Escaping the Lock-in to Pesticide Use: Do Vietnamese Farmers Respond to Flower Strips as a Restoration Practice or Pest Management Action?
Abstract
:1. Introduction
2. Methods
2.1. Integration Models for Flower Strips and Pest Management with Related Indicators
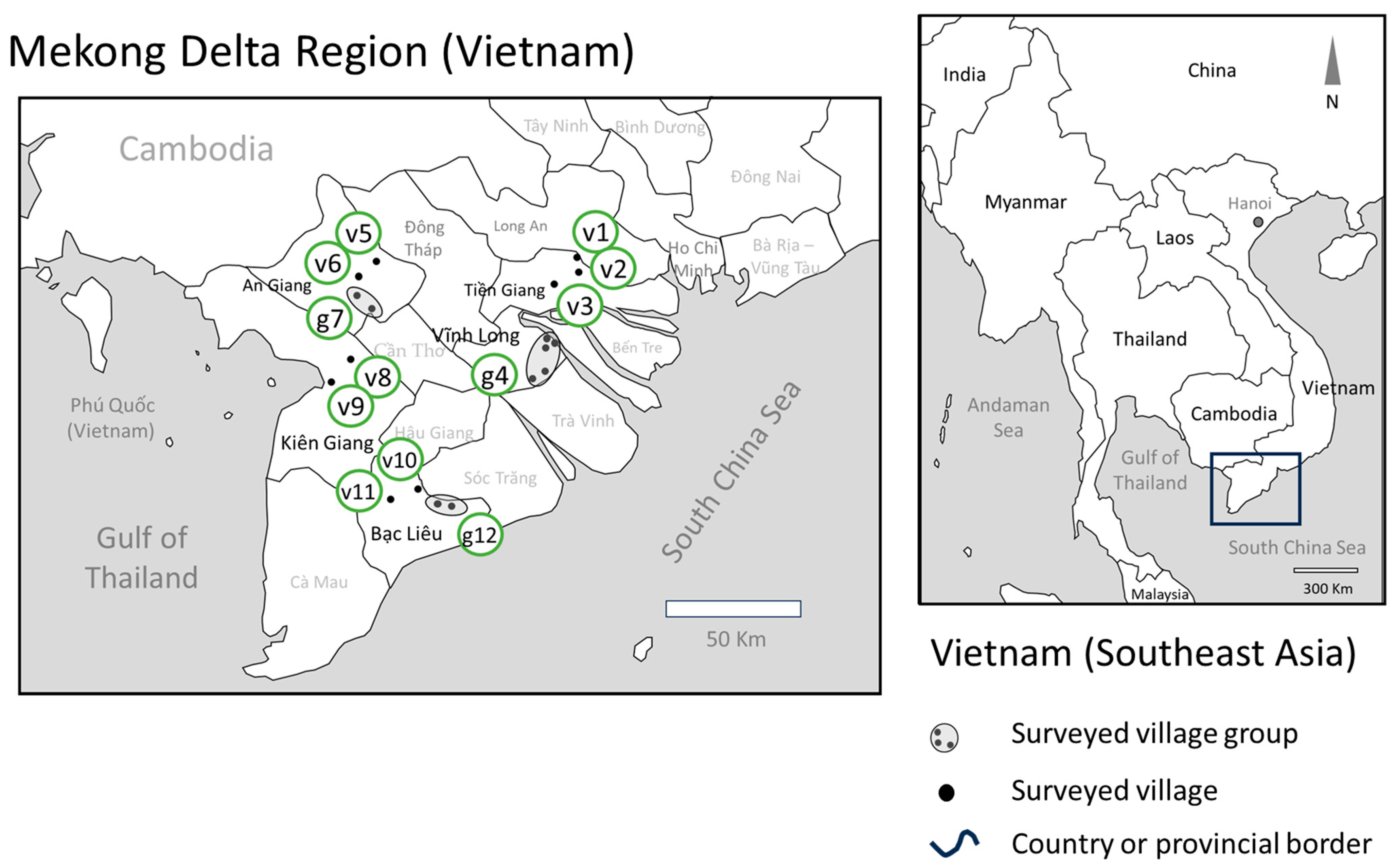
2.2. Study Sites
2.3. Data Collection
2.4. Data Analyses
3. Results
3.1. Farmer Profiles
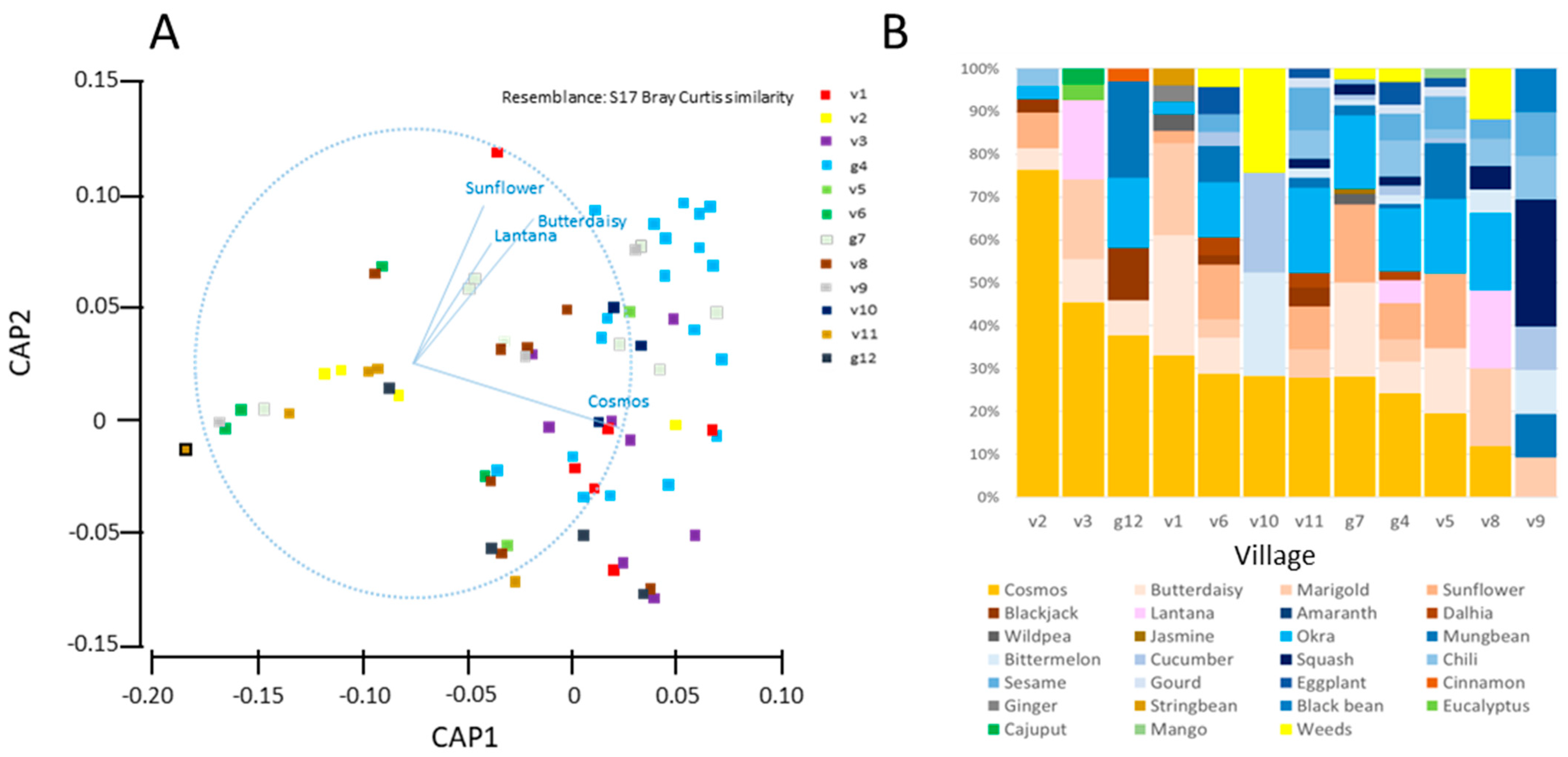
3.2. Adoption of Ecological Engineering
3.3. Agronomic Practices
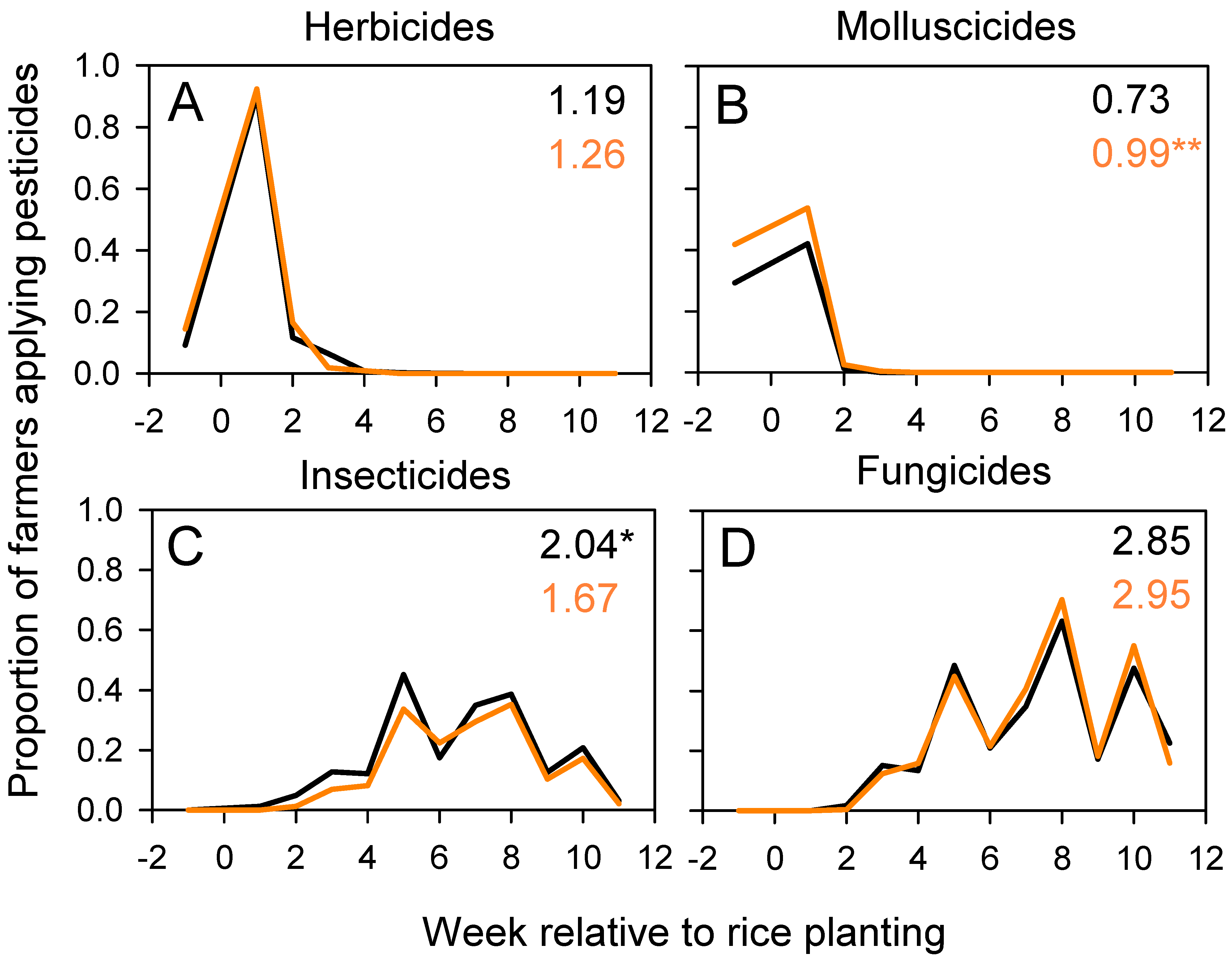
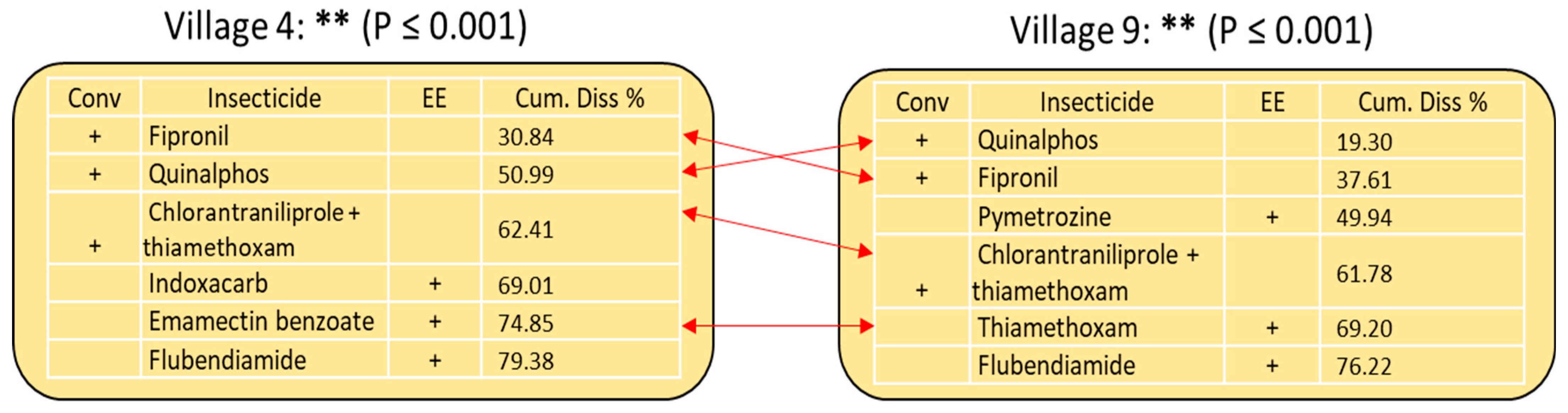
3.4. Pest, Disease, and Weed Management
3.5. Predictors of Pesticide Use
3.6. Predictors of Reported Rice Yields
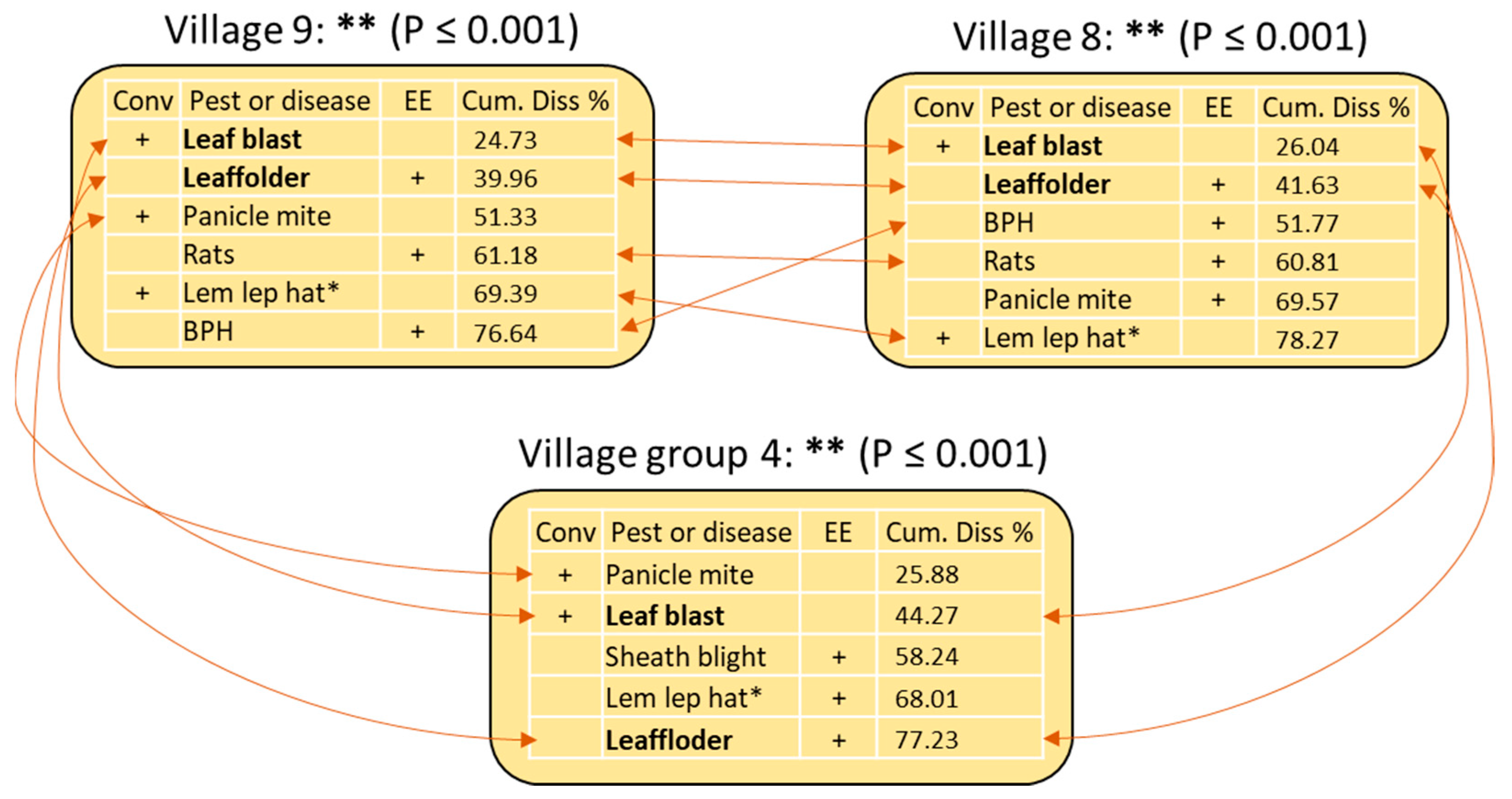
3.7. Pest and Disease Concerns
4. Discussion
4.1. Flower Strips and Insect Management Options
4.2. Other Factors Determining the Success of Flower Strips
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Pesticides Use, Pesticides Trade and Pesticides Indicators: Global, Regional and Country Trends, 1990–2020 FAOSTAT Analytical Brief 46; FAO: Rome, Italy, 2022. [Google Scholar]
- Pretty, J.; Bharucha, Z.P. Integrated pest management for sustainable intensification of agriculture in Asia and Africa. Insects 2015, 6, 152–182. [Google Scholar] [CrossRef] [PubMed]
- Flor, R.J.; Maat, H.; Hadi, B.A.R.; Kumar, V.; Castilla, N. Do field-level practices of Cambodian farmers prompt a pesticide lock-in? Field Crops Res. 2019, 235, 68–78. [Google Scholar] [CrossRef]
- Bakker, L.; van der Werf, W.; Tittonell, P.A.; Wyckhuys, K.A.G.; Bianchi, F.J.J.A. Neonicotinoids in global agriculture: Evidence for a new pesticide threadmill? Ecol. Soc. 2020, 25. [Google Scholar] [CrossRef]
- Flor, R.J.; Maat, H.; Hadi, B.A.R.; Then, R.; Kraus, E.; Chhay, K. How do stakeholder interactions in Cambodian rice farming villages contribute to a pesticide lock-in? Crop Prot. 2020, 135, 104799. [Google Scholar] [CrossRef]
- Matteson, P.C. Integrated pest management in tropical Asian irrigated rice. Annu. Rev. Entomol. 2000, 45, 549–574. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Heong, K.L.; Sanchez-Bayo, F.; Bianchi, F.J.J.A.; Lundgren, J.G.; Bentley, J.W. Ecological illiteracy can deepen farmers’ pesticide dependency. Environ. Res. Lett. 2019, 14, 093004. [Google Scholar] [CrossRef]
- Huan, N.H.; Mai, V.; Escalada, M.M.; Heong, K.L. Changes in rice farmers’ pest management in the Mekong Delta, Vietnam. Crop Prot. 1999, 18, 557–563. [Google Scholar] [CrossRef]
- Flor, R.J.; Tuan, L.A.; Hung, N.V.; My Phung, N.T.; Connor, M.; Stuart, A.M.; Sander, B.O.; Wehmeyer, H.; Cao, B.T.; Tchale, H.; et al. Unpacking the processes that catalyzed the adoption of best management practices for lowland irrigated rice in the Mekong Delta. Agronomy 2021, 11, 1707. [Google Scholar] [CrossRef]
- Escalada, M.; Heong, K.; Huan, N.; Chien, H. Changes in rice farmers’ pest management beliefs and practices in Vietnam: An analytical review of survey data from 1992 to 2007. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; International Rice Research Institute: Los Baños, Philippines, 2009; pp. 447–456. [Google Scholar]
- Ali, M.P.; Bari, M.N.; Haque, S.S.; Kabir, M.M.M.; Afrin, S.; Nowrin, F.; Islam, M.S.; Landis, D.A. Establishing next-generation pest control services in rice fields: Eco-agriculture. Sci. Rep. 2019, 9, 10180. [Google Scholar] [CrossRef]
- Gurr, G.M.; Lu, Z.; Zheng, X.; Xu, H.; Zhu, P.; Chen, G.; Yao, X.; Cheng, J.; Zhu, Z.; Catindig, J.L.; et al. Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nat. Plants 2016, 2, 16014. [Google Scholar] [CrossRef]
- Horgan, F.G.; Crisol Martínez, E.; Stuart, A.M.; Bernal, C.C.; de Cima Martín, E.; Almazan, M.L.P.; Ramal, A.F. Effects of vegetation strips, fertilizer levels and varietal resistance on the integrated management of arthropod biodiversity in a tropical rice ecosystem. Insects 2019, 10, 328. [Google Scholar] [CrossRef]
- Horgan, F.G.; Vu, Q.; Mundaca, E.A.; Crisol-Martínez, E. Restoration of rice ecosystem services: ‘ecological engineering for pest management’ incentives and practices in the Mekong Delta Region of Vietnam. Agronomy 2022, 12, 1042. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, P.; Gurr, G.M.; Zheng, X.; Chen, G.; Heong, K.L. Rice pest management by ecological engineering: A pioneering attempt in China. In Rice Planthoppers: Ecology, Management, Socio Economics and Policy; Heong, K.L., Cheng, J., Escalada, M.M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 161–178. [Google Scholar]
- Shanker, C.; Chintagunta, L.; Muthusamy, S.; Vailla, S.; Srinivasan, A.; Katti, G. Flora surrounding rice fields as a source of alternative prey for coccinellids feeding on the pests of rice. Eur. J. Entomol. 2018, 115, 364–371. [Google Scholar] [CrossRef]
