Green Synthesis of Silver and Titanium Oxide Nanoparticles Using Tea and Eggshell Wastes, Their Characterization, and Biocompatibility Evaluation
Abstract
:1. Introduction
2. Materials and Methods
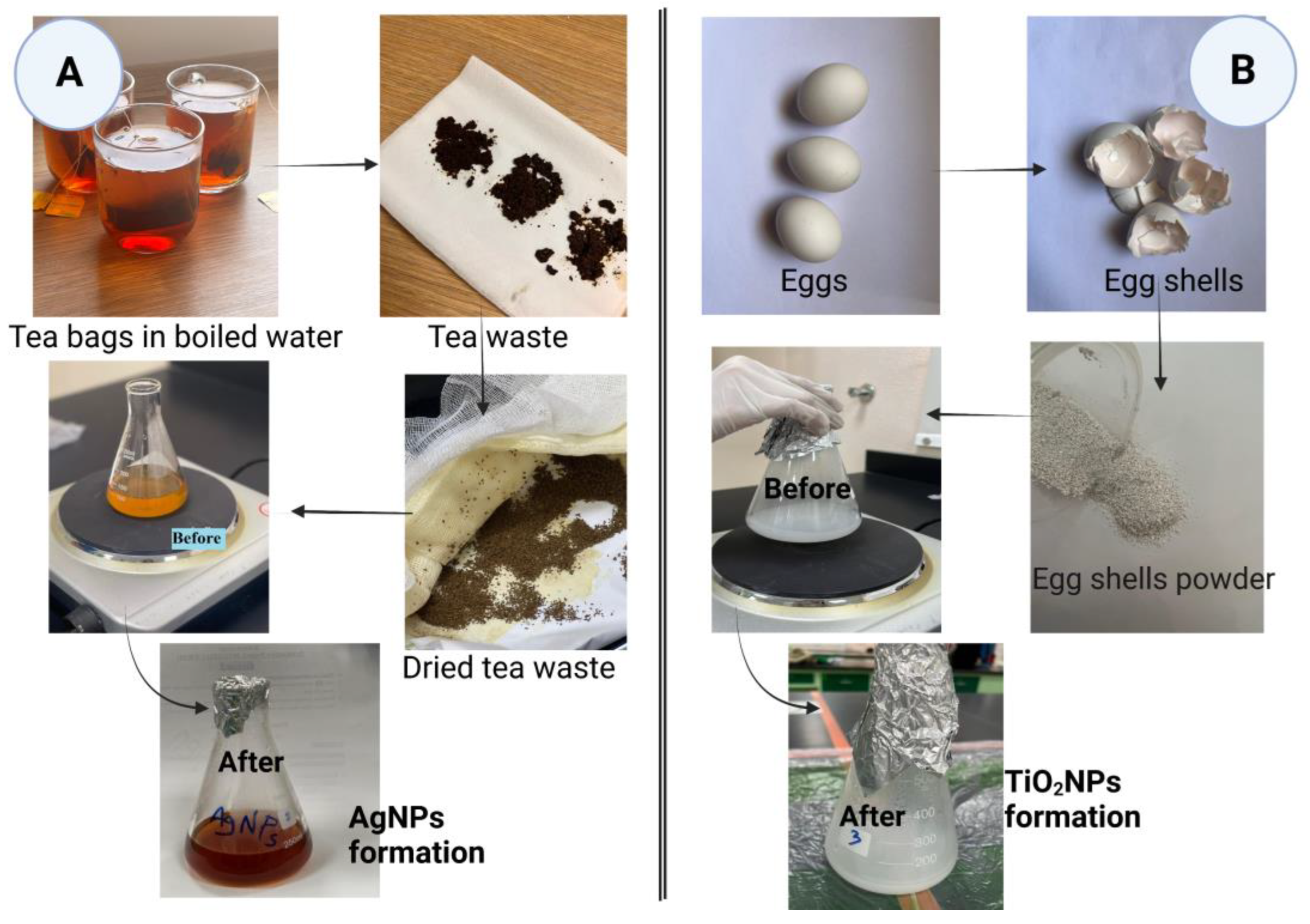
2.1. Preparation of Tea and Eggshell Waste Extracts
2.2. Green Synthesis of Nanoparticles Using Waste Products
2.3. Characterization of Green AgNPs and TiO2NPs
2.4. Cytotoxicity Assessment of NPs Using SRB
3. Results and Discussion
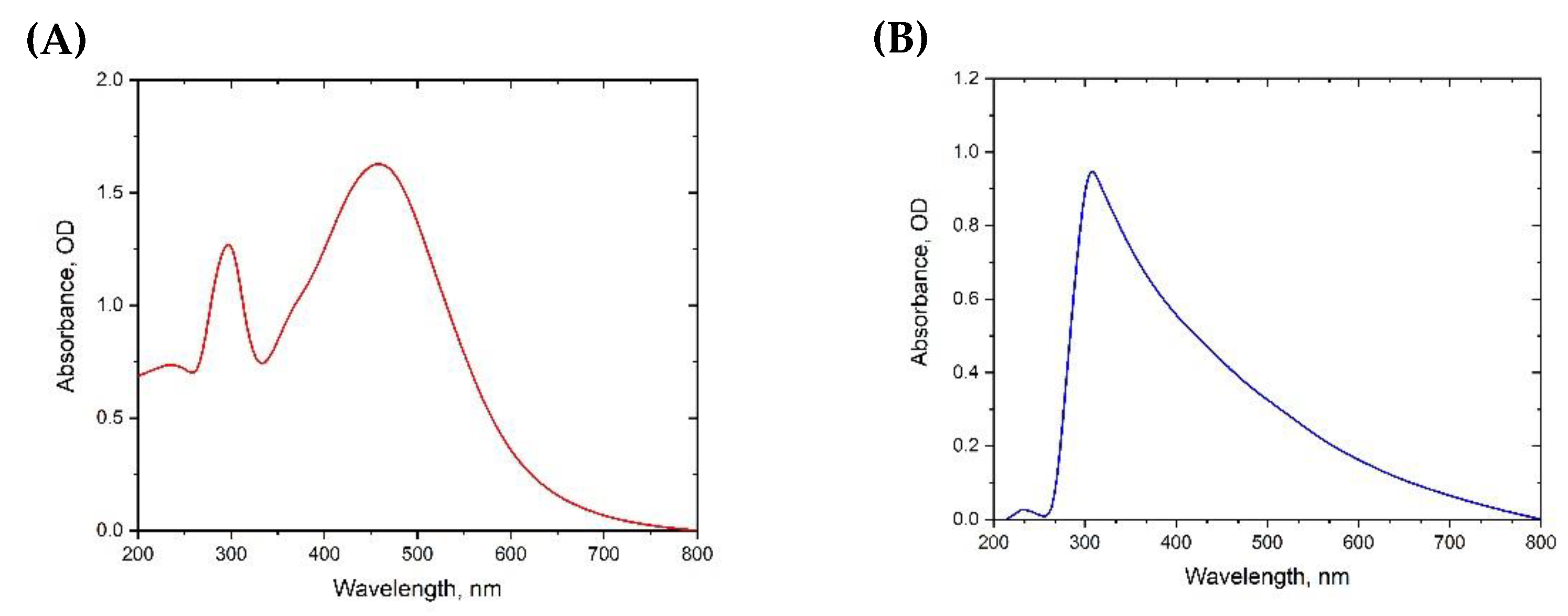
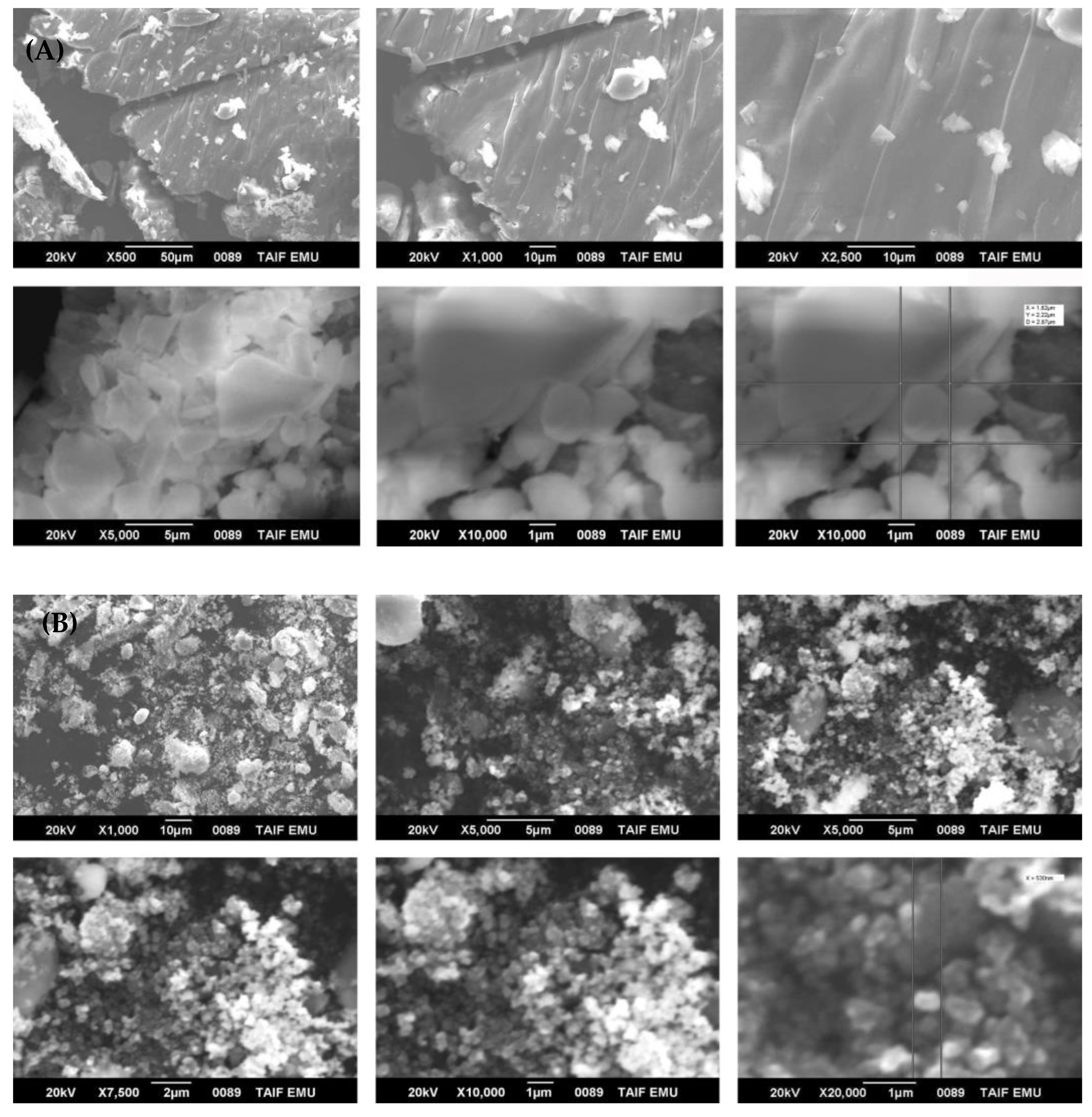
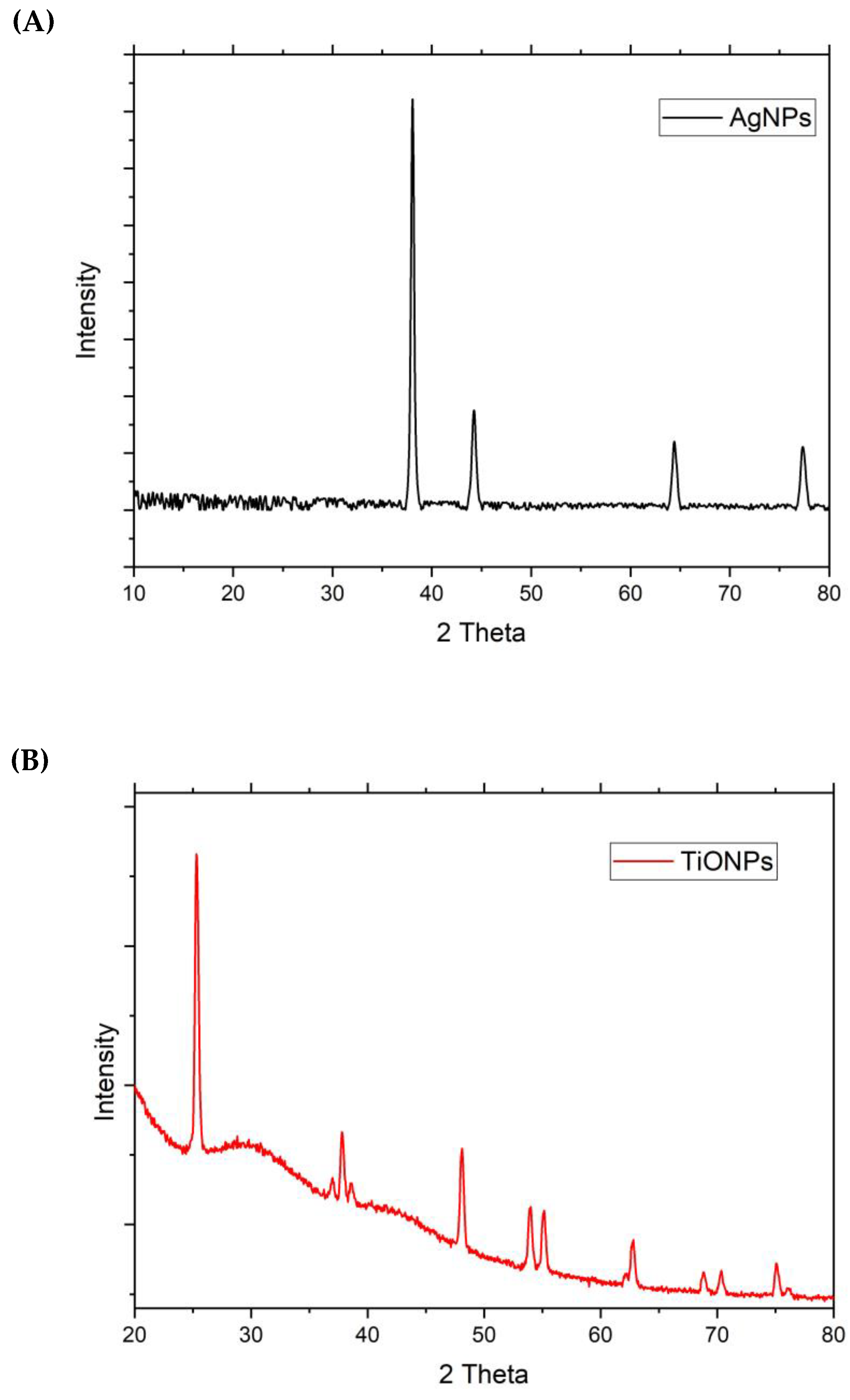
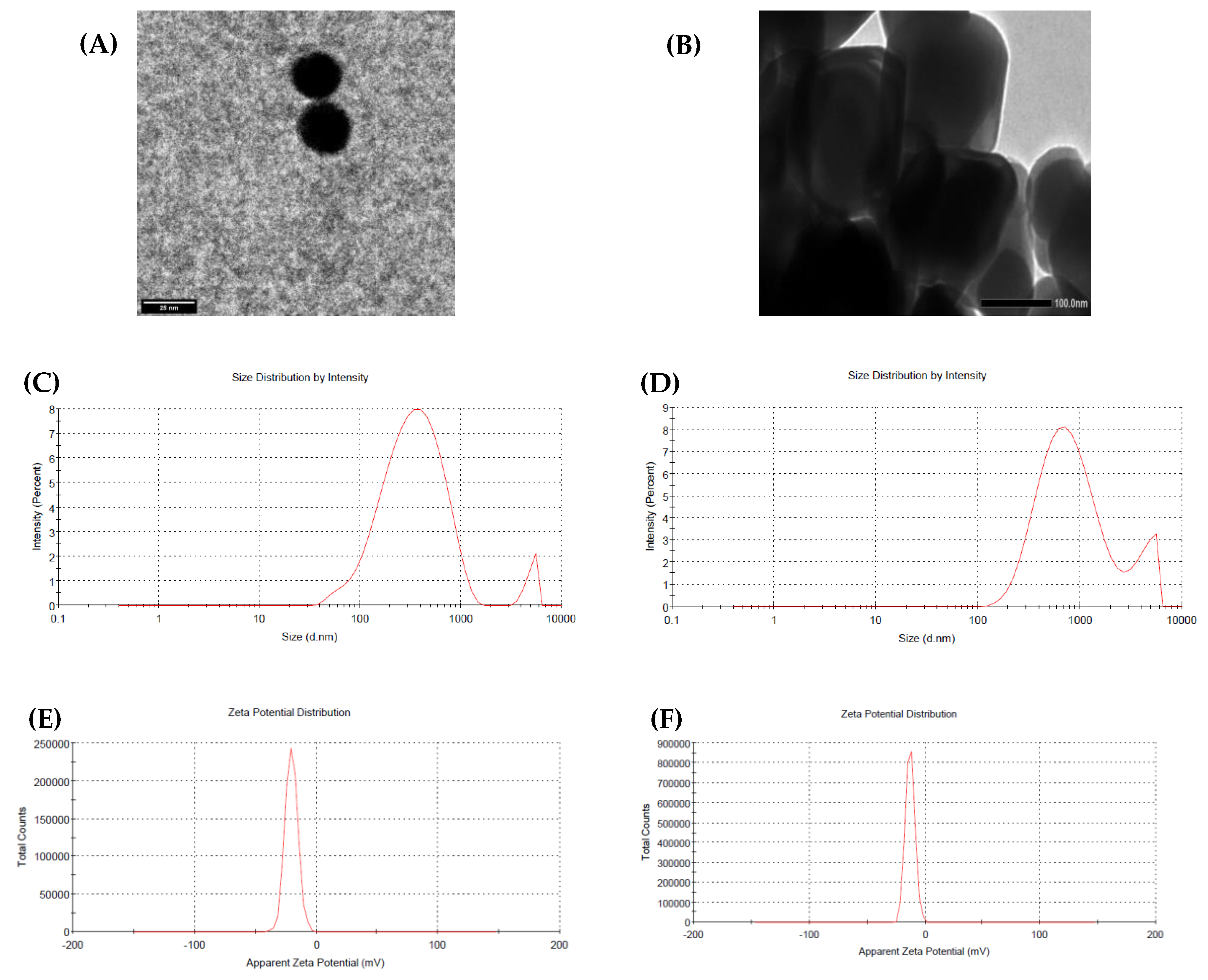
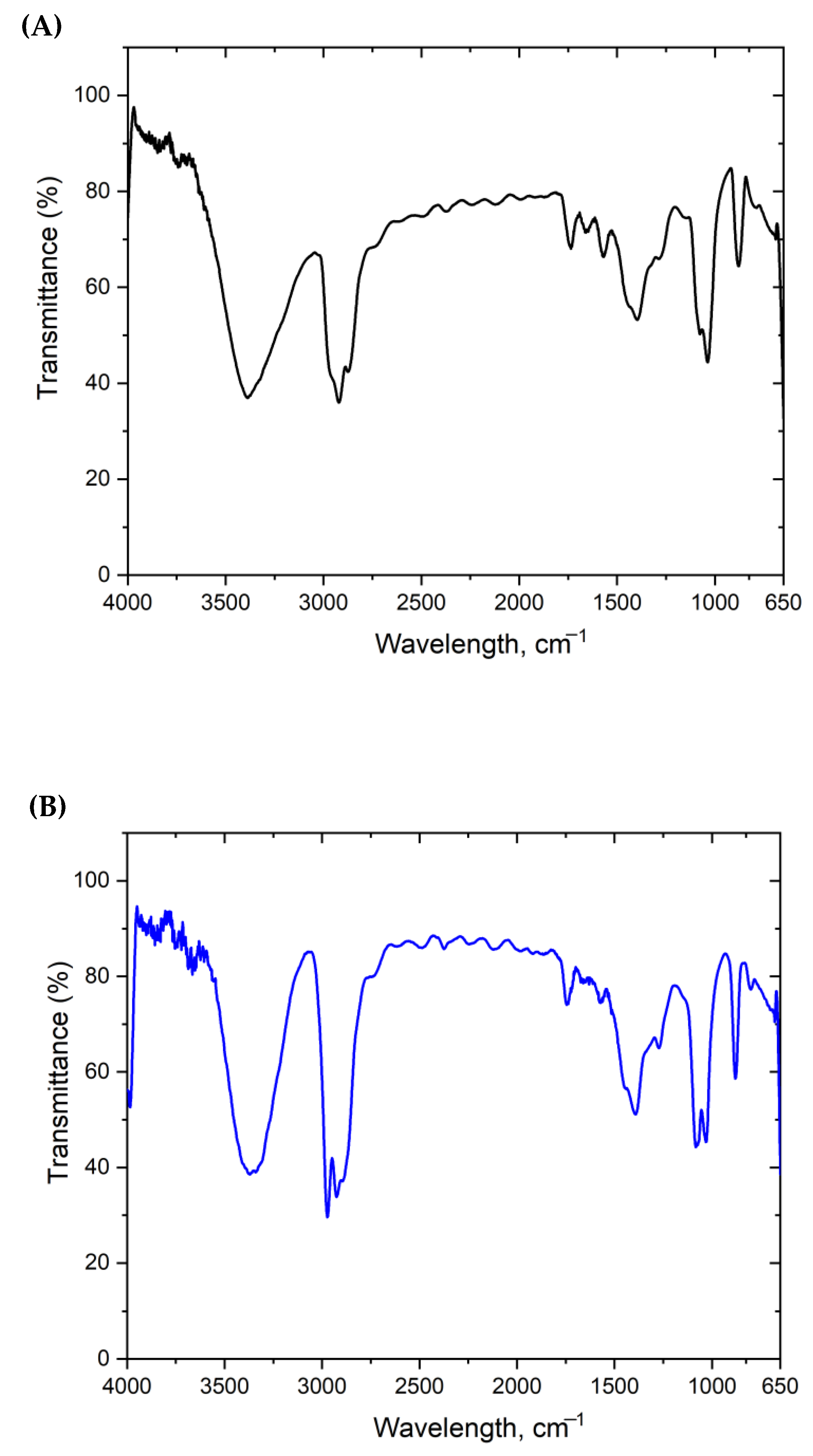
3.1. Characterization of Nanoparticles (NPs)
