Spatio-Temporal Modeling of COVID-19 Spread in Relation to Urban Land Uses: An Agent-Based Approach
Abstract
:1. Introduction
- Develop an ABM by integrating with an improved susceptible–asymptomatic–symptomatic–on treatment–aggravated infection–recovered–dead (SASOARD) model in the GIS framework to simulate the spatio-temporal spread of COVID-19.
- Incorporate the heterogeneous interactions within the community in the model.
- Design the proposed model based on demographic data and different types of urban land use to track the spatial location of agents, their mobility, and the spatial spread of the disease.
- Evaluate the effects of various intervention scenarios, including complete lockdown, partial lockdown, social distancing, restriction on social gatherings, and vaccination.
- Investigate the effect of urban land use on the COVID-19 outbreak.
- Identify vulnerable populations and disease-prone areas, which should be prioritized for more targeted and effective policies.
2. Materials and Methods
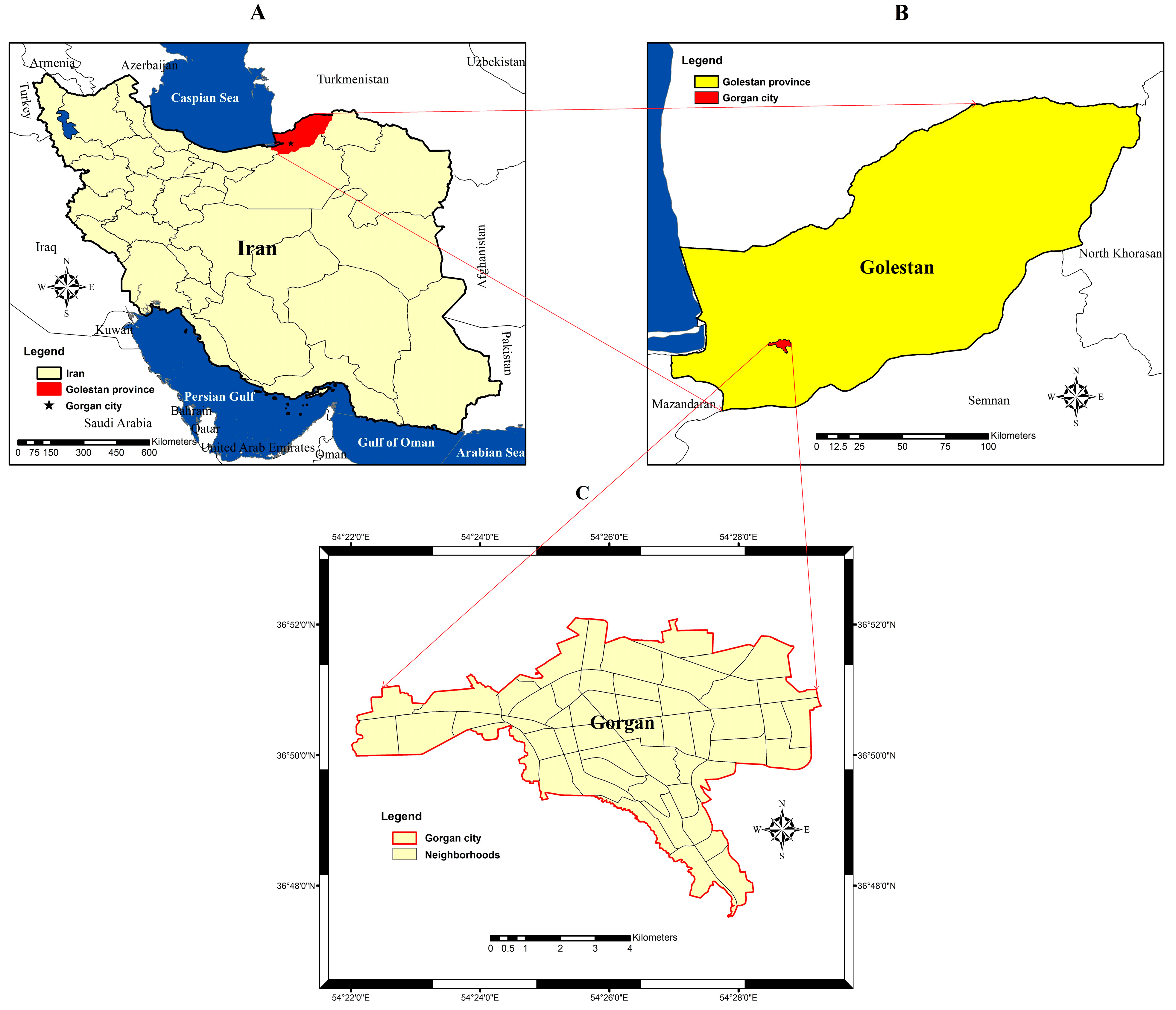
2.1. Study Area
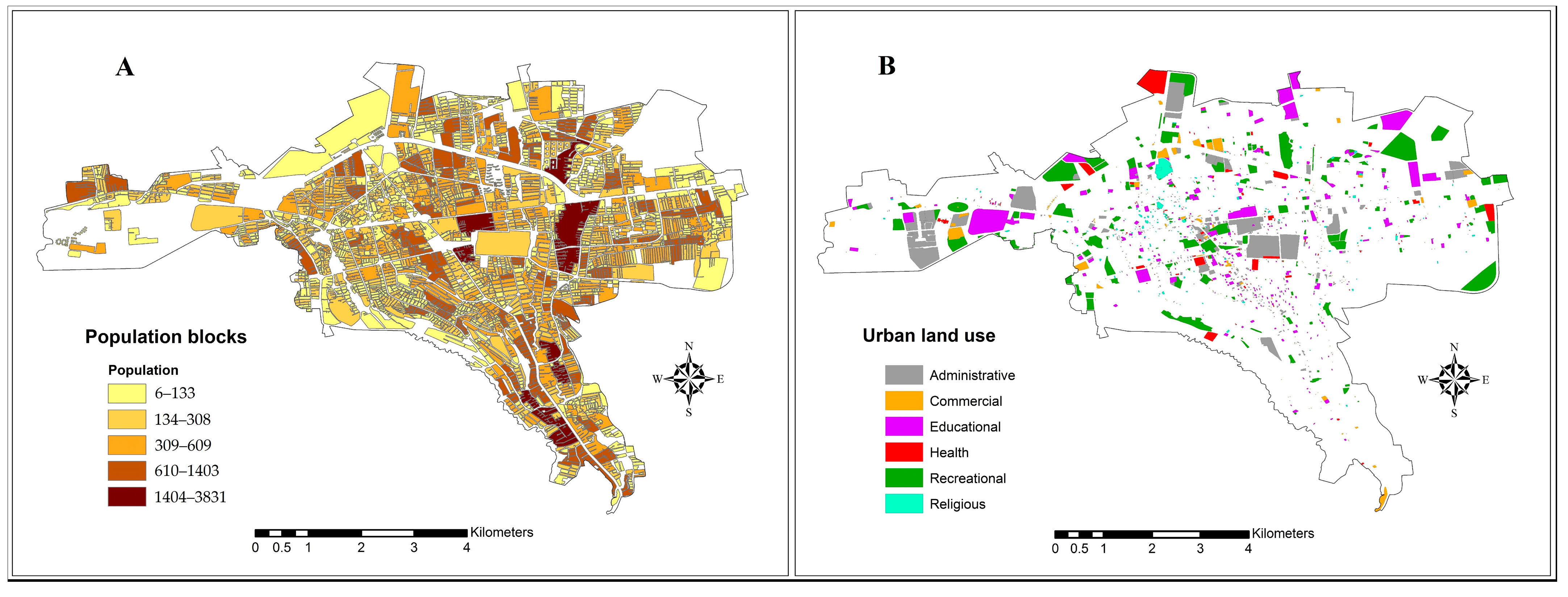
2.2. Data Collection and Preparation
2.3. Model Development
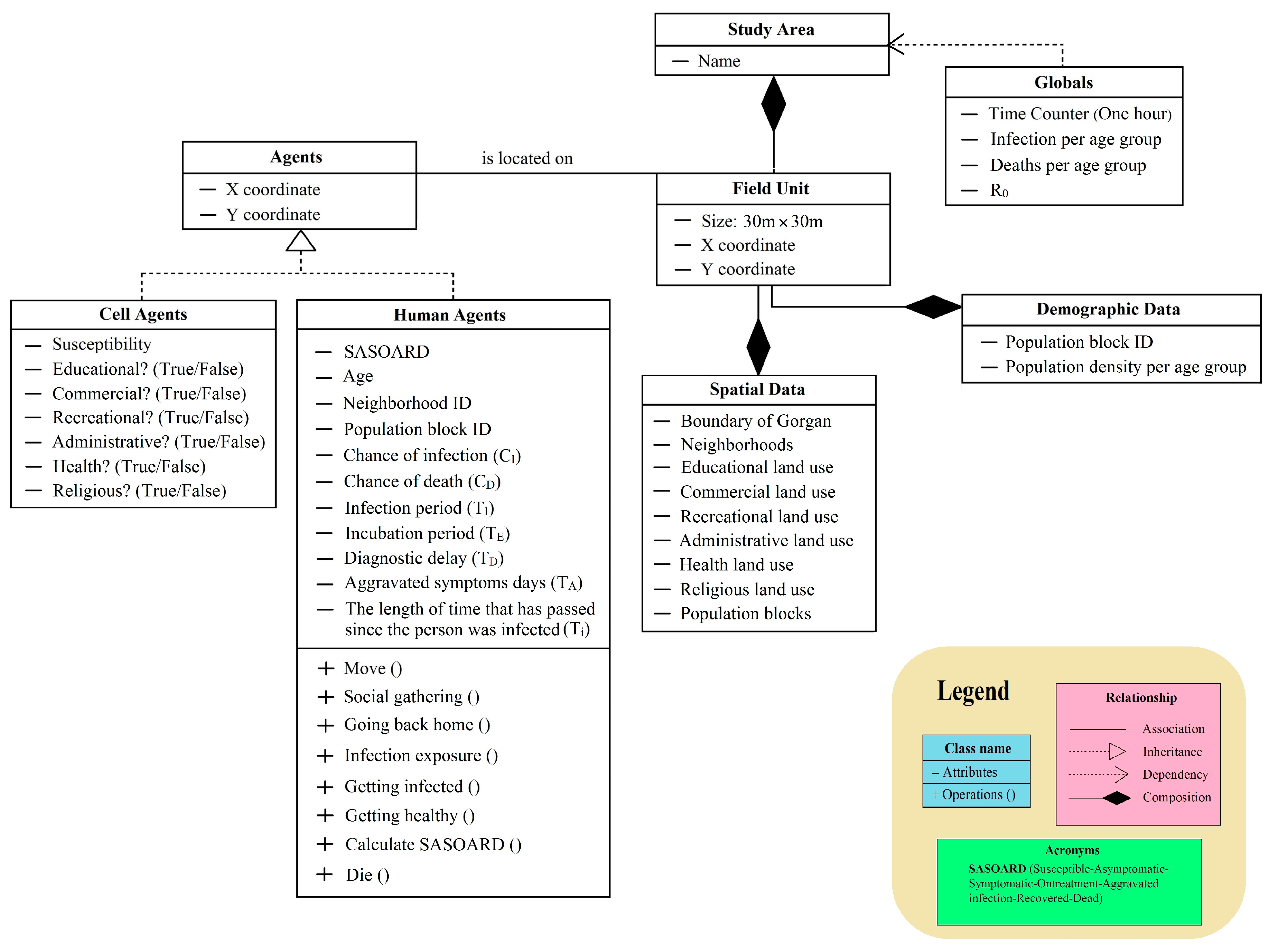