- Zhu, P.; Zheng, X.; Zhang, F.; Xu, H.; Yang, Y.; Chen, G.; Lu, Z.; Johnson, A.C.; Gurr, G.M. Quantifying the respective and additive effects of nectar plant crop borders and withholding insecticides on biological control of pests in subtropical rice. J. Pest Sci. 2018, 91, 575–584. [Google Scholar] [CrossRef]
- Sattler, C.; Schrader, J.; Flor, R.J.; Keo, M.; Chhun, S.; Choun, S.; Hadi, B.A.; Settele, J. Reducing pesticides and increasing crop diversification offer ecological and economic benefits for farmers—A case study in Cambodian rice fields. Insects 2021, 12, 267. [Google Scholar] [CrossRef]
- Babendreier, D.; Hou, M.; Tang, R.; Zhang, F.; Vongsabouth, T.; Win, K.K.; Kang, M.; Peng, H.; Song, K.; Annamalai, S.; et al. Biological control of lepidopteran pests in rice: A multi-nation case study from Asia. J. Integr. Pest Manag. 2020, 11, 5. [Google Scholar] [CrossRef]
- Wang, P.; Li, M.J.; Bai, Q.R.; Ali, A.; Desneux, N.; Dai, H.J.; Zang, L.S. Performance of Trichogramma japonicum as a vector of Beauveria bassiana for parasitizing eggs of rice striped stem borer, Chilo Suppressalis. Entomol. Gen. 2021, 41, 147–155. [Google Scholar] [CrossRef] [PubMed]
- D’Annolfo, R.; Gemmill-Herren, B.; Amudavi, D.; Shiraku, H.W.; Piva, M.; Garibaldi, L.A. The effects of agroecological farming systems on smallholder livelihoods: A case study on push–pull system from Western Kenya. Int. J. Agric. Sustain. 2021, 19, 56–70. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Kennedy, J.F. Encapsulating biocontrol bacteria with starch as a safe and edible biopolymer to alleviate plant diseases: A review. Carbohydr. Polym. 2022, 302, 120384. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, X.; Li, Z. Substitution or complementarity: Why do rice farmers use a mix of biopesticides and chemical pesticides in China? Pest Manag. Sci. 2022, 78, 1630–1639. [Google Scholar] [CrossRef]
- Bao, J.; Zhuo, C.; Zhang, D.; Li, Y.; Hu, F.; Li, H.; Su, Z.; Liang, Y.; He, H. Potential applicability of a cyanobacterium as a biofertilizer and biopesticide in rice fields. Plant Soil. 2021, 463, 97–112. [Google Scholar] [CrossRef]
- Seo, Y.; Umeda, S. Evaluating farm management performance by the choice of pest-control sprayers in rice farming in Japan. Sustainability 2021, 13, 2618. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M. A novel encapsulation of Streptomyces fulvissimus Uts22 by spray drying and its biocontrol efficiency against Gaeumannomyces graminis, the causal agent of take-all disease in wheat. Pest Manag. Sci. 2021, 77, 4357–4364. [Google Scholar] [CrossRef]
- Thar, S.P.; Ramilan, T.; Farquharson, R.J.; Chen, D. Identifying potential for decision support tools through farm systems typology analysis coupled with participatory research: A case for smallholder farmers in Myanmar. Agriculture 2021, 11, 516. [Google Scholar] [CrossRef]
- Manalo, J.A., IV; Pasiona, S.P.; Bautista, A.M.F. Understanding the complexities in the adoption of the Rice Crop Manager tool in the Philippines. Int. J. Agric. Sustain. 2022, 20, 381–392. [Google Scholar] [CrossRef]
- Horgan, F.G.; Ramal, A.F.; Villegas, J.M.; Jamoralin, A.; Bernal, C.C.; Perez, M.O.; Pasang, J.M.; Naredo, A.I.; Almazan, M.L.P. Effects of bund crops and insecticide treatments on arthropod diversity and herbivore regulation in tropical rice fields. J. Appl. Entomol. 2017, 141, 587–599. [Google Scholar] [CrossRef]
- Lu, Z.-X.; Zhu, P.-Y.; Gurr, G.M.; Zheng, X.-S.; Read, D.M.Y.; Heong, K.-L.; Yang, Y.-J.; Xu, H.-X. Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: Prospects for enhanced use in agriculture. Insect Sci. 2014, 21, 1–12. [Google Scholar] [CrossRef]
- Vu, Q.; Ramal, A.F.; Villegas, J.M.; Jamoralin, A.; Bernal, C.C.; Pasang, J.M.; Almazan, M.L.P.; Ramp, D.; Settele, J.; Horgan, F.G. Enhancing the parasitism of insect herbivores through diversification of habitat in Philippine rice fields. Paddy Water Environ. 2018, 16, 379–390. [Google Scholar] [CrossRef]
- Zhu, P.; Gurr, G.M.; Lu, Z.; Heong, K.; Chen, G.; Zheng, X.; Xu, H.; Yang, Y. Laboratory screening supports the selection of sesame (Sesamum indicum) to enhance Anagrus spp. parasitoids (Hymenoptera: Mymaridae) of rice planthoppers. Biol. Control 2013, 64, 83–89. [Google Scholar] [CrossRef]
- Zhu, P.; Lu, Z.; Heong, K.; Chen, G.; Zheng, X.; Xu, H.; Yang, Y.; Nicol, H.I.; Gurr, G.M. Selection of nectar plants for use in ecological engineering to promote biological control of rice pests by the predatory bug, Cyrtorhinus lividipennis, (Heteroptera: Miridae). PLoS ONE 2014, 9, e108669. [Google Scholar] [CrossRef]
