Comparative Study of UV Radiation Resistance and Reactivation Characteristics of E. coli ATCC 8739 and Native Strains: Implications for Water Disinfection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organisms, Culturing Medium, and Pretreatment Incubation
2.2. UV Device Description and Dose Calculation
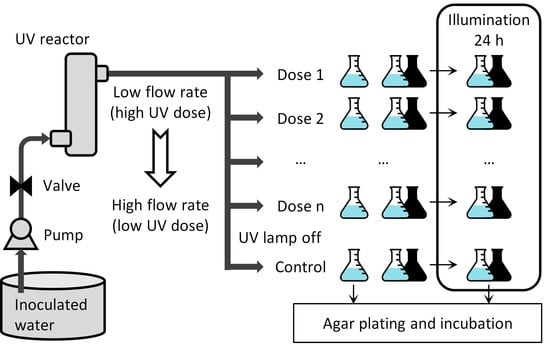
2.3. Experimental Procedure
2.4. Analysis of the Results
3. Results
3.1. Inactivation Kinetics
3.2. Effect of the Post-Treatment Incubation on the Treatment Efficacy
3.3. Percentage of Photoreactivation of the Different Bacterial Strains
4. Discussion
4.1. Native Bacteria vs. ATCC Isolates
4.2. Kinetic Model for E. coli
4.3. Kinetic Modeling Parameters
4.4. Reactivation Processes for E. coli
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNICEF; WHO. Progress on Drinking Water, Sanitation and Hygiene 2017; WHO: Geneve, Switzerland, 2017. [Google Scholar]
- United Nations. Global Sustainable Development Report 2016 Edition; United Nations: New York, NY, USA, 2016. [Google Scholar]
- Rodríguez-Chueca, J.; Ormad, M.P.; Mosteo, R.; Ovelleiro, J.L. Kinetic Modeling of Escherichia coli and Enterococcus sp. Inactivation in Wastewater Treatment by Photo-Fenton and H2O2/UV–Vis Processes. Chem. Eng. Sci. 2015, 138, 730–740. [Google Scholar] [CrossRef]
- Guidelines for Water Reuse|US EPA. Available online: https://www.epa.gov/waterreuse/guidelines-water-reuse (accessed on 29 August 2022).
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneve, Switzerland, 2011; ISBN 9789241548151. [Google Scholar]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirnie, M.; Linden, K.G.; Malley, J.P., Jr. Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface Water Treatment Rule. Environ. Prot. 2006, 2, 1–436. [Google Scholar]
- Council, N.R. Drinking Water and Health, Volume 7: Disinfectants and Disinfectant by-Products; National Academies Press: Washington, DC, USA, 1987; Volume 7. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The Problem of Drinking Water Access: A Review of Disinfection Technologies with an Emphasis on Solar Treatment Methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef]
- EPA. Manual de Factores de Exposición 2011 Edición (Final); EPA: Washington, DC, USA, 2011. [Google Scholar]
- Von Sonntag, C.; Schuchmann, H.P. UV Disinfection of Drinking Water and By-Product Formation- Some Basic Considerations. Aqua-J. Water Supply Res. Technol. 1992, 41, 67–74. [Google Scholar]
- Noguera-Oviedo, K.; Aga, D.S. Lessons Learned from More than Two Decades of Research on Emerging Contaminants in the Environment. J. Hazard Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation Credit of UV Radiation for Viruses, Bacteria and Protozoan (Oo)Cysts in Water: A Review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Tosa, K.; Hirata, T. Photoreactivation of Enterohemorrhagic Escherichia coli Following UV Disinfection. Water Res. 1999, 33, 361–366. [Google Scholar] [CrossRef]
- Ramos, Y.; Alonso, G. Evaluación de La Resistencia a Agentes Desinfectantes de Bacterias Aisladas de Ambientes Naturales. Rev. De La Soc. Venez. De Microbiol. 2011, 31, 130–137. [Google Scholar]
- Marugán, J.; van Grieken, R.; Sordo, C.; Cruz, C. Kinetics of the Photocatalytic Disinfection of Escherichia coli Suspensions. Appl. Catal B 2008, 82, 27–36. [Google Scholar] [CrossRef]