3.2. Biocompatibility of Green Synthesized AgNPs and TiO2NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Nour, K.M.; Eftaiha, A.; Al-Wartgan, A.; Ammar, R.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Irvani, S.; Korbekandi, H.; Mirmohammadi, S.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Vishwanath, R.; Negi, B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Curr. Opin. Green Sustain. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of metal nanoparticles via microbial enzymes: A mechanistic approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef] [Green Version]
- Sportelli, M.C.; Izzi, M.; Annalisa, V.; Clemente, M.; Picca, R.A.; Ancona, A.; Cioffi, N. The pros and cons of the use of laser ablation synthesis for the production of silver nano-antimicrobials. Antibiotics 2018, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Chopra, H.; Bibi, S.; Singh, I.; Hasan, M.M.; Khan, M.S.; Yousafi, Q.; Baig, A.A.; Rahman, M.M.; Islam, F.; Emran, T.B.; et al. Green Metallic Nanoparticles: Biosynthesis to Applications. Front. Bioeng. Biotechnol. 2022, 10, 874742. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Moreno-Vásquez, M.J.; Lagarda-Díaz, I.; Barreras-Urbina, C.G.; Armenta-Villegas, L.; Olguín-Moreno, A.; Tapia-Hernández, J.A. Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health. J. Nanomater. 2022, 2022, 8874003. [Google Scholar] [CrossRef]
- Debnath, B.; Haldar, D.; Purkait, M.K. Potential and sustainable utilization of tea waste: A review on present status and future trends. Environ. Chem. Eng. 2021, 9, 106179. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [Green Version]
- Su, L.J.; Arab, L. Tea consumption and the reduced risk of colon cancer—Results from a national prospective cohort study. Public Health Nutr. 2002, 5, 419–425. