling soil phosphorus: Major restructuring of soil P fractions over four decades in an aggrading forest. Oecologia 2006, 150, 259–271. [Google Scholar] [CrossRef]
- IGAC. Estudio General de Suelos y Zonificación de tierras: Departamento de Caquetá, Escala 1:100.000; Imprenta Nacional de Colombia: Bogotá, Colombia, 2014; ISBN 78 958 8323 73-2.
- Dane, J.H.; Hopmans, J.W.; Topp, G.C. Pressure plate extractor. Methods Soil Anal. Part 2002, 4, 688–690. [Google Scholar]
- Grossman, R.B.; Reinsch, T.G. 2.1 Bulk Density and Linear Extensibility; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 201–228. [Google Scholar]
- Condron, L.M.; Goh, K.M.; Newman, R.H. Nature and distribution of soil phosphorus as revealed by a sequential extraction method followed by 31P nuclear magnetic resonance analysis. J. Soil Sci. 1985, 36, 199–207. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Dick, W.A.; Tabatabai, M.A. Determination of Orthophosphate in Aqueous Solutions Containing Labile Organic and Inorganic Phosphorus Compounds. J. Environ. Qual. 1977, 6, 82–85. [Google Scholar] [CrossRef]
- Cross, A.F.; Schlesinger, W.H. A literature review and evaluation of the. Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1996; pp. 961–1010. [Google Scholar]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Hopmans, J.W.; Rolston, D.E.; Baer, S.G.; Six, J. Determining soil carbon stock changes: Simple bulk density corrections fail. Agric. Ecosyst. Environ. 2009, 134, 251–256. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J. Vis. Exp. 2013, 15, 50961. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husson, F.; Josse, J.; Le, S.; Maintainer, J.M. Package “FactoMineR”. Multivar. Explor. Data Anal. Data Min. 2016, 96, 698. [Google Scholar]
- Kassambara, A.; Mundt, F. Package ‘factoextra’. Extr. Vis. Results Multivar. Data Anal. 2017, 76, 71–74. [Google Scholar]
- Wickham, H.; Chang, W. Package “ggplot2”. Creat. Elegant Data Vis. Using Gramm. Graph. Version 2016, 2, 1–189. [Google Scholar]
- Dray, S.; Dufour, A.B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. 2021. Available online: https://www.r-project.org/ (accessed on 21 September 2021).
- RStudio Team. RStudio: Integrated Development Environment for R. 2021. Available online: https://www.kdnuggets.com/2011/03/rstudio-ide-for-r.html#:~:text=a%20new%2C%20free%20and%20open,environment%20(IDE)%20for%20R (accessed on 21 September 2021).