- Maghsoodi, M.; Lowry, G.L.; Smith, I.M.; Snow, S.D. Evaluation of Parameters Governing Dark and Photo-Repair in UVC-Irradiated Escherichia coli. Environ. Sci. 2022, 8, 407–418. [Google Scholar] [CrossRef]
- Vélez-Colmenares, J.J.; Acevedo, A.; Nebot, E. Effect of Recirculation and Initial Concentration of Microorganisms on the Disinfection Kinetics of Escherichia coli. Desalination 2011, 280, 20–26. [Google Scholar] [CrossRef]
- Nyangaresi, P.O.; Qin, Y.; Chen, G.; Zhang, B.; Lu, Y.; Shen, L. Effects of Single and Combined UV-LEDs on Inactivation and Subsequent Reactivation of E. coli in Water Disinfection. Water Res. 2018, 147, 331–341. [Google Scholar] [CrossRef]
- Verhulst, A.J.; Cappuyns, A.M.; van Derlinden, E.; Bernaerts, K.; van Impe, J.F. Analysis of the Lag Phase to Exponential Growth Transition by Incorporating Inoculum Characteristics. Food Microbiol. 2011, 28, 656–666. [Google Scholar] [CrossRef]
- Forster, B.; Arango Pinedo, C. Bacteriological Examination of Waters: Membrane Filtration Protocol; American Society for Microbiology: Washington, DC, USA, 2016. [Google Scholar]
- Romero-Martínez, L.; Moreno-Andrés, J.; Acevedo-Merino, A.; Nebot, E. Improvement of Ballast Water Disinfection Using a Photocatalytic (UV-C + TiO2) Flow-through Reactor for Saltwater Treatment. J. Chem. Technol. Biotechnol. 2014, 89, 1203–1210. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Romero-Martínez, L.; Acevedo-Merino, A.; Nebot, E. UV-Based Technologies for Marine Water Disinfection and the Application to Ballast Water: Does Salinity Interfere with Disinfection Processes? Sci. Total Environ. 2017, 581–582, 144–152. [Google Scholar] [CrossRef]
- Figawa. Ultraviolet Disinfection in Water Treatment; Technical Report 01|08—Revised Version of Technical; Figawa: Köln, Germany, 2009. [Google Scholar]
- Bolton, J.R. Calculation of Ultraviolet Fluence Rate Distributions in an Annular Reactor: Significance of Refraction and Reflection. Water Res. 2000, 34, 3315–3324. [Google Scholar] [CrossRef]
- Guan, W.; Fan, X.; Yan, R. Effect of Combination of Ultraviolet Light and Hydrogen Peroxide on Inactivation of Escherichia coli O157:H7, Native Microbial Loads, and Quality of Button Mushrooms. Food Control 2013, 34, 554–559. [Google Scholar] [CrossRef]
- Lindenauer, K.G.; Darby, J.L. Ultraviolet Disinfection of Wastewater: Effect of Dose on Subsequent Photoreactivation. Water Res. 1994, 28, 805–817. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Herremans, C.H.; van Impe, J.F. Structural Model Requirements to Describe Microbial Inactivation during a Mild Heat Treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Schabert, F.A.; Henn, C.; Engel, A. Native Escherichia coli OmpF Porin Surfaces Probed by Atomic Force Microscopy. Science 1995, 268, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Fiorentino, A.; Anselmo, A. Advanced Treatment of Urban Wastewater by UV Radiation: Effect on Antibiotics and Antibiotic-Resistant E. coli Strains. Chemosphere 2013, 92, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Koutchma, T.; Parisi, B.; Larkin, J.; Forney, L.J. Ultraviolet Inactivation Kinetics of Escherichia coli and Yersinia pseudotuberculosis in Annular Reactors. J. Food Sci. 2007, 72, E271–E278. [Google Scholar] [CrossRef] [PubMed]
- Coohill, T.P.; Sagripanti, J.L. Overview of the Inactivation by 254 Nm Ultraviolet Radiation of Bacteria with Particular Relevance to Biodefense. Photochem. Photobiol. 2008, 84, 1084–1090. [Google Scholar] [CrossRef]