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Sun, L.; Yu, H.; Ni, Q.X.; Risch, H.A.; Gao, Y.T. Green tea drinking and risk of pancreatic cancer: A large-scale, population-based case-control study in urban Shanghai. Cancer Epidemiol. 2012, 36, e354–e358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negishi, H.; Xu, J.W.; Ikeda, K.; Njelekela, M.; Nara, Y.; Yamori, Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J. Nutr. 2004, 134, 38–42. [Google Scholar] [PubMed] [Green Version]
- Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Merlino, L.; Mudano, A.S.; Burma, M.; Folsom, A.R.; Saag, K.G. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2002, 46, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Date, C.; Wakai, K.; Fukui, M.; Tamakoshi, A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann. Intern. Med. 2006, 144, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.Y.; Nemzer, B.V.; Combet, E.; Yashin, Y.I. Determination of the Chemical Composition of Tea by Chromatographic Methods: A Review. J. Food Res. 2015, 4, 56. [Google Scholar] [CrossRef] [Green Version]
- Rolim, W.R.; Pelegrino, M.T.; de Araújo Lima, B.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.B. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Appl. Surf. Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- Onitsuka, S.; Hamada, T.; Okamura, H. Preparation of antimicrobial gold and silver nanoparticles from tea leaf extracts. Colloids Surf. B. 2019, 173, 242–248. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT: Livestock Primary. Available online: https://www.fao.org/statistics/en/ (accessed on 2 September 2020).