2.3.1. Overview
2.3.2. Design Concepts
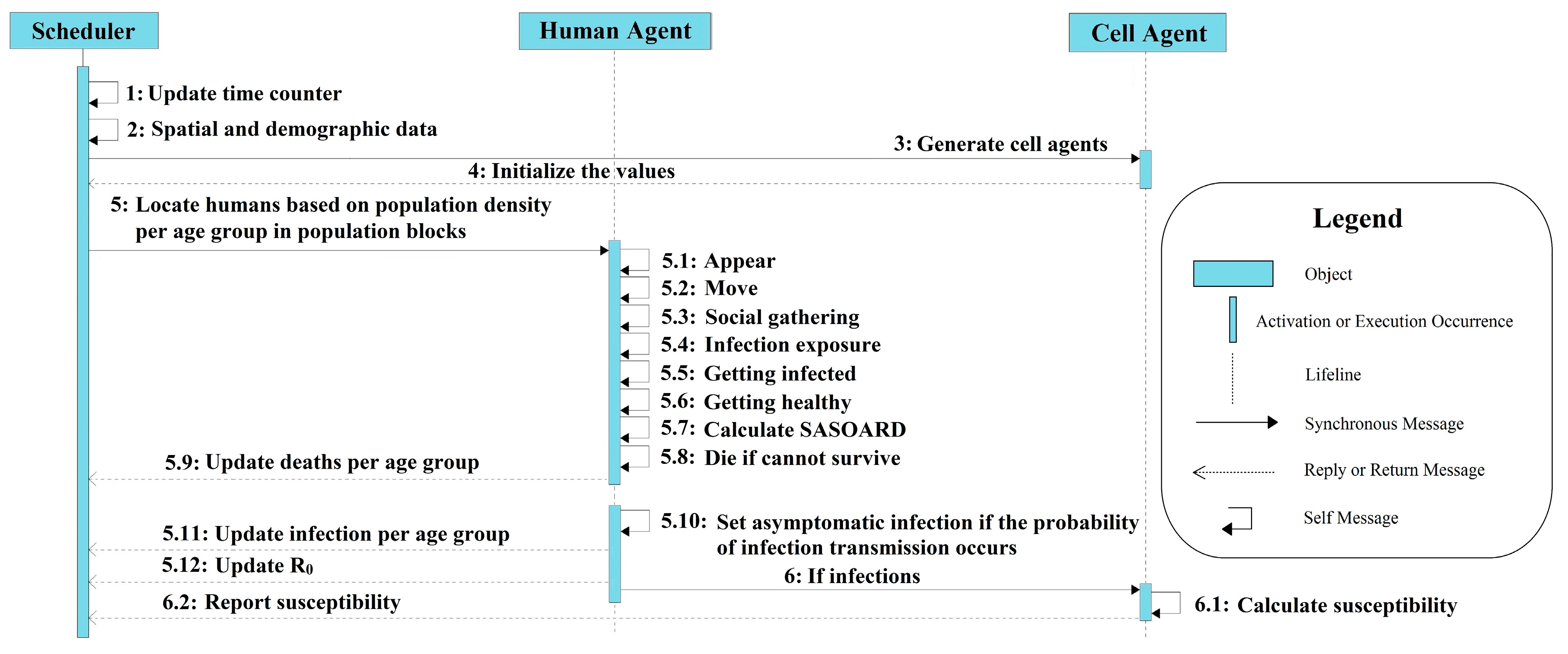
2.3.3. Details
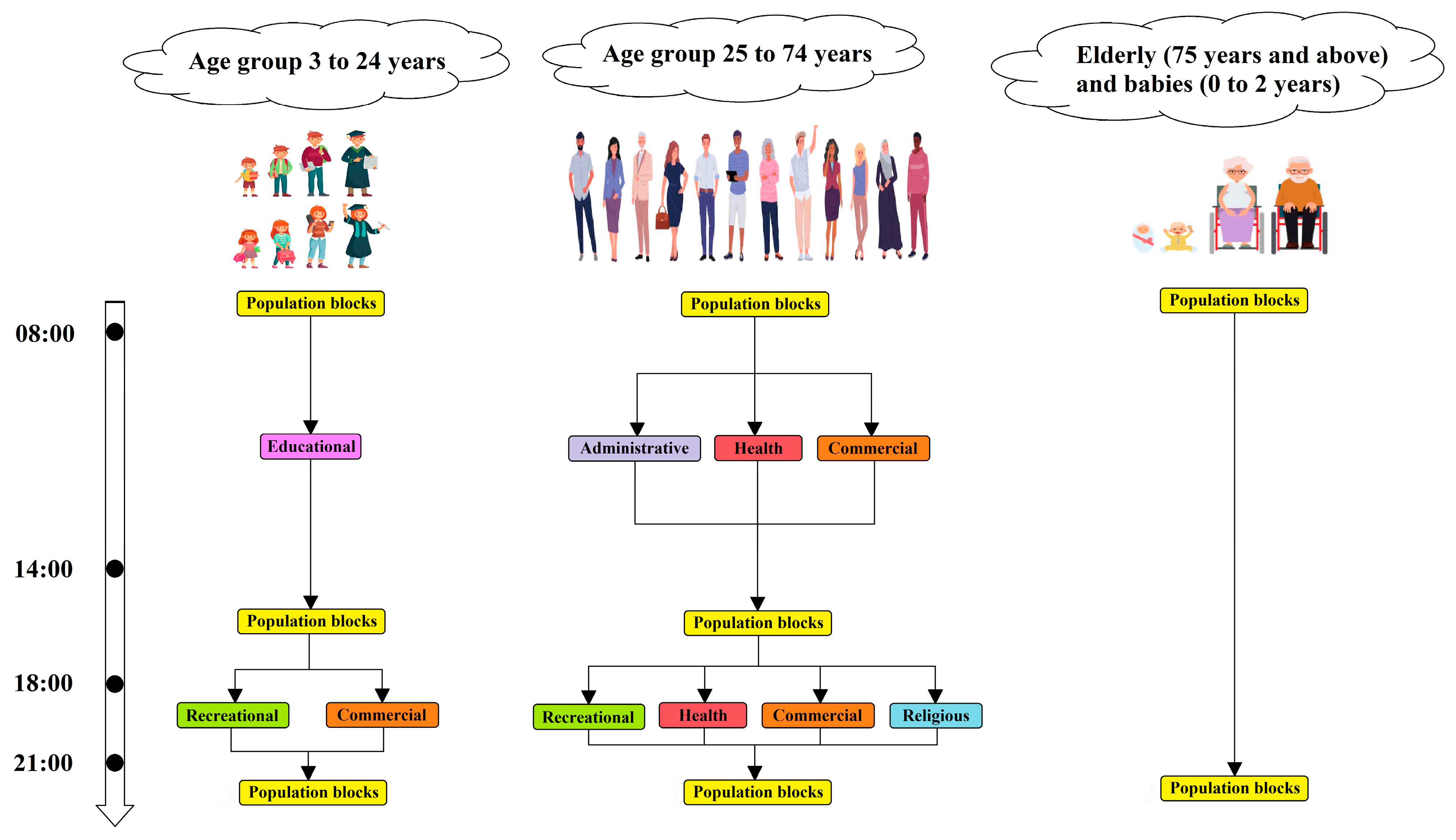
Human Mobility
Social Gatherings
Disease Transmission
SASOARD Model
Susceptibility Map
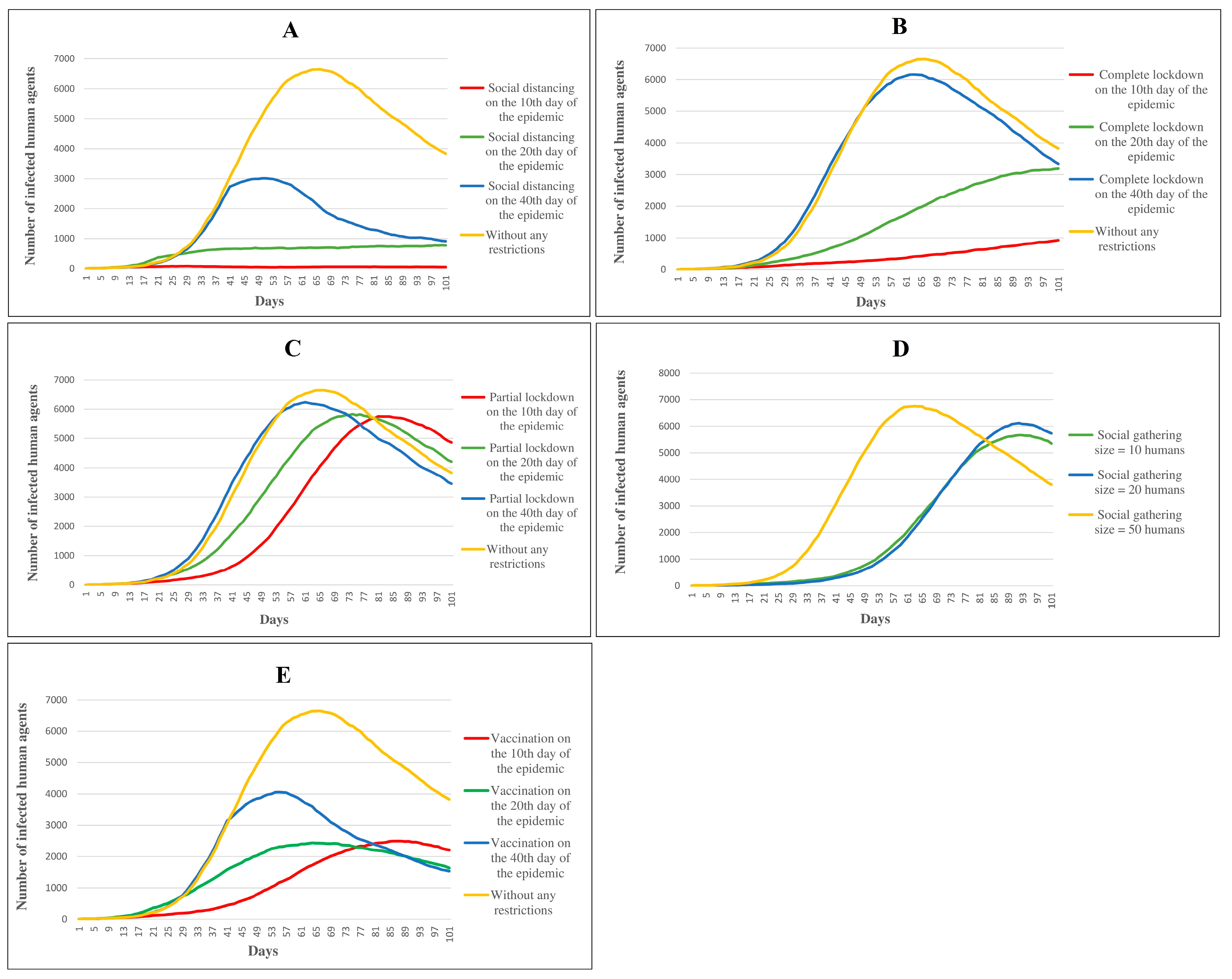
Social Distancing
Complete Lockdown
Partial Lockdown
Restriction in Social Gatherings
Vaccination
2.4. Model Verification
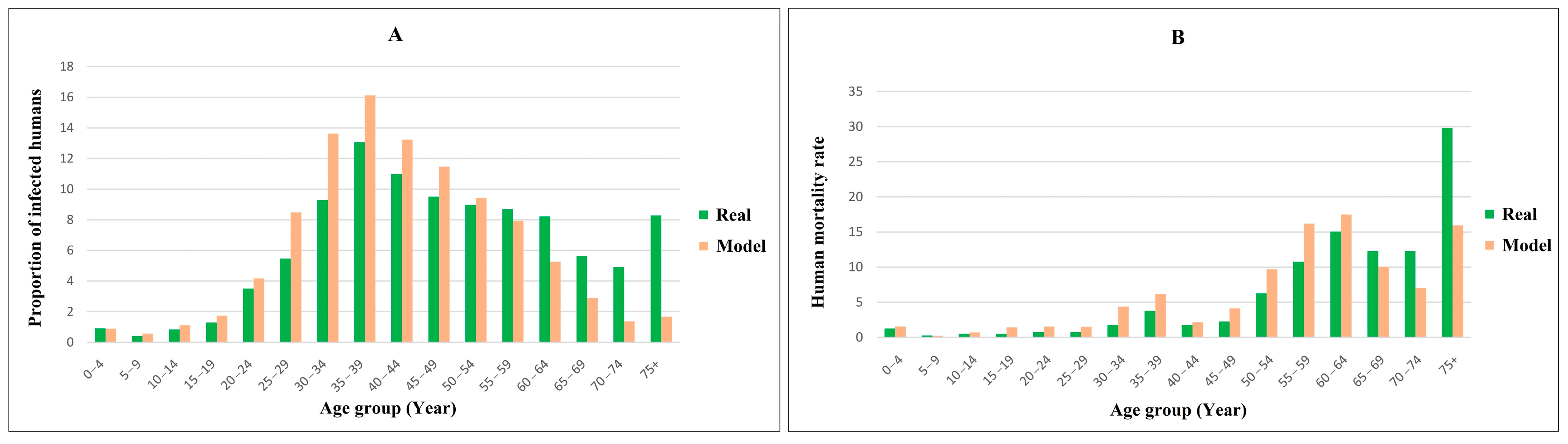
2.5. Model Validation
3. Results
3.1. Control Scenarios
3.2. Model Verification
3.3. Model Validation
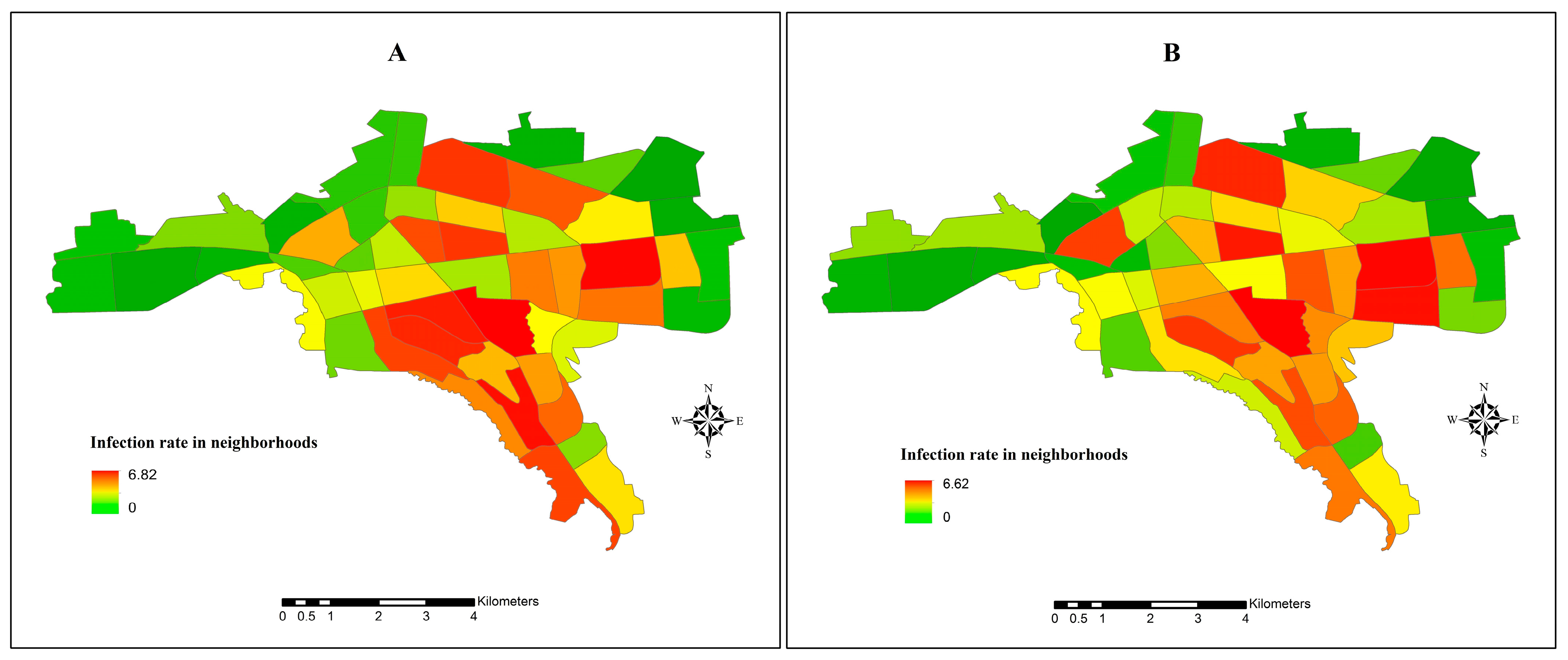
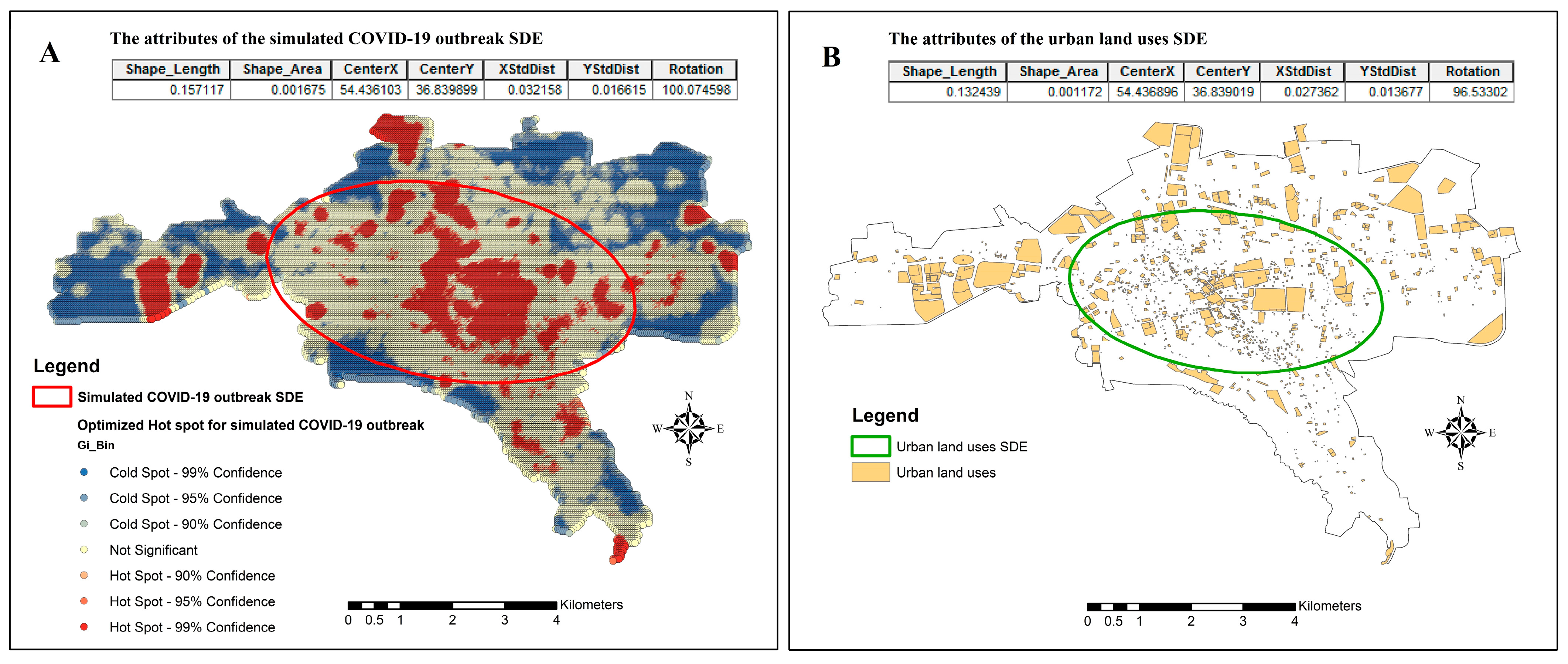
3.4. Spatial Spread of COVID-19
3.5. Temporal Trend of COVID-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Our World in Data 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 7 January 2023).
- World Health Organization. Novel Coronavirus (2019-nCoV) Situation Reports; World Health Organization: Geneva, Switzerland, 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 4 April 2020).
- Babaie, E.; Alesheikh, A.A.; Tabasi, M. Spatial prediction of human brucellosis (HB) using a GIS-based adaptive neuro-fuzzy inference system (ANFIS). Acta Trop. 2021, 220, 105951. [Google Scholar] [CrossRef] [PubMed]