- Soltangheisi, A.; Withers, P.J.A.; Pavinato, P.S.; Cherubin, M.R.; Rossetto, R.; Do Carmo, J.B.; Rocha, G.C.; Martinelli, L.A. Improving phosphorus sustainability of sugarcane production in Brazil. GCB Bioenergy 2019, 11, 1444–1455. [Google Scholar] [CrossRef]
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.A.; Teles, A.P.B.; Herrera, W.F.B. Legacy phosphorus and no tillage agriculture in tropical oxisols of the Brazilian savanna. Sci. Total Environ. 2016, 542, 1050–1061. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Xu, Z.H. Impacts of grassland afforestation with coniferous trees on soil phosphorus dynamics and associated microbial processes: A review. For. Ecol. Manage. 2008, 255, 396–409. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Ding, Y.Z.; Li, Z.A.; Zou, B. Low-molecular weight organic acids and their ecological roles in soil. Soils 2005, 37, 243–250. [Google Scholar]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C.; Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Armenteras, D.; Rudas, G.; Rodriguez, N.; Sua, S.; Romero, M. Patterns and causes of deforestation in the Colombian Amazon. Ecol. Indic. 2006, 6, 353–368. [Google Scholar] [CrossRef]
- Murad, C.A.; Pearse, J. Landsat study of deforestation in the Amazon region of Colombia: Departments of Caquetá and Putumayo. Remote Sens. Appl. Soc. Environ. 2018, 11, 161–171. [Google Scholar] [CrossRef]
- Rueda, B.L.; McRoberts, K.C.; Blake, R.W.; Nicholson, C.F.; Valentim, J.F.; Fernandes, E.C.M. Nutrient status of cattle grazing systems in the western brazilian amazon. Cogen Food Agri. 2020, 6, 1722350. [Google Scholar] [CrossRef]
- Juo, A.S.R.; Manu, A. Chemical dynamics in slash-and-burn agriculture. Agric. Ecosyst. Environ. 1996, 58, 49–60. [Google Scholar] [CrossRef]
- Numata, I.; Chadwick, O.A.; Roberts, D.A.; Schimel, J.P.; Sampaio, F.F.; Leonidas, F.C.; Soares, J.V. Temporal nutrient variation in soil and vegetation of post-forest pastures as a function of soil order, pasture age, and management, Rondônia, Brazil. Agric. Ecosyst. Environ. 2007, 118, 159–172. [Google Scholar] [CrossRef]
- Martínez, J.; Cajas, Y.S.; León, J.D.; Osorio, N.W. Silvopastoral systems enhance soil quality in grasslands of Colombia. Appl. Environ. Soil Sci. 2014, 2014, 359736. [Google Scholar] [CrossRef] [Green Version]
- Zin Battisti, L.F.; Schmitt Filho, A.L.; Loss, A.; Sinisgalli, P.A. de A. Soil chemical attributes in a high biodiversity silvopastoral system. Acta Agronómica 2018, 67, 486–493. [Google Scholar] [CrossRef]