- Faridi, H.; Arabhosseini, A. Application of eggshell wastes as valuable and utilizable products: A review. Res. Agric. Eng. 2018, 64, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The eggshell: Structure, composition and mineralization. Front. Biosci. 2012, 17, 1266–1280. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, M.M.C.; Hincke, T.M. Recent Patents on Eggshell: Shell and Membrane Applications. Recent Pat. Food Nutr. Agric. 2012, 3, 1–8. [Google Scholar] [CrossRef]
- Chakraborty, S.; De, S.D. Eggshell: An alternative, cheap, bioavailable source of calcium in human diet. Res. Rev. J. Dairy Sci. Technol. 2019, 8, 25–33. [Google Scholar]
- Jalu, R.G.; Chamada, T.A.; Kasirajan, R. Calcium oxide nanoparticles synthesis from hen eggshells for removal of lead (Pb (II)) from aqueous solution. Environ. Chall. 2021, 4, 100193. [Google Scholar] [CrossRef]
- Ramya, S.; Vijayakumar, S.; Vidhya, E.; Bukhari, N.A.; Hatamleh, A.A.; Nilavukkarasi, M.; Pham, T.H. TiO2 nanoparticles derived from egg shell waste: Eco synthesis, characterization, biological and photocatalytic applications. Environ. Res. 2022, 214, 113829. [Google Scholar] [CrossRef] [PubMed]
- Whiley, H.; Ross, K. Salmonella and eggs: From production to plate. Int. J. Environ. Res. Public Health 2015, 12, 2543–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aswathi, V.P.; Meera, S.; Maria, C.A.; Nidhin, M. Green synthesis of nanoparticles from biodegradable waste extracts and their applications: A critical review. Nanotechnol. Environ. Eng. 2022, 8, 377–397. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Al Sufyani, N.M. Comparative Analysis of nanosilver Particles synthesized by different approaches and their antimicrobial efficacy. J. Nanomater. 2021, 2021, 2204776. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Alyamani, A.A.; Hussien, N.A.; Morsi, M.M.; Alhumaidi, A. Integrated Approaches for Adsorption and Incorporation Testing of Green-Synthesized TiO2NPs Mediated by Seed-Priming Technology in Punica granatum L. Agronomy 2022, 12, 1601. [Google Scholar] [CrossRef]
- Swathi, N.; Sandhiya, D.; Rajeshkumar, S.; Lakshmi, T. Green synthesis of titanium dioxide nanoparticles using Cassia fistula and its antibacterial activity. Int. J. Res. Pharm. Sci. 2019, 10, 856–860. [Google Scholar] [CrossRef]
- Ahmad, W.; Jaiswal, K.K.; Soni, S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg. Nano Met. Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Raja, S.; Ramesh, V.; Thivaharan, V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab. J. Chem. 2017, 10, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A.; Nofal, S.M.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol. Lett. 2018, 291, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Katha, P.S.; Ahmed, Z.; Alam, R.; Saha, B.; Acharjee, A.; Rahman, M.S. Efficiency analysis of eggshell and tea waste as Low cost adsorbents for Cr removal from wastewater sample. S. Afr. J. Chem. Eng. 2021, 37, 186–195. [Google Scholar] [CrossRef]
- Valavanidis, A. Tea, the Most Popular Beverage Worldwide, is Beneficial to Human Health. Stud. Antioxid. Polyphenolic Const. Epidemiol. Evid. Dis. Prev. 2019, 1, 1–35. Available online: https://www.chem-tox-ecotox.org/ScientificReview (accessed on 14 May 2023).