- Trường, Đ.T.; Anh, N.T.Q.; Anh, N.T.N.; Ly, Đ.K.K.; Spangenberg, J.H.; Settele, J.; Tekken, V.; Rodríguez-Labajos, B. Policy analysis approaches in implementing the ecological engineering in Vietnam: Experiences from LEGATO—An interdisciplinary project. VNU J. Sci. Policy Manag. Stud. 2016, 32, 49–67. [Google Scholar]
- Vo, H.T.; Yabe, M.; Nguyễn, T.T.; Huỳnh, V.K. Environmental efficiency of ecologically engineered rice production in the Mekong Delta of Vietnam. J. Fac. Agric. Kyushu. Univ. 2015, 60, 493–500. [Google Scholar]
- Van Meel, P. Multiple approaches to enhance communication between rice farmers, rural service providers and scientists. Basic. Outlooks Pest Manag. 2008, 19, 260–263. [Google Scholar]
- Heong, K.; Escalada, M.; Chien, H.; Cuong, L. Restoration of rice landscape biodiversity by farmers in Vietnam through education and motivation using media. Surv. Perspect. Integr. Environ. Soc. 2014, 7, 2. [Google Scholar]
- Comberti, C.; Thornton, T.F.; Wyllie de Echeverria, V.; Patterson, T. Ecosystem services or services to ecosystems? Valuing cultivation and reciprocal relationships between humans and ecosystems. Glob. Environ. Chang. 2015, 34, 247–262. [Google Scholar] [CrossRef]
- Bottrell, D.G.; Schoenly, K.G. Resurrecting the ghost of green revolutions past: The brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia-Pac. Entomol. 2012, 15, 122–140. [Google Scholar] [CrossRef]
- Wu, J.; Ge, L.; Liu, F.; Song, Q.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2020, 65, 409–429. [Google Scholar] [CrossRef]
- Radermacher, N.; Hartke, T.R.; Villareal, S.; Scheu, S. Spiders in rice-paddy ecosystems shift from aquatic to terrestrial prey and use carbon pools of different origin. Oecologia 2020, 192, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Huan, N.H.; Chien, H.V.; Quynh, P.V.; Tan, P.S.; Du, P.V.; Escalada, M.M.; Heong, K.L. Motivating rice farmers in the Mekong Delta to modify pest management and related practices through mass media. Int. J. Pest Manag. 2008, 54, 339–346. [Google Scholar] [CrossRef]
- Settle, W.H.; Ariawan, H.; Astuti, E.T.; Cahyana, W.; Hakim, A.L.; Hindayana, D.; Lestari, A.S. Managing tropical rice pests through conservation of generalist natural enemies and alternative prey. Ecology 1996, 77, 1975–1988. [Google Scholar] [CrossRef]
- Horgan, F.G.; Ramal, A.F.; Villegas, J.M.; Almazan, M.L.P.; Bernal, C.C.; Jamoralin, A.; Pasang, J.M.; Orboc, G.; Agreda, V.; Arroyo, C. Ecological engineering with high diversity vegetation patches enhances bird activity and ecosystem services in Philippine rice fields. Reg. Environ. Change 2017, 17, 1355–1367. [Google Scholar] [CrossRef]
- Zhu, P.; Zheng, X.; Johnson, A.C.; Chen, G.; Xu, H.; Zhang, F.; Yao, X.; Heong, K.; Lu, Z.; Gurr, G.M. Ecological engineering for rice pest suppression in China. A review. Agron. Sustain. Dev. 2022, 42, 69. [Google Scholar]
- Berg, H.; Ekman Söderholm, A.; Söderström, A.-S.; Tam, N.T. Recognizing wetland ecosystem services for sustainable rice farming in the Mekong Delta, Vietnam. Sustain. Sci. 2017, 12, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.D.; van Halsema, G.; Hellegers, P.J.G.J.; Ludwig, F.; Wyatt, A. Questioning triple rice intensification on the Vietnamese Mekong Delta floodplains: An environmental and economic analysis of current land-use trends and alternatives. J. Environ. Manag. 2018, 217, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Linh, T.B.; Guong, V.T.; Tran, V.T.T.; Van Khoa, L.; Olk, D.; Cornelis, W.M. Effects of crop rotation on properties of a Vietnam clay soil under rice-based cropping systems in small-scale farmers’ fields. Soil Res. 2017, 55, 162–171. [Google Scholar] [CrossRef]
- Berg, H. Pesticide use in rice and rice–fish farms in the Mekong Delta, Vietnam. Crop Prot. 2001, 20, 897–905. [Google Scholar] [CrossRef]
- Stone, L.; Campbell, J. The use and misuse of surveys in international development: An experiment from Nepal. Hum. Organ. 2008, 43, 27–37. [Google Scholar] [CrossRef]
- Schattman, R.E.; Méndez, V.E.; Merrill, S.C.; Zia, A. Mixed methods approach to understanding farmer and agricultural advisor perceptions of climate change and adaptation in Vermont, United States. Agroecol. Sustain. Food Syst. 2018, 42, 121–148. [Google Scholar] [CrossRef]
- Blair, A.; Stewart, P.; Kross, B.; Ogilvie, L.; Burmeister, L.; Ward, M.; Zahm, S. Comparison of two techniques to obtain information on pesticide use from Iowa farmers by interview. J. Agric. Saf. Health 1997, 3, 229. [Google Scholar] [CrossRef]