- Townsend, A.R.; Asner, G.P.; Cleveland, C.C.; Lefer, M.E.; Bustamante, M.M.C. Unexpected changes in soil phosphorus dynamics along pasture chronosequences in the humid tropics. J. Geophys. Res. Atmos. 2002, 107, LBA 34-1–LBA 34-9. [Google Scholar] [CrossRef] [Green Version]
- Motavalli, P.P.; Miles, R.J. Soil phosphorus fractions after 111 years of animal manure and fertilizer applications. Biol. Fertil. Soils 2002, 36, 35–42. [Google Scholar] [CrossRef]
- McGill, W.B.; Cole, C.V. Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 1981, 26, 267–286. [Google Scholar] [CrossRef]
- Ge, Z.; Rubio, G.; Lynch, J.P. The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: Results from a geometric simulation model. Plant Soil 2000, 218, 159–171. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C. V An exploratory method for fractionation of organic phosphorus from grassland soils. Soil Sci. 1978, 125, 95–101. [Google Scholar] [CrossRef]
- Dinesh, R.; Ghoshal Chaudhuri, S.; Sheeja, T.E. Soil biochemical and microbial indices in wet tropical forests: Effects of deforestation and cultivation. J. Plant Nutr. Soil Sci. 2004, 167, 24–32. [Google Scholar] [CrossRef]
- Lilienfein, J.; Wilcke, W.; Ayarza, M.A.; Vilela, L.; Do Carmo Lima, S.; Zech, W. Chemical fractionation of phosphorus, sulphur, and molybdenum in Brazilian savannah Oxisols under different land use. Geoderma 2000, 96, 31–46. [Google Scholar] [CrossRef]
- Moore, A.; Reddy, K.R. Role of Eh and pH on Phosphorus Geochemistry in Sediments of Lake Okeechobee, Florida. J. Environ. Qual. 1994, 23, 955–964. [Google Scholar] [CrossRef]
- Wright, C.J.; Coleman, D.C. Cross-site comparison of soil microbial biomass, soil nutrient status, and nematode trophic groups. Pedobiologia. 2000, 44, 2–23. [Google Scholar] [CrossRef]
- Gatiboni, L.C.; Schmitt, D.E.; Tiecher, T.; Veloso, M.G.; dos Santos, D.R.; Kaminski, J.; Brunetto, G. Plant uptake of legacy phosphorus from soils without P fertilization. Nutr. Cycl. Agroecosyst. 2021, 119, 139–151. [Google Scholar] [CrossRef]
- Fonte, S.J.; Nesper, M.; Hegglin, D.; Velásquez, J.E.; Ramirez, B.; Rao, I.M.; Bernasconi, S.M.; Bünemann, E.K.; Frossard, E.; Oberson, A. Pasture degradation impacts soil phosphorus storage via changes to aggregate-associated soil organic matter in highly weathered tropical soils. Soil Biol. Biochem. 2014, 68, 150–157. [Google Scholar] [CrossRef]
- Navarrete, D.; Sitch, S.; Aragão, L.E.O.C.; Pedroni, L. Conversion from forests to pastures in the Colombian Amazon leads to contrasting soil carbon dynamics depending on land management practices. Glob. Change Biol. 2016, 22, 3503–3517. [Google Scholar] [CrossRef] [PubMed] [Green Version]