- Razavi-Termeh, S.V.; Sadeghi-Niaraki, A.; Choi, S.M. Spatio-temporal modelling of asthma-prone areas using a machine learning optimized with metaheuristic algorithms. Geocarto Int. 2022, 37, 9917–9942. [Google Scholar] [CrossRef]
- Tabasi, M.; Alesheikh, A.A.; Babaie, E.; Hatamiafkoueieh, J. Spatiotemporal Surveillance of COVID-19 Based on Epidemiological Features: Evidence from Northeast Iran. Sustainability 2022, 14, 12189. [Google Scholar] [CrossRef]
- Masrur, A.; Yu, M.; Luo, W.; Dewan, A. Space-time patterns, change, and propagation of COVID-19 risk relative to the intervention scenarios in Bangladesh. Int. J. Environ. Res. Public Health 2020, 17, 5911. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Russo, L.; Tsakris, A.; Siettos, C. Data-based analysis, modelling and forecasting of the COVID-19 outbreak. PLoS ONE 2020, 15, 0230405. [Google Scholar] [CrossRef]
- Barlow, N.S.; Weinstein, S.J. Accurate closed-form solution of the SIR epidemic model. Phys. D Nonlinear Phenom. 2020, 408, 132540. [Google Scholar] [CrossRef]