- Glik, D.C.; Parker, K.; Hategikamana, G.M.B. Integrating qualitative and quantitative survey techniques. Int. Q. Community Health Educ. 2005, 25, 115–133. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance; Department of Statistics, University of Auckland: Auckland, New Zealand, 2005; Volume 26, pp. 32–46. [Google Scholar]
- Clarke, K.; Gorley, R. PRIMER: Getting Started with v6; PRIMER-E Ltd.: Plymouth, UK, 2005; Volume 931, p. 932. [Google Scholar]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Chi, T.T.N.; Anh, T.T.T.; Paris, T.; Duy, L.; Loan, D.T.; Lang, N.T. Farmers’ feedback on rice varieties tested under farmer-managed trials. Omonrice 2015, 20, 93–108. [Google Scholar]
- Lang, N.T.; Buu, B.C. Rice genetic resource conservation and utilization in the Mekong Delta. Omonrice 2011, 18, 22–33. [Google Scholar]
- Vang, P.T.K.; Lang, N.T.; Chau, L.M.; He, T.N. Determination of the presence of brown planthopper resistance genes (Nilaparvata lugens Stål.) in rice (Oryza sativa L.). Int. J. Environ. Agric. Biotechnol. 2020, 5, 796–804. [Google Scholar] [CrossRef]
- Gallagher, K.D.; Kenmore, P.E.; Sogawa, K. Judicial use of insecticides deter planthopper outbreaks and extend the life of resistant varieties in Southeast Asian rice. In Planthoppers: Their Ecology and Management; Denno, R.F., Perfect, T.J., Eds.; Springer: Boston, MA, USA, 1994; pp. 599–614. [Google Scholar]
- Cuong, N.L.; Ben, P.T.; Phuong, L.T.; Chau, L.M.; Cohen, M.B. Effect of host plant resistance and insecticide on brown planthopper Nilaparvata lugens (Stål) and predator population development in the Mekong Delta, Vietnam. Crop Prot. 1997, 16, 707–715. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Raupp, M.J.; Parker, R.D.; Kerns, D.; Eubanks, M.D. Neonicotinoid insecticides alter induced defenses and increase susceptibility to spider mites in distantly related crop plants. PLoS ONE 2013, 8, e62620. [Google Scholar]
- Cohen, E. Pesticide-mediated homeostatic modulation in arthropods. Pestic. Biochem. Physiol. 2006, 85, 21–27. [Google Scholar] [CrossRef]
- Chintalapati, P.; Katti, G.; Puskur, R.R.; Nagella Venkata, K. Neonicotinoid-induced resurgence of rice leaffolder, Cnaphalocrocis medinalis (Guénee). Pest Manag. Sci. 2016, 72, 155–161. [Google Scholar] [CrossRef]
- Singh, S.; Kushwaha, K.; Sharma, P.; Singh, S. Resurgence of rice leaf folder Cnaphalocrocis medinalis Guenee due to application of phorate granules. Haryana J. Agron. 1995, 57, 366–372. [Google Scholar]
- Phung, N.T.M.; Brown, P.R.; Leung, L.K.-P. Changes in population abundance, reproduction and habitat use of the rice-field rat, Rattus argentiventer, in relation to rice-crop growth stage in a lowland rice agroecosystem in Vietnam. Wildl. Res. 2012, 39, 250–257. [Google Scholar] [CrossRef]
- Joshi, R.; Antolin, M.; Marquez, L.; Duque, U.; Sebastian, L. Predation on Pomacea and radix snails during non-rice growing periods by the Philippine ricefield rat, Rattus tanezumi Temminck. Philipp. J. Crop Sci. 2006, 31, 41–51. [Google Scholar]
- Deguine, J.-P.; Aubertot, J.-N.; Flor, R.J.; Lescourret, F.; Wyckhuys, A.G.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]
- Horgan, F.G.; Kudavidanage, E.P. Use and avoidance of pesticides as responses by farmers to change impacts in rice ecosystems of southern Sri Lanka. Environ. Manag. 2020, 65, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.; Aldosari, F. Agricultural extension in Asia: Constraints and options for improvement. J. Anim. Plant Sci. 2013, 23, 619–632. [Google Scholar]
- Taylor, M.; Bhasme, S. Model farmers, extension networks and the politics of agricultural knowledge transfer. J. Rural Stud. 2018, 64, 1–10. [Google Scholar] [CrossRef]
- Banks, J.E.; Laubmeier, A. Compatibility of biological control and pesticides mediated by arthropod movement behavior and field spatial scale. Biol. Control 2023, 177, 105125. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Zou, Y.; Wanger, T.C.; Zhou, W.; Gc, Y.D.; Lu, Y. Agro-ecology science relates to economic development but not global pesticide pollution. J. Environ. Manag. 2022, 307, 114529. [Google Scholar] [CrossRef]
- Calvo-Agudo, M.; Tooker, J.F.; Dicke, M.; Tena, A. Insecticide-contaminated honeydew: Risks for beneficial insects. Biol. Rev. 2022, 97, 664–678. [Google Scholar] [CrossRef]

| Village 1 | Farm Type 2 | Season 3 | Rotating Crops (%) | Length of Fallow (Days) 4 | Age of Seedlings (Days) 4 | Nitrogen (Kg ha−1) 4 | Potassium (Kg ha−1) 4 | Phosphorus (Kg ha−1) 4 | Perceived Yields (Tons ha−1) 4 |
|---|---|---|---|---|---|---|---|---|---|
| v1 | C | 1 | 11.11 | 15.56 (2.32) abc | 5.56 (1.27) b | 66.97 (12.66) abc | 33.90 (6.18) bc | 49.84 (12.26) ab | 5.55 (6.20) bc |
| 2 | 21.00 (2.51) | 5.56 (1.27) | 53.51 (15.57) | 33.56 (4.51) | 28.73 (6.94) | 8.22 (3.29) | |||