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Rajput, D.; Paul, S.; Gupta, A. Green Synthesis of Silver Nanoparticles Using Waste Tea Leaves. Adv. Nan. Res 2020, 3, 1–14. [Google Scholar] [CrossRef]
- Bekele, E.T.; Gonfa, B.A.; Sabir, F.K. Use of Different Natural Products to Control Growth of Titanium Oxide Nanoparticles in Green Solvent Emulsion, Characterization, and Their Photocatalytic Application. Bioinorg. Chem. Appl. 2021, 2021, 6626313. [Google Scholar] [CrossRef]
- Waleczek, M.; Dendooven, J.; Dyachenko, P.; Petrov, A.Y.; Eich, M.; Blick, R.H.; Detavernier, C.; Nielsch, K.; Furlan, K.P.; Zierold, R. Influence of Alumina Addition on the Optical Properties and the Thermal Stability of Titania Thin Films and Inverse Opals Produced by Atomic Layer Deposition. Nanomaterials 2021, 11, 1053. [Google Scholar] [CrossRef]
- Lakkappa, B.A.; Jasmith, S.C.; Prabhuodeyara, M.G. Effect of concentration and pH on the size of silver nanoparticles synthesized by green chemistry. Int. J. Med. Chem. 2017, 3, 5444. [Google Scholar]
- Saeb, A.T.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci. World J. 2014, 2014, 704708. [Google Scholar] [CrossRef] [Green Version]
- Al Sufyani, N.M.; Hussien, N.A.; Hawsawi, Y.M. Characterization and anticancer potential of silver nanoparticles biosynthesized from Olea chrysophylla and Lavandula dentata leaf extracts on HCT116 colon cancer cells. J. Nanomater. 2019, 2019, 7361695. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, C.A.; Silambarasan, D.; Sarika, R.; Selvam, V. Synthesis and characterization of TiO2. Mater. Today Proc. 2022, 64, 1793–1797. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int. J. Electrochem. Sci. 2012, 7, 4871–4888. [Google Scholar] [CrossRef]
- Sakthivel, S.; Hidalgo, M.C.; Bahnemann, D.W.; Geissen, S.U.; Murugesan, V.; Vogelpohl, A. A fine route to tune the photocatalytic activity of TiO2. Appl. Catal. B 2006, 63, 31–40. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Bama, K.; Bhavani, M.; Jegatheeswaran, S.; Ambika, S.; Sangili, A.; Nithya, P.; Sumathi, R. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photochem. Photobiol. 2017, 171, 117–124. [Google Scholar]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. Influence of pH, Precursor Concentration, Growth Time, and Temperature on the Morphology of ZnO Nanostructures Grown by the Hydrothermal Method. J. Nanomater. 2011, 2011, 269692. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.H.; Lee, S.; Hwang, Y.S. Characterization of Silver Nanoparticles under Environmentally Relevant Conditions Using Asymmetrical Flow Field-Flow Fractionation (AF4). PLoS ONE 2015, 10, e0143149. [Google Scholar] [CrossRef]
- Domingos, R.F.; Baalousha, M.A.; Ju-Nam, Y.; Reid, N.; Tufenkji, N.; Lead, J.R.; Leppard, G.G.; Wilkinson, K.J. Characterizing manufactured nanoparticles in the Environment: Multimethod determination of particle sizes. Environ. Sci. Technol. 2009, 43, 7277–7284. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Chieng, B.W.; Nishibuchi, M.; Radu, S. Synthesis of silver nanoparticles by using tea leaf extract from Camellia sinensis. Int. J. Nanomed. 2012, 7, 4263–4267. [Google Scholar]
- Senthilkumar, S.R.; Sivakumar, T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci. 2014, 6, 461–465. [Google Scholar]
- Dubey, S.P.; Sillanpaa, M.; Varma, R.S. Reduction of hexavalent chromium using Sorbaria sorbifolia aqueous leaf extract. Appl. Sci. 2017, 7, 715. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Wang, Y.; Xi, X.; Li, H.; Wei, X. Using FTIR spectra and pattern recognition for discrimination of tea varieties. Int. J. Biol. Macromol. 2015, 78, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Huang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles by green tea extract and their degradation of malachite. Ind. Crops Prod. 2013, 51, 342–347. [Google Scholar] [CrossRef]
- Wu, D.; Bird, M.R. The interaction of protein and polyphenol species in ready to drink black tea liquor production. J. Food Process Eng. 2010, 33, 481–505. [Google Scholar] [CrossRef]
- Islam, M.A.; Nikoloutsou, Z.; Sakkas, V.; Papatheodorou, M.; Albanis, T. Statistical optimisation by combination of response surface methodology and desirability function for removal of azo dye from aqueous solution. J. Environ. Anal. Chem. 2010, 90, 497–509. [Google Scholar] [CrossRef]
- Engin, B.; Demirtas, H.; Eken, M. Temperature effects on egg shells investigated by XRD, IR and ESR techniques. Radiat. Phys. Chem. 2006, 75, 268–277. [Google Scholar] [CrossRef]
- Eswararao, Y.; Niju, S.; Begum, K.M.M.S.; Anantharaman, N.; Raj, S.M. Transesterification of jatropha oil using a mixture of natural shells as solid catalyst. Biofuels 2016, 7, 345–351. [Google Scholar] [CrossRef]