- Weissman, G.E.; Crane-Droesch, A.; Chivers, C.; Luong, T.; Hanish, A.; Levy, M.Z.; Lubken, J.; Becker, M.; Draugelis, M.E.; Anesi, G.L.; et al. Locally informed simulation to predict hospital capacity needs during the COVID-19 pandemic. Ann. Intern. Med. 2020, 173, 21–28. [Google Scholar] [CrossRef]
- Kuniya, T. Prediction of the epidemic peak of coronavirus disease in Japan, 2020. J. Clin. Med. 2020, 9, 789. [Google Scholar] [CrossRef]
- Tuite, A.R.; Fisman, D.N.; Greer, A.L. Mathematical modelling of COVID-19 transmission and mitigation strategies in the population of Ontario, Canada. Can. Med. Assoc. J. 2020, 192, E497–E505. [Google Scholar] [CrossRef]
- Kelly, R.A.; Jakeman, A.J.; Barreteau, O.; Borsuk, M.E.; ElSawah, S.; Hamilton, S.H.; Henriksen, H.J.; Kuikka, S.; Maier, H.R.; Rizzoli, A.E.; et al. Selecting among five common modelling approaches for integrated environmental assessment and management. Environ. Model. Softw. 2013, 47, 159–181. [Google Scholar] [CrossRef]
- Germann, T.C.; Kadau, K.; Longini, I.M., Jr.; Macken, C.A. Mitigation strategies for pandemic influenza in the United States. Proc. Natl. Acad. Sci. USA 2006, 103, 5935–5940. [Google Scholar] [CrossRef]