| 3 | 17.11 (2.08) | 5.56 (1.27) | 57.68 (16.80) | 32.49 (4.95) | 26.34 (7.92) | 5.45 (7.23) | |||
| EE | 1 | 11.11 | 17.28 (1.17) | 5.89 (0.78) | 99.60 (10.17) | 37.31 (5.49) | 72.25 (14.45) | 6.07 (9.16) | |
| 2 | 25.33 (3.37) | 5.89 (0.78) | 92.93 (8.62) | 40.27 (5.34) | 73.21 (13.80) | 7.94 (2.22) | |||
| 3 | 21.61 (2.63) | 5.89 (0.78) | 89.83 (7.88) | 40.08 (5.61) | 60.13 (10.27) | 6.06 (9.26) | |||
| v2 | C | 1 | 20.00 | 13.00 (1.33) a | 3.25 (0.25) a | 79.51 (9.22) abc | 27.90 (4.91) b | 35.21 (8.64) a | 4.88 (9.42) a |
| 2 | 16.11 (2.00) | 3.25 (0.25) | 86.48 (12.37) | 38.17 (12.46) | 51.29 (19.88) | 5.87 (6.38) | |||
| 3 | 14.44 (1.00) | 3.25 (0.25) | 82.14 (9.86) | 27.90 (4.91) | 35.98 (8.59) | 4.71 (2.33) | |||
| EE | 1 | 50.00 | 17.50 (2.50) | 3.00 (0.00) | 103.81 (8.89) | 26.75 (3.75) | 68.75 (20.25) | 5.59 (0.43) | |
| 2 | 16.25 (1.25) | 14.00 (11.00) | 103.81 (8.89) | 26.75 (3.75) | 68.75 (20.25) | 6.24 (0.41) | |||
| 3 | 17.50 (2.50) | 3.00 (0.00) | 103.81 (8.89) | 26.75 (3.75) | 68.75 (20.25) | 6.02 (0.86) | |||
| v3 | C | 1 | 25.00 | 21.25 (5.15) c | 3.25 (0.25) a | 45.33 (3.40) ab | 26.39 (11.47) bc | 33.07 (1.72) a | 6.77 (5.67) bc |
| 2 | 60.00 (0.00) | 3.25 (0.25) | 44.01 (4.31) | 24.18 (11.95) | 30.86 (2.31) | 8.60 (4.00) | |||
| 3 | 11.25 (1.25) | 3.25 (0.25) | 43.94 (4.36) | 24.07 (11.98) | 30.75 (2.38) | 6.67 (3.22) | |||
| EE | 1 | 27.27 | 18.64 (2.25) | 3.00 (0.00) | 88.06 (6.46) | 40.77 (5.66) | 51.03 (4.34) | 5.29 (8.14) | |
| 2 | 40.91 (4.56) | 3.00 (0.00) | 77.03 (8.65) | 41.15 (6.71) | 45.58 (5.52) | 7.70 (7.26) | |||
| 3 | 12.91 (0.91) | 3.00 (0.00) | 89.14 (5.87) | 41.87 (5.89) | 50.88 (4.36) | 6.18 (9.24) | |||
| g4 | C | 1 | 3.23 | 20.65 (0.95) bc | 2.84 (0.07) a | 86.59 (3.49) abc | 35.59 (3.01) bc | 72.57 (2.92) ab | 5.47 (6.10) bc |
| 2 | 29.84 (1.68) | 2.84 (0.07) | 85.51 (3.52) | 30.51 (1.97) | 72.92 (2.90) | 6.70 (5.13) | |||
| 3 | 20.00 (0.40) | 2.84 (0.07) | 85.79 (4.88) | 34.16 (4.83) | 74.08 (5.02) | 5.46 (5.09) | |||
| EE | 1 | 0.00 | 16.72 (0.45) | 2.79 (0.43) | 95.61 (3.14) | 43.44 (3.25) | 60.85 (5.69) | 6.30 (5.11) | |
| 2 | 24.31 (1.31) | 2.79 (0.43) | 90.36 (3.69) | 49.55 (4.74) | 51.90 (2.96) | 7.78 (0.21) | |||
| 3 | 18.45 (0.56) | 2.79 (0.43) | 91.05 (3.28) | 42.47 (3.40) | 52.00 (4.03) | 6.47 (1.14) | |||
| v5 | C | 1 | 0.00 | 15.53 (1.40) ab | 3.93 (0.64) ab | 129.69 (12.41) d | 54.56 (6.76) bc | 63.99 (6.46) ab | 5.88 (2.14) b |
| 2 | 18.33 (1.67) | 3.93 (0.64) | 135.95 (10.64) | 51.08 (6.83) | 63.62 (6.31) | 6.93 (3.19) | |||
| 3 | 18.33 (1.05) | 3.93 (0.64) | 135.67 (10.78) | 51.96 (7.00) | 62.70 (6.60) | 5.95 (3.19) | |||
| EE | 1 | 14.29 | 20.00 (0.00) | 6.00 (1.41) | 130.18 (8.96) | 47.93 (6.55) | 54.05 (4.49) | 5.47 (3.37) | |
| 2 | 29.29 (5.39) | 5.57 (1.27) | 134.20 (7.60) | 55.30 (6.54) | 58.03 (4.53) | 6.77 (3.21) | |||
| 3 | 19.29 (0.71) | 5.57 (1.27) | 129.37 (7.61) | 51.02 (5.08) | 57.98 (4.53) | 5.78 (2.14) | |||
| v6 | C | 1 | 0.00 | 14.00 (1.53) ab | 4.00 (1.21) ab | 94.98 (19.96) bc | 54.87 (14.87) bc | 51.86 (6.95) ab | 5.63 (4.12) bc |
| 2 | 16.67 (1.67) | 4.00 (1.21) | 104.64 (21.77) | 54.87 (14.87) | 55.06 (8.11) | 7.12 (0.37) | |||
| 3 | 16.67 (1.05) | 4.00 (1.21) | 101.23 (21.22) | 58.03 (14.25) | 67.83 (18.42) | 5.42 (0.22) | |||
| EE | 1 | 0.00 | 15.00 (1.83) | 5.17 (1.54) | 84.88 (11.44) | 41.95 (7.59) | 53.77 (8.66) | 5.55 (4.34) | |
| 2 | 20.83 (2.39) | 5.17 (1.54) | 104.44 (12.10) | 43.38 (7.29) | 63.30 (8.25) | 7.74 (0.35) | |||
| 3 | 17.50 (1.12) | 5.17 (1.54) | 103.08 (12.56) | 43.06 (7.42) | 59.99 (7.50) | 6.08 (0.23) | |||
| g7 | C | 1 | 0.00 | 22.77 (1.45) ab | 3.00 (0.00) ab | 107.57 (8.25) cd | 50.16 (5.15) c | 78.27 (9.86) b | 6.13 (0.26) c |
| 2 | 20.08 (0.98) | 3.69 (0.69) | 107.59 (8.87) | 50.16 (5.15) | 82.88 (12.89) | 7.93 (0.24) | |||
| 3 | 22.00 (1.33) | 3.69 (0.69) | 108.57 (8.48) | 52.60 (6.52) | 78.98 (10.11) | 5.93 (0.22) | |||
| EE | 1 | 0.00 | 15.75 (1.21) | 4.17 (1.06) | 121.07 (6.57) | 53.09 (7.28) | 91.47 (8.75) | 6.92 (2.22) | |
| 2 | 18.25 (1.57) | 4.17 (1.06) | 121.07 (6.57) | 66.14 (19.35) | 91.47 (8.75) | 8.36 (2.25) | |||
| 3 | 16.00 (1.66) | 4.17 (1.06) | 121.07 (6.57) | 50.59 (7.79) | 91.47 (8.75) | 6.28 (2.30) | |||
| v8 | C | 1 | 0.00 | 21.00 (0.87) bc | 3.00 (0.00) a | 63.44 (2.04) a | 31.72 (1.02) bc | 63.44 (2.04) ab | 5.81 (3.27) b |
| 2 | 21.05 (0.72) | 3.00 (0.00) | 69.87 (1.89) | 34.93 (0.95) | 69.87 (1.89) | 7.05 (9.24) | |||
| 3 | 21.58 (0.77) | 3.00 (0.00) | 62.48 (1.22) | 31.24 (0.61) | 62.48 (1.22) | 5.80 (2.16) | |||
| EE | 1 | 0.00 | 19.50 (0.90) | 3.00 (0.00) | 65.60 (2.32) | 32.80 (1.16) | 65.60 (2.32) | 5.53 (5.16) | |
| 2 | 26.36 (3.57) | 3.00 (0.00) | 72.73 (2.28) | 36.36 (1.14) | 72.73 (2.28) | 6.88 (2.27) | |||