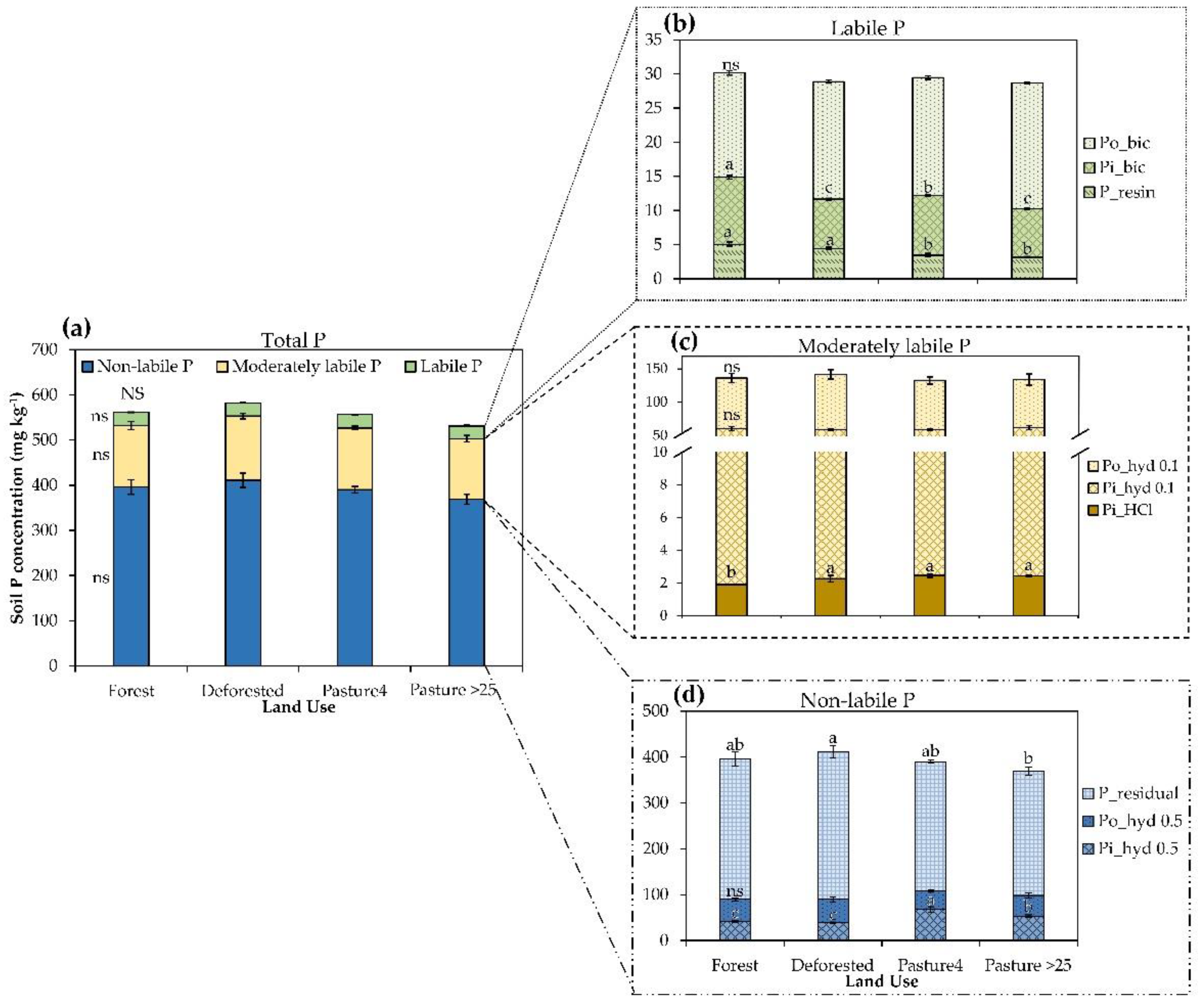

| P (mg kg−1) | ||||||
|---|---|---|---|---|---|---|
| Soil Depth (cm) | P Lability | P Fraction | Land Use | |||
| Forest | Deforested | Pasture4 | Pasture >25 | |||
| 0–10 | Labile | P_resin | 5.6 ± 0.3 a | 5.5 ± 0.2 a | 4.4 ± 0.5 ab | 3.7 ± 0.2 b |
| Pi_bic | 11.6 ± 0.3 a | 8.3 ± 0.6 b | 11.9 ± 0.5 a | 8.9 ± 0.3 b | ||
| Po_bic | 19.6 ± 1.3 ns | 24.7 ± 1.1 | 22.7 ± 1.0 | 21.3 ± 1.8 | ||
| Total | 37.1 ± 5.1 ns | 38.5 ± 2.9 | 39.0 ± 3.4 | 32.3 ± 1.5 | ||
| Moderately labile | Pi_hyd 0.1 | 60.5 ± 2.7 ns | 62.3 ± 2.7 | 57.3 ± 2.8 | 59.5 ± 1.7 | |
| Po_hyd 0.1 | 65.3 ± 2.6 b | 85.0 ± 7.7 ab | 103.8 ± 14.6 a | 74.1 ± 7.3 ab | ||
| Pi_HCl | 2.0 ± 0.1 b | 2.1 ± 0.1 b | 2.6 ± 0.1 a | 2.6 ± 0.1 a | ||
| Total | 133.5 ± 16.3 ns | 149.8 ± 14.7 | 164.0 ± 35.2 | 139.3 ± 28.7 | ||
| Non-labile | Pi_hyd 0.5 | 44.4 ± 2.4 c | 50.8 ± 6.4 bc | 82.3 ± 8.4 a | 58.7 ± 4.8 b | |
| Po_hyd 0.5 | 49.8 ± 3.1 ab | 52.1 ± 5.4 a | 31.4 ± 2.1 b | 47.7 ± 7.2 ab | ||
| P_residual | 285.7 ± 4.4 ns | 301.7 ± 14.9 | 274.8 ± 13.8 | 271.8 ± 4.4 | ||
| Total | 381.0 ± 20.9 ns | 379.3 ± 23.1 | 370.1 ± 18.1 | 378.2 ± 22.5 | ||
| 10–20 | Labile | P_resin | 5.1 ± 0.3 a | 4.1 ± 0.1 b | 3.2 ± 0.1 c | 3.1 ± 0.1 c |
| Pi_bic | 10.1 ± 0.3 a | 7.1 ± 0.2 c | 8.6 ± 0.2 b | 6.9 ± 0.1 c | ||
| Po_bic | 14.9 ± 1.2 ns | 15.1 ± 1.2 | 18.4 ± 1.4 | 15.1 ± 1.2 | ||
| Total | 30.1 ± 3.7 ns | 32.4 ± 8.0 | 30.2 ± 3.5 | 25.1 ± 2.7 | ||
| Moderately labile | Pi_hyd 0.1 | 56.3 ± 4.4 ns | 52.5 ± 1.8 | 60.9 ± 2.3 | 59.3 ± 3.4 | |
| Po_hyd 0.1 | 74.6 ± 7.4 ab | 98.70 ± 11.0 a | 53.3 ± 4.4 b | 61.2 ± 10.5 b | ||
| Pi_HCl | 1.8 ± 0.1 c | 1.9 ± 0.1 bc | 2.4 ± 0.1 a | 2.3 ± 0.1 ab | ||
| Total | 132.6 ± 15.2 ns | 153.5 ± 27.3 | 116.7 ± 11.0 | 122.8 ± 18.7 | ||
| Non-labile | Pi_hyd 0.5 | 43.5 ± 2.6 bc | 38.0 ± 0.6 c | 70.4 ± 6.3 a | 52.4 ± 3.9 b | |
| Po_hyd 0.5 | 47.1 ± 4.9 ns | 47.1 ± 2.0 | 47.9 ± 3.2 | 43.8 ± 5.2 | ||
| P_residual | 289.0 ± 11.4 ab | 308.5 ± 7.7 a | 285.4 ± 5.5 ab | 247.5 ± 23.3 b | ||
| Total | 379.7 ± 31.7 ab | 393.6 ± 16.2 ab | 403.7 ± 22.5 a | 343.7 ± 57.6 b | ||
| 20–30 | Labile | P_resin | 4.3 ± 0.1 a | 3.9 ± 0.1 a | 3.1 ± 0.1 b | 3.0 ± 0.1 b |
| Pi_bic | 9.5 ± 0.7 a | 6.8 ± 0.2 bc | 7.9 ± 0.4 b | 6.9 ± 0.1 c | ||
| Po_bic | 11.9 ± 0.6 ns | 13.4 ± 0.6 | 14.4 ± 1.5 | 15.1 ± 1.2 | ||
| Total | 28.4 ± 5.9 a | 24.4 ± 1.9 ab | 25.4 ± 3.7 ab | 21.9 ± 2.5 b | ||