- Longini, I.M., Jr.; Nizam, A.; Xu, S.; Ungchusak, K.; Hanshaoworakul, W.; Cummings, D.A.; Halloran, M.E. Containing pandemic influenza at the source. Science 2005, 309, 1083–1087. [Google Scholar] [CrossRef]
- Banisch, S. Markov Chain Aggregation for Agent-Based Models; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Bouchnita, A.; Jebrane, A. A hybrid multi-scale model of COVID-19 transmission dynamics to assess the potential of non-pharmaceutical interventions. Chaos Solitons Fractals 2020, 138, 109941. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, S.; Zheng, Z.; Lu, J. Optimizing spatial allocation of COVID-19 vaccine by agent—Based spatiotemporal simulations. GeoHealth 2021, 5, e2021GH000427. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, M.; Alesheikh, A.A.; Sofizadeh, A.; Saeidian, B.; Pradhan, B.; AlAmri, A. A spatio-temporal agent-based approach for modeling the spread of zoonotic cutaneous leishmaniasis in northeast Iran. Parasites Vectors 2020, 13, 572. [Google Scholar] [CrossRef]
- Gharakhanlou, N.M.; Hooshangi, N. Spatio-temporal simulation of the novel coronavirus (COVID-19) outbreak using the agent-based modeling approach (case study: Urmia, Iran). Inform. Med. Unlocked 2020, 20, 100403. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, R.; Pałka, P.; Wendland, A. Agent-based modeling as a tool for predicting the spatial-temporal diffusion of the COVID-19 pandemic. In Proceedings of the 2021 IEEE International Conference on Industrial Engineering and Engineering Management (IEEM), Singapore, 11–15 December 2021. [Google Scholar]
- COVID-19 Cases Data in Golestan Province, 2020–2021: Iranian Ministry of Health, Center for Disease Control and Prevention (CDC) of Golestan Province; Unpublished Data. 2021. Available online: https://goums.ac.ir/index.php?slc_lang=en&sid=200 (accessed on 28 February 2021).
- Census Data and Land Use Data in Golestan Province, 2020–2021: Statistical Center of Iran, Deputy of Statistics and Information of Golestan Province. 2021. Available online: https://amar.golestanmporg.ir/ (accessed on 10 February 2022).
- Tabasi, M.; Alesheikh, A.A.; Kalantari, M.; Babaie, E.; Mollalo, A. Spatial Modeling of COVID-19 Prevalence Using Adaptive Neuro-Fuzzy Inference System. ISPRS Int. J. Geo-Inf. 2022, 11, 499. [Google Scholar] [CrossRef]
- Cuevas, E. An agent-based model to evaluate the COVID-19 transmission risks in facilities. Comput. Biol. Med. 2020, 121, 103827. [Google Scholar] [CrossRef]