| 3 | 19.55 (0.81) | 3.00 (0.00) | 65.45 (1.81) | 32.73 (0.91) | 65.45 (1.81) | 5.73 (5.11) | |||
| v97 | C | 1 | 0.00 | - | - | - | - | - | - |
| 2 | 26.94 (1.67) bc | 3.00 (0.00) a | 63.22 (3.52) a | 31.61 (1.76) bc | 63.22 (3.52) ab | 6.79 (3.20) b | |||
| 3 | 19.72 (0.28) | 3.00 (0.00) | 59.52 (2.52) | 29.76 (1.26) | 59.52 (2.52) | 5.99 (9.21) | |||
| EE | 1 | 0.00 | - | - | - | - | - | - | |
| 2 | 27.27 (3.53) | 3.00 (0.00) | 66.91 (5.53) | 33.45 (2.76) | 66.91 (5.53) | 6.32 (6.16) | |||
| 3 | 19.09 (0.61) | 3.00 (0.00) | 68.36 (4.75) | 34.18 (2.37) | 68.36 (4.75) | 5.80 (8.27) | |||
| v10 | C | 1 | 0.00 | 28.00 (4.36) d | 3.00 (0.00) a | 89.76 (5.50) ab | 44.50 (6.14) bc | 75.93 (10.95) ab | 5.83 (9.17) bc |
| 2 | 76.92 (11.76) | 3.00 (0.00) | 80.43 (7.25) | 36.74 (5.09) | 81.93 (10.90) | 7.76 (0.17) | |||
| 3 | 55.00 (6.35) | 3.00 (0.00) | 42.17 (8.06) | 26.24 (4.28) | 45.21 (9.03) | 5.78 (2.14) | |||
| EE | 1 | 11.11 | - | - | - | - | - | - | |
| 2 | 87.86 (17.14) | 3.00 (0.00) | 70.87 (11.26) | 37.85 (11.08) | 55.84 (17.21) | 7.29 (0.15) | |||
| 3 | 79.29 (9.09) | 3.00 (0.00) | 66.04 (11.17) | 29.66 (10.79) | 53.11 (15.43) | 5.32 (6.23) | |||
| v117 | C | 1 | 100.00 | 30.00 (0.00) e | 3.00 (0.00) a | 104.83 (17.58) bc | 11.33 (2.64) a | 43.53 (12.60) ab | 6.33 (0.27) bc |
| 2 | - | - | - | - | - | - | |||
| 3 | - | - | - | - | - | - | |||
| EE | 1 | 100.00 | 30.00 (0.00) | 3.00 (0.00) | 82.76 (9.92) | 13.50 (1.00) | 73.30 (5.62) | 6.50 (2.31) | |
| 2 | - | - | - | - | - | - | |||
| 3 | - | - | - | - | - | - | |||
| g12 | C | 1 | 0.00 | 14.75 (0.57) ab | 3.05 (0.14) a | 93.76 (7.69) abc | 45.35 (5.42) bc | 60.96 (5.62) ab | 5.97 (3.11) bc |
| 2 | 16.10 (1.19) | 3.10 (0.16) | 112.63 (9.07) | 49.08 (5.71) | 67.89 (5.87) | 6.91 (1.13) | |||
| 3 | 23.75 (1.53) | 3.05 (0.14) | 98.09 (8.03) | 44.36 (5.17) | 61.54 (5.70) | 6.54 (8.13) | |||
| EE | 1 | 0.00 | 16.00 (1.00) | 3.00 (0.00) | 51.25 (11.78) | 20.18 (4.66) | 48.72 (15.18) | 5.64 (1.17) | |
| 2 | 19.50 (2.03) | 3.00 (0.00) | 70.32 (19.21) | 22.69 (3.97) | 70.51 (24.29) | 7.00 (0.20) | |||
| 3 | 20.50 (3.20) | 3.00 (0.00) | 49.77 (11.56) | 20.16 (4.75) | 44.79 (11.27) | 6.07 (9.27) | |||
| Season 5,7 | 57.726 *** | 0.371 ns | 5.086 ** | 2.816 ns | 3.999 * | 319.341 *** | |||
| Season × Village 5,7 | 13.509 *** | 0.807 ns | 4.028 *** | 0.523 ns | 2.089 ** | 6.174 *** | |||
| Season × Farm type 5,7 | 0.176 ns | 0.490 ns | 0.034 ns | 0.677 ns | 0.150 ns | 2.029 ns | |||
| Season × Village × Farm type 5,7 | 2.663 *** | 0.891 ns | 0.282 ns | 0.698 ns | 0.754 ns | 1.647* | |||
| Village 6 | 9.558 *** | 5.332 *** | 10.936 *** | 3.250 *** | 3.491 *** | 5.528 *** | |||
| Farm type 6 | 0.001 ns | 1.058 ns | 2.830 ns | 0.022 ns | 2.170 ns | 1.744 ns | |||
| Village × Farm type 6 | 3.343 *** | 0.596 ns | 4.196 *** | 2.485 ** | 2.446 * | 5.265 *** | |||
| Village 1 | Farm Type 2 | Season 3 | Herbicide Applications (Number) 4 | Molluscicide Applications (Number) 4 | Fungicide Applications (Number) 4 | Insecticide Applications (Number) 4 | Time of First Insecticide Application (Days) 4 | Pesticide Applications (Number) 4 |
|---|---|---|---|---|---|---|---|---|
| v1 | C | 1 | 1.11 (0.20) a | 0.89 (0.20) | 2.00 (0.60) a | 1.56 (0.56) ab | 26.00 (5.10) ab | 5.56 (1.17) a |
| 2 | 1.11 (0.20) | 0.89 (0.20) | 2.67 (0.41) | 2.33 (0.47) | 28.75 (5.07) | 7.00 (1.82) | ||
| 3 | 1.11 (0.20) | 1.00 (0.17) | 2.33 (0.50) | 2.11 (0.48) | 26.67 (4.22) | 6.56 (0.99) | ||
| EE | 1 | 1.50 (0.12) | 1.33 (0.11) | 2.72 (0.32) | 1.72 (0.32) | 40.00 (2.04) | 7.28 (1.52) | |
| 2 | 1.50 (0.12) | 1.33 (0.16) | 2.78 (0.33) | 1.94 (0.27) | 37.15 (2.54) | 7.56 (1.53) | ||
| 3 | 1.50 (0.12) | 1.22 (0.13) | 2.61 (0.33) | 1.67 (0.30) | 42.31 (2.87) | 7.00 (1.54) | ||
| v2 | C | 1 | 2.20 (0.20) b | 0.80 (0.25) | 2.50 (0.22) a | 2.00 (0.45) ab | 26.38 (5.49) ab | 6.82 (1.93) a |
| 2 | 2.10 (0.18) | 0.60 (0.27) | 2.50 (0.22) | 2.10 (0.43) | 28.25 (5.50) | 6.64 (1.90) | ||
| 3 | 2.20 (0.20) | 0.80 (0.25) | 2.50 (0.22) | 1.80 (0.44) | 27.63 (5.42) | 6.64 (1.90) | ||
| EE | 1 | 2.00 (0.00) | 1.00 (0.00) | 3.50 (0.50) | 1.50 (1.50) | 40.00 (0.00) | 8.00 (1.00) | |
| 2 | 1.33 (0.67) | 0.67 (0.33) | 2.33 (0.20) | 1.33 (0.88) | 40.00 (0.00) | 5.67 (1.85) | ||
| 3 | 2.00 (0.00) | 1.00 (0.00) | 3.50 (0.50) | 1.50 (1.50) | 40.00 (0.00) | 8.00 (1.00) | ||
| v3 | C | 1 | 1.25 (0.25) a | 1.00 (0.00) | 4.50 (0.29) ab | 1.75 (0.25) ab | 35.00 (4.56) ab | 8.50 (1.29) a |
| 2 | 1.25 (0.25) | 1.00 (0.00) | 4.50 (0.29) | 1.25 (0.75) | 40.00 (5.00) | 8.00 (1.71) | ||
| 3 | 1.25 (0.25) | 1.00 (0.00) | 4.50 (0.29) | 1.00 (0.58) | 42.50 (2.50) | 7.75 (1.48) | ||