| Moderately labile | Pi_hyd 0.1 | 55.7 ± 2.6 ns | 49.5 ± 1.5 | 52.4 ± 1.6 | 59.3 ± 3.4 | |
| Po_hyd 0.1 | 57.8 ± 5.3 ns | 78.7 ± 5.7 | 54.3 ± 9.1 | 61.2 ± 10.5 | ||
| Pi_HCl | 1.9 ± 0.1 c | 2.0 ± 0.1 bc | 2.2 ± 0.1 ab | 2.3 ± 0.1 ab | ||
| Total | 115.5 ± 12.1 ns | 130.2 ± 13.3 | 112.9 ± 13.1 | 128.7 ± 20.9 | ||
| Non-labile | Pi_hyd 0.5 | 43.1 ± 3.0 b | 35.9 ± 1.8 b | 63.8 ± 6.4 a | 52.4 ± 3.9 b | |
| Po_hyd 0.5 | 36.4 ± 0.5 ns | 47.1 ± 4.1 | 40.4 ± 3.2 | 43.8 ± 5.2 | ||
| P_residual | 285.9 ± 11.0 ab | 331.5 ± 16.7 a | 278.7 ± 5.1 b | 247.5 ± 23.3 b | ||
| Total | 358.5 ± 16.4 b | 428.2 ± 59.6 a | 383.0 ± 15.6 b | 378.9 ± 27.4 b | ||
| 30–40 | Labile | P_resin | 4.1 ± 0.1 a | 3.8 ± 0.17 a | 3.1 ± 0.1 b | 3.0 ± 0.1 b |
| Pi_bic | 8.1 ± 0.5 a | 6.4 ± 0.1 b | 6.6 ± 0.2 b | 6.1 ± 0.1 b | ||
| Po_bic | 12.0 ± 1.0 b | 12.4 ± 0.6 ab | 13.5 ± 1.1 ab | 16.7 ± 3.1 a | ||
| Total | 24.2 ± 2.3 ab | 22.7 ± 1.6 b | 23.2 ± 2.9 b | 31.1 ± 14.1 a | ||
| Moderately labile | Pi_hyd 0.1 | 54.9 ± 4.6 ns | 60.6 ± 5.3 | 52.6 ± 1.9 | 53.3 ± 0.7 | |
| Po_hyd 0.1 | 79.4 ± 7.4 ns | 70.3 ± 11.8 | 83.8 ± 7.6 | 88.9 ± 11.2 | ||
| Pi_HCl | 1.9 ± 0.1 b | 2.0 ± 0.03 b | 2.2 ± 0.1 a | 2.3 ± 0.1 a | ||
| Total | 131.8 ± 13.5 ns | 133.1 ± 18.7 | 153.5 ± 40.1 | 144.4 ± 27.5 | ||
| Non-labile | Pi_hyd 0.5 | 37.3 ± 1.5 b | 30.6 ± 3.0 b | 50.1 ± 1.6 a | 50.3 ± 2.7 a | |
| Po_hyd 0.5 | 51.4 ± 6.0 ns | 52.2 ± 6.1 | 39.8 ± 3.2 | 44.0 ± 7.6 | ||
| P_residual | 312.3 ± 9.8 a | 304.5 ± 15.8 ab | 280.8 ± 4.6 b | 280.4 ± 4.0 b | ||
| Total | 401.0 ± 24.4 ns | 399.5 ± 57.5 | 384.7 ± 26.5 | 374.8 ± 19.2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavarro-Bermeo, J.P.; Arruda, B.; Mora-Motta, D.A.; Bejarano-Herrera, W.; Ortiz-Morea, F.A.; Somenahally, A.; Silva-Olaya, A.M. Responses of Soil Phosphorus Fractions to Land-Use Change in Colombian Amazon. Sustainability 2022, 14, 2285. https://doi.org/10.3390/su14042285
Chavarro-Bermeo JP, Arruda B, Mora-Motta DA, Bejarano-Herrera W, Ortiz-Morea FA, Somenahally A, Silva-Olaya AM. Responses of Soil Phosphorus Fractions to Land-Use Change in Colombian Amazon. Sustainability. 2022; 14(4):2285. https://doi.org/10.3390/su14042285
Chicago/Turabian StyleChavarro-Bermeo, Juan P., Bruna Arruda, Dúber A. Mora-Motta, Wilfrand Bejarano-Herrera, Fausto A. Ortiz-Morea, Anil Somenahally, and Adriana M. Silva-Olaya. 2022. "Responses of Soil Phosphorus Fractions to Land-Use Change in Colombian Amazon" Sustainability 14, no. 4: 2285. https://doi.org/10.3390/su14042285