- Dascalu, M.; Malita, M.; Barbilian, A.; Franti, E.; Stefan, G.M. Enhanced cellular automata with autonomous agents for covid-19 pandemic modeling. Rom. J. Inf. Sci. Technol. 2020, 23, S15–S27. [Google Scholar]
- Kai, D.; Goldstein, G.; Morgunov, A.; Nangalia, V.; Rotkirch, A. Universal masking is urgent in the COVID-19 pandemic: SEIR and agent based models, empirical validation, policy recommendations. arXiv 2020, arXiv:2004.13553. [Google Scholar]
- Kontokosta, C.E.; Hong, B.; Bonczak, B.J. Measuring sensitivity to social distancing behavior during the COVID-19 pandemic. Sci. Rep. 2022, 12, 16350. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Perrone, M.G.; Borelli, M.; Palmisani, J.; Di Gilio, A.; Piscitelli, P.; Miani, A. Airborne transmission route of COVID-19: Why 2 meters/6 feet of inter-personal distance could not be enough. Int. J. Environ. Res. Public Health 2020, 17, 2932. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Wu, Y.; Mei, S.; Ye, C.; Zou, X.; Zhang, Z.; Liu, X.; Wei, L.; Truelove, S.A.; Zhang, T.; et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: A retrospective cohort study. Lancet Infect. Dis. 2020, 20, 911–919. [Google Scholar] [CrossRef]