| EE | 1 | 1.20 (0.13) | 0.90 (0.18) | 3.30 (0.37) | 2.20 (0.47) | 28.14 (4.62) | 7.60 (1.69) | |
| 2 | 1.18 (0.12) | 1.00 (0.00) | 3.36 (0.34) | 2.27 (0.49) | 25.71 (4.00) | 7.82 (1.66) | ||
| 3 | 1.20 (0.13) | 1.00 (0.00) | 3.30 (0.37) | 2.10 (0.50) | 25.83 (4.73) | 7.60 (1.65) | ||
| g4 | C | 1 | 1.06 (0.04) a | 0.90 (0.08) | 3.81 (0.13) a | 1.74 (0.12) a | 34.60 (1.08) b | 7.52 (1.18) a |
| 2 | 1.06 (0.04) | 0.80 (0.10) | 3.74 (0.11) | 1.77 (0.12) | 35.00 (1.11) | 7.35 (1.19) | ||
| 3 | 1.16 (0.07) | 0.84 (0.09) | 3.77 (0.12) | 1.68 (0.13) | 35.21 (1.26) | 7.45 (1.23) | ||
| EE | 1 | 1.10 (0.06) | 1.66 (0.12) | 3.10 (0.20) | 0.90 (0.18) | 41.37 (2.48) | 6.76 (1.38) | |
| 2 | 1.10 (0.06) | 1.66 (0.12) | 3.07 (0.20) | 0.90 (0.14) | 40.80 (2.42) | 6.72 (1.34) | ||
| 3 | 1.14 (0.07) | 1.52 (0.15) | 3.00 (0.17) | 0.69 (0.12) | 44.29 (1.62) | 6.34 (1.24) | ||
| v5 | C | 1 | 1.13 (0.09) a | 1.07 (0.07) | 3.67 (0.23) a | 3.00 (0.22) bc | 30.43 (3.45) ab | 8.87 (1.40) a |
| 2 | 1.13 (0.09) | 1.07 (0.07) | 3.73 (0.25) | 3.07 (0.21) | 33.50 (2.76) | 9.00 (1.38) | ||
| 3 | 1.20 (0.11) | 1.07 (0.07) | 3.87 (0.24) | 3.20 (0.34) | 28.71 (4.43) | 9.33 (1.54) | ||
| EE | 1 | 1.50 (0.22) | 1.33 (0.21) | 3.00 (0.37) | 1.33 (0.33) | 33.75 (2.39) | 6.57 (1.07) | |
| 2 | 1.57 (0.20) | 1.43 (0.20) | 3.00 (0.31) | 1.86 (0.34) | 40.83 (3.52) | 7.67 (1.89) | ||
| 3 | 1.57 (0.20) | 1.43 (0.20) | 3.00 (0.31) | 1.43 (0.37) | 39.00 (3.32) | 7.72 (1.99) | ||
| v6 | C | 1 | 1.17 (0.17) a | 1.33 (0.21) | 3.67 (0.33) ab | 2.17 (0.40) ab | 38.75 (3.75) ab | 8.33 (1.56) a |
| 2 | 1.17 (0.17) | 1.17 (0.17) | 3.50 (0.43) | 2.17 (0.31) | 40.00 (4.56) | 8.00 (1.73) | ||
| 3 | 1.17 (0.17) | 1.17 (0.17) | 3.67 (0.33) | 2.00 (0.45) | 38.75 (3.75) | 8.00 (1.68) | ||
| EE | 1 | 1.33 (0.21) | 1.00 (0.00) | 3.83 (0.31) | 1.50 (0.43) | 39.00 (4.00) | 7.67 (1.67) | |
| 2 | 1.33 (0.21) | 1.00 (0.00) | 3.83 (0.31) | 1.50 (0.56) | 30.40 (5.35) | 7.67 (1.67) | ||
| 3 | 1.33 (0.21) | 1.00 (0.00) | 3.83 (0.31) | 1.33 (0.42) | 36.00 (2.92) | 7.50 (1.56) | ||
| g7 | C | 1 | 1.54 (0.14) a | 1.08 (0.08) | 2.62 (0.58) a | 2.00 (0.47) abc | 18.70 (2.89) ab | 7.23 (1.06) a |
| 2 | 1.54 (0.14) | 1.08 (0.08) | 2.69 (0.60) | 2.08 (0.50) | 18.70 (2.89) | 7.38 (1.11) | ||
| 3 | 1.54 (0.14) | 1.08 (0.08) | 2.77 (0.61) | 2.08 (0.47) | 18.70 (2.89) | 7.46 (1.10) | ||
| EE | 1 | 1.33 (0.14) | 1.00 (0.00) | 3.00 (0.46) | 2.42 (0.43) | 39.20 (5.44) | 7.75 (1.86) | |
| 2 | 1.33 (0.14) | 1.00 (0.00) | 3.17 (0.39) | 2.50 (0.42) | 38.70 (3.62) | 8.00 (1.80) | ||
| 3 | 1.33 (0.14) | 1.00 (0.00) | 3.17 (0.39) | 2.42 (0.48) | 37.00 (4.24) | 7.92 (1.83) | ||
| v8 | C | 1 | 1.33 (0.13) a | 0.87 (0.09) | 3.33 (0.40) a | 2.20 (0.28) abc | 26.57 (3.09) ab | 6.11 (1.87) a |
| 2 | 1.37 (0.11) | 0.84 (0.09) | 3.42 (0.34) | 2.16 (0.28) | 29.06 (3.03) | 7.79 (1.56) | ||
| 3 | 1.37 (0.11) | 0.89 (0.07) | 3.47 (0.32) | 1.95 (0.26) | 29.28 (3.24) | 7.68 (1.51) | ||
| EE | 1 | 1.60 (0.16) | 1.22 (0.15) | 3.40 (0.27) | 2.00 (0.21) | 37.50 (2.67) | 7.36 (1.81) | |
| 2 | 1.55 (0.16) | 1.27 (0.14) | 3.45 (0.25) | 2.00 (0.30) | 36.70 (3.65) | 8.27 (1.41) | ||
| 3 | 1.55 (0.16) | 1.27 (0.14) | 3.45 (0.25) | 1.64 (0.28) | 38.33 (2.20) | 7.91 (1.44) | ||
| v9 | C | 1 | - | - | - | - | - | - |
| 2 | 1.05 (0.05) a | 0.68 (0.13) | 4.05 (0.09) a | 1.68 (0.17) a | 36.67 (1.26) b | 7.47 (1.26) a | ||
| 3 | 1.11 (0.07) | 0.74 (0.13) | 4.11 (0.11) | 1.53 (0.18) | 37.50 (1.36) | 7.47 (1.31) | ||
| EE | 1 | - | - | - | - | - | - | |
| 2 | 1.55 (0.16) | 1.18 (0.12) | 3.36 (0.28) | 1.73 (0.30) | 37.70 (4.02) | 7.82 (1.46) | ||
| 3 | 1.55 (0.16) | 1.18 (0.12) | 3.36 (0.28) | 1.27 (0.27) | 38.13 (2.49) | 7.36 (1.47) | ||
| v10 | C | 1 | 1.38 (0.18) a | 1.13 (0.23) | 5.00 (0.57) b | 4.75 (0.56) d | 26.29 (3.21) a | 12.25 (1.00) b |
| 2 | 1.43 (0.13) | 0.76 (0.15) | 5.00 (0.39) | 4.29 (0.37) | 26.59 (1.84) | 11.48 (1.71) | ||
| 3 | 1.24 (0.14) | 0.48 (0.13) | 4.90 (0.40) | 4.29 (0.38) | 26.82 (1.79) | 10.90 (1.78) | ||
| EE | 1 | - | - | - | - | - | - | |
| 2 | 1.56 (0.18) | 0.89 (0.11) | 3.89 (0.75) | 3.11 (0.68) | 28.50 (3.23) | 9.44 (1.30) | ||
| 3 | 1.78 (0.15) | 0.56 (0.18) | 3.56 (0.65) | 3.11 (0.68) | 31.25 (3.93) | 9.00 (1.13) | ||
| v11 | C | 1 | 0.00 (0.00) a | 0.00 (0.00) | 3.80 (0.37) a | 2.40 (0.75) ab | 35.00 (0.00) b | 6.20 (1.07) a |
| 2 | - | - | - | - | - | - | ||
| 3 | - | - | - | - | - | - | ||
| EE | 1 | 0.20 (0.20) | 0.00 (0.00) | 4.60 (0.03) | 2.20 (0.86) | 38.33 (3.33) | 7.00 (1.00) | |
| 2 | - | - | - | - | - | - | ||
| 3 | - | - | - | - | - | - | ||
| g12 | C | 1 | 1.35 (0.11) a | 1.00 (0.07) | 3.35 (0.28) a | 3.00 (0.19) c | 30.53 (1.62) ab | 8.70 (1.45) a |
| 2 | 1.40 (0.11) | 0.95 (0.09) | 3.35 (0.31) | 2.95 (0.27) | 30.79 (1.84) | 8.65 (1.45) | ||