- Oguma, K.; Katayama, H.; Ohgaki, S. Spectral Impact of Inactivating Light on Photoreactivation of Escherichia coli. J. Environ. Eng. Sci. 2005, 4, S1–S6. [Google Scholar] [CrossRef]
- Guo, M.; Huang, J.; Hu, H.; Liu, W.; Yang, J. UV Inactivation and Characteristics after Photoreactivation of Escherichia coli with Plasmid: Health Safety Concern about UV Disinfection. Water Res. 2012, 46, 4031–4036. [Google Scholar] [CrossRef]
- Harris, G.D.; Adams, V.D.; Sorensen, D.L.; Curtis, M.S. Ultraviolet Inactivation of Selected Bacteria and Viruses with Photoreactivation of the Bacteria. Water Res. 1987, 21, 687–692. [Google Scholar] [CrossRef]
- Xiong, P.; Hu, J. Inactivation/Reactivation of Antibiotic-Resistant Bacteria by a Novel UVA/LED/TiO2 System. Water Res. 2013, 47, 4547–4555. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Application of Ultraviolet Light-Emitting Diodes (UV-LEDs) for Water Disinfection: A Review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef]
- Leung, Y.H.; Xu, X.; Ma, A.P.Y.; Liu, F.; Ng, A.M.C.; Shen, Z.; Gethings, L.A.; Guo, M.Y.; Djurišic, A.B.; Lee, P.K.H.; et al. Toxicity of ZnO and TiO2 to Escherichia coli Cells. Sci. Rep. 2016, 6, 35243. [Google Scholar] [CrossRef] [Green Version]
- Bohrerova, Z.; Linden, K.G. Standardizing Photoreactivation: Comparison of DNA Photorepair Rate in Escherichia coli Using Four Different Fluorescent Lamps. Water Res. 2007, 41, 2832–2838. [Google Scholar] [CrossRef]






| Part | Parameter and Abbreviation | Value | |
|---|---|---|---|
| UV Lamp | Total power | P | 6 W |
| Power at 254 nm | P254 | 2 W | |
| Length | L | 18.9 cm | |
| Quartz sleeve | Outer radius | rQ | 1.2 cm |
| Thickness | e | 1.6 mm | |
| Transmittance | TQ | 0.94 | |
| Reactor case | Inner radius | rR | 2.55 cm |
| Organism | Treatment | k ± SE (cm2 mJ−1) | Log (S0) ± SE | Log (Sres) ± SE | R2 | RMSE | D3 (mJ cm−2) ± SE |
|---|---|---|---|---|---|---|---|
| E. coli (ATCC 8739) | 0 days | 0.54 ± 0.03 | 0.00 ± 0.10 | −4.67 ± 0.16 | 0.972 | 0.302 | 12.8 ± 1.3 |
| 1 day (dark) | 0.50 ± 0.08 | −0.18 ± 0.10 | −4.94 ± 0.36 | 0.937 | 0.511 | 12.1 ± 1.8 | |
| 1 day (light) | 0.50 ± 0.02 | 0.08 ± 0.09 | −3.99 ± 0.11 | 0.990 | 0.166 | 14.4 ± 1.2 | |
| E. coli (native) | 0 days | 0.52 ± 0.04 | −0.17 ± 0.16 | −4.45 ± 0.21 | 0.935 | 0.381 | 12.6 ± 2.0 |
| 1 day (dark) | 0.65 ± 0.08 | 0.06 ± 0.29 | −5.04 ± 0.35 | 0.882 | 0.673 | 10.8 ± 2.6 | |
| 1 day (light) | 0.29 ± 0.05 | −0.29 ± 0.20 | −2.92 ± 0.35 | 0.922 | 0.259 | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duque-Sarango, P.; Romero-Martínez, L.; Pinos-Vélez, V.; Sánchez-Cordero, E.; Samaniego, E. Comparative Study of UV Radiation Resistance and Reactivation Characteristics of E. coli ATCC 8739 and Native Strains: Implications for Water Disinfection. Sustainability 2023, 15, 9559. https://doi.org/10.3390/su15129559
Duque-Sarango P, Romero-Martínez L, Pinos-Vélez V, Sánchez-Cordero E, Samaniego E. Comparative Study of UV Radiation Resistance and Reactivation Characteristics of E. coli ATCC 8739 and Native Strains: Implications for Water Disinfection. Sustainability. 2023; 15(12):9559. https://doi.org/10.3390/su15129559
Chicago/Turabian StyleDuque-Sarango, Paola, Leonardo Romero-Martínez, Verónica Pinos-Vélez, Esteban Sánchez-Cordero, and Esteban Samaniego. 2023. "Comparative Study of UV Radiation Resistance and Reactivation Characteristics of E. coli ATCC 8739 and Native Strains: Implications for Water Disinfection" Sustainability 15, no. 12: 9559. https://doi.org/10.3390/su15129559