- Nakanot, T.; Ikawani, N.I.; Ozimek, L. Chemical composition of chicken eggshell and shell membranes. Poultry Sci. 2003, 82, 510–514. [Google Scholar] [CrossRef]
- Nikhil, P.; Sudheer, P.; Joy, P.; Sruthi, T.; Vangalpati, M. Synthesis and characterization of nanoparticles using egg shell. Mater. Today Proc. 2020, 26, 3246–3249. [Google Scholar] [CrossRef]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. S7), 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, H.; Minhajat, R.; Muis, M.; Umar, B.T.; Cangara, M.H.; Zainuddin, A.A. Review of the Benefits of Eggshell Content in Body Tissue Structure Repair. Mal. J. Med. Health Sci. 2023, 19, 278–285. [Google Scholar] [CrossRef]
- Chellappa, M.; Anjaneyulu, U.; Manivasagam, G.; Vijayalakshmi, U. Preparation and evaluation of the cytotoxic nature of TiO2 nanoparticles by direct contact method. Int. J. Nanomed. 2015, 10 (Suppl. S2), 31–41. [Google Scholar] [CrossRef] [Green Version]
- Jannathul Firdhouse, M.; Lalitha, P. Biosynthesis of Silver Nanoparticles and Its Applications. J. Nanotechnol. 2015, 2015, 829526. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Al Sufyani, N.M.; Hussien, N.A.; Hawsawi, Y.M. Cytotoxic effect of synthesized silver nanoparticles on normal human gingival fibroblast GF01 cells. In Proceedings of the 2021 International Conference of Women in Data Science at Taif University (WiDSTaif), Taif, Saudi Arabia, 30–31 March 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Butler, K.S.; Peeler, D.J.; Casey, B.J.; Dair, B.J.; Elespuru, R.K. Silver nanoparticles: Correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis 2015, 30, 577–591. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.; Lee, S.; Kim, K.; Saha, S.; Yang, G.-M.; Choi, H.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef]
- Braydich-Stolle, L.; Hussain, S.M.; Schlager, J.J.; Hoffman, M.C. In vitro cytotoxicty of nanoparticles in mammalian germline stem cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Bhakat, K.K.; Mokkapati, S.K.; Boldogh, I.; Hazra, T.K.; Mitra, S. Acetylation of human 8-oxoguanine-DNA glycosylase by p300 and its role in 8-oxoguanine repair in vivo. Mol. Cell Biol. 2006, 26, 1654–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diakowska, D.; Lewandowski, A.; Kopec, W.; Diakowski, W.; Chrzanowska, T. Oxidative DNA damage and total antioxidant status in serum of patients with esophageal squamous cell carcinoma. Hepatogastroenterology 2007, 78, 1701–1704. [Google Scholar]
- Paz-Elizur, T.; Sevilya, Z.; Leitner-Dagan, Y.; Elinger, D.; Roisman, L.C.; Livneh, Z. DNA repair of oxidative DNA damage in human carcinogenesis: Potential application for cancer risk assessment and prevention. Cancer Lett. 2008, 266, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.M.; Frazier, J.M. Involvement of apoptosis in hydrazine induced toxicity in rat primary hepatocytes. Toxicol. In Vitro 2003, 17, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Osborne, N.N. Oxidative-induced apoptosis to an immortalized ganglion cell line is caspase independent but involves the activation of poly (ADP-ribose) polymerase and apoptosis-inducing factor. Brain Res. 2008, 188, 35–43. [Google Scholar] [CrossRef]
- Liang, S.H.; Clarke, M.F. Regulation of p53 location. Eur. J. Biochem. 2001, 289, 2779–2783. [Google Scholar] [CrossRef]
- Sherr, C.J. Principles of tumor suppression. Cell 2004, 11, 235–246. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Malki, J.S.; Hussien, N.A.; Akkad, L.M.; Al Thurmani, S.O.; Al Motiri, A.E. Green Synthesis of Silver and Titanium Oxide Nanoparticles Using Tea and Eggshell Wastes, Their Characterization, and Biocompatibility Evaluation. Sustainability 2023, 15, 11858. https://doi.org/10.3390/su151511858
Al Malki JS, Hussien NA, Akkad LM, Al Thurmani SO, Al Motiri AE. Green Synthesis of Silver and Titanium Oxide Nanoparticles Using Tea and Eggshell Wastes, Their Characterization, and Biocompatibility Evaluation. Sustainability. 2023; 15(15):11858. https://doi.org/10.3390/su151511858
Chicago/Turabian StyleAl Malki, Jamila S., Nahed Ahmed Hussien, Lamia M. Akkad, Shatha O. Al Thurmani, and Anhal E. Al Motiri. 2023. "Green Synthesis of Silver and Titanium Oxide Nanoparticles Using Tea and Eggshell Wastes, Their Characterization, and Biocompatibility Evaluation" Sustainability 15, no. 15: 11858. https://doi.org/10.3390/su151511858
APA StyleAl Malki, J. S., Hussien, N. A., Akkad, L. M., Al Thurmani, S. O., & Al Motiri, A. E. (2023). Green Synthesis of Silver and Titanium Oxide Nanoparticles Using Tea and Eggshell Wastes, Their Characterization, and Biocompatibility Evaluation. Sustainability, 15(15), 11858. https://doi.org/10.3390/su151511858