| 3 | 1.35 (0.11) | 1.00 (0.07) | 3.00 (0.26) | 3.00 (0.23) | 29.05 (1.36) | 8.35 (1.44) | ||
| EE | 1 | 1.10 (0.10) | 1.00 (0.00) | 3.50 (0.34) | 3.40 (0.27) | 27.50 (2.27) | 9.00 (1.52) | |
| 2 | 1.20 (0.13) | 1.00 (0.00) | 3.60 (0.34) | 3.50 (0.27) | 29.50 (2.83) | 9.30 (1.58) | ||
| 3 | 1.10 (0.10) | 1.00 (0.00) | 3.60 (0.43) | 3.70 (0.30) | 28.50 (2.59) | 9.40 (1.69) | ||
| Season 5,7 | 0.748 ns | 4.129 * | 1.017 ns | 3.694 * | 1.153 ns | 2.353 ns | ||
| Season × Village 5,7 | 1.338 ns | 1.995 ** | 1.302 ns | 1.373 ns | 0.933 ns | 2.311 *** | ||
| Season × Farm type 5,7 | 0.686 ns | 1.569 ns | 0.189 ns | 0.242 ns | 1.487 ns | 0.453 ns | ||
| Season × Village × Farm type 5,7 | 0.213 ns | 0.431 ns | 1.175 ns | 0.901 ns | 0.953 ns | 1.173 ns | ||
| Village 6 | 4.387 *** | 1.347 ns | 3.705 *** | 11.690 *** | 3.896 *** | 5.255 *** | ||
| Farm type 6 | 0.651 ns | 6.258 ** | 0.034 ns | 2.219 ns | 7.923 *** | 0.003 ns | ||
| Village × Farm type 6 | 1.578 ns | 3.393 *** | 1.563 ns | 2.691 ** | 3.878 *** | 1.236 ns | ||
| Dependent Variable | Model Summary | Sequential Tests | ||||||
|---|---|---|---|---|---|---|---|---|
| AICc | R2 | No. of Predictors | Predictor Variable | Pseudo-F | p-Value | % Var. expl 1 | % Cum. Var 2 | |
| Insecticide use | 3584.9 | 0.226 | 6 | Village | 44,813 | 0.002 | 10.94 | 10.94 |
| Variety | 46,817 | 0.002 | 5.57 | 16.51 | ||||
| Phosphorus | 28,162 | 0.002 | 2.7 | 19.21 | ||||
| Farm type | 17,930 | 0.002 | 1.68 | 20.89 | ||||
| Nitrogen | 11,228 | 0.003 | 1.02 | 21.91 | ||||
| Variety Res. Pest | 7632 | 0.008 | 0.68 | 22.59 | ||||
| Herbicide use | 2345.5 | 0.331 | 4 | Village | 74,909 | 0.002 | 26.83 | 26.83 |
| Variety | 22,128 | 0.002 | 4.99 | 31.82 | ||||
| Variety Res. Pest | 8711 | 0.010 | 0.79 | 32.61 | ||||
| Farm type | 5117 | 0.0259 | 0.46 | 33.07 | ||||
| Molluscicide use | 3016.8 | 0.234 | 6 | Village | 66,759 | 0.002 | 9.84 | 9.84 |
| Farm type | 64,939 | 0.002 | 7.71 | 17.55 | ||||
| Potassium | 20,882 | 0.002 | 2.22 | 19.77 | ||||
| Variety | 15,838 | 0.002 | 1.66 | 21.43 | ||||
| Education | 5793 | 0.014 | 1.06 | 22.49 | ||||
| Farming experience | 4932 | 0.029 | 0.48 | 22.97 | ||||
| Age | 4144 | 0.048 | 0.41 | 23.38 | ||||
| Fungicide use | 3174.7 | 0.242 | 9 | Village | 42,965 | 0.002 | 8.12 | 8.12 |
| Variety | 37,696 | 0.002 | 7.11 | 15.23 | ||||
| Education | 27,317 | 0.002 | 3.07 | 18.3 | ||||
| Nitrogen | 16,771 | 0.002 | 1.76 | 20.06 | ||||
| Age | 12,930 | 0.002 | 1.41 | 21.47 | ||||
| Farm type | 8288 | 0.004 | 0.84 | 22.31 | ||||
| Phosphorous | 7234 | 0.008 | 0.77 | 23.08 | ||||
| Type of planting | 5712 | 0.02 | 0.57 | 23.65 | ||||
| Rice area | 5570 | 0.014 | 0.56 | 24.21 | ||||
| Pesticide use | 3058.8 | 0.233 | 6 | Village | 39,579 | 0.002 | 12.07 | 12.07 |
| Variety | 19,577 | 0.002 | 4.87 | 16.94 | ||||
| Phosphorous | 18,577 | 0.002 | 2.16 | 19.1 | ||||
| Education | 15,933 | 0.004 | 1.72 | 20.82 | ||||
| Age | 15,836 | 0.002 | 1.68 | 22.5 | ||||
| Nitrogen | 7541 | 0.010 | 0.77 | 23.27 | ||||
| Yield | 3080.2 | 0.231 | 6 | Type of planting | 65,227 | 0.002 | 11.97 | 11.97 |
| Variety | 59,718 | 0.002 | 4.87 | 16.84 | ||||
| Village | 12,836 | 0.002 | 4.54 | 21.38 | ||||
| Fallow | 6867 | 0.012 | 0.67 | 22.05 | ||||
| Farm type | 5792 | 0.014 | 0.56 | 22.61 | ||||
| Phosphorous | 4664 | 0.029 | 0.45 | 23.06 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horgan, F.G.; Vu, Q.; Mundaca, E.A.; Dabholkar, S.; Davis, M.; Settele, J.; Crisol-Martínez, E. Escaping the Lock-in to Pesticide Use: Do Vietnamese Farmers Respond to Flower Strips as a Restoration Practice or Pest Management Action? Sustainability 2023, 15, 12508. https://doi.org/10.3390/su151612508
Horgan FG, Vu Q, Mundaca EA, Dabholkar S, Davis M, Settele J, Crisol-Martínez E. Escaping the Lock-in to Pesticide Use: Do Vietnamese Farmers Respond to Flower Strips as a Restoration Practice or Pest Management Action? Sustainability. 2023; 15(16):12508. https://doi.org/10.3390/su151612508
Chicago/Turabian StyleHorgan, Finbarr G., Quynh Vu, Enrique A. Mundaca, Shweta Dabholkar, Mark Davis, Josef Settele, and Eduardo Crisol-Martínez. 2023. "Escaping the Lock-in to Pesticide Use: Do Vietnamese Farmers Respond to Flower Strips as a Restoration Practice or Pest Management Action?" Sustainability 15, no. 16: 12508. https://doi.org/10.3390/su151612508
APA StyleHorgan, F. G., Vu, Q., Mundaca, E. A., Dabholkar, S., Davis, M., Settele, J., & Crisol-Martínez, E. (2023). Escaping the Lock-in to Pesticide Use: Do Vietnamese Farmers Respond to Flower Strips as a Restoration Practice or Pest Management Action? Sustainability, 15(16), 12508. https://doi.org/10.3390/su151612508