- Moore_Neighborhood. Available online: https://en.wikipedia.org/wiki/Moore_neighborhood (accessed on 20 February 2023).
- Kerr, C.C.; Stuart, R.M.; Mistry, D.; Abeysuriya, R.G.; Rosenfeld, K.; Hart, G.R.; Núñez, R.C.; Cohen, J.A.; Selvaraj, P.; Hagedorn, B.; et al. Covasim: An agent-based model of COVID-19 dynamics and interventions. PLoS Comput. Biol. 2021, 17, e1009149. [Google Scholar] [CrossRef]
- World Health Organization. Getting the COVID-19 Vaccine. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/getting-the-covid-19-vaccine (accessed on 31 March 2021).
- Xiang, X.; Kennedy, R.; Madey, G.; Cabaniss, S. Verification and validation of agent-based scientific simulation models. In Proceedings of the Agent-Directed Simulation Conference, San Diego, CA, USA, 14–17 April 2005; Volume 47, p. 55. [Google Scholar]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. In Perspectives on Spatial Data Analysis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 127–145. [Google Scholar]
- Mitchell, A. The ESRI Guide to GIS Analysis; Esri Press: Redlands, CA, USA, 2005; Volume 2, Available online: https://www.esri.com/en-us/esri-press/browse/the-esri-guide-to-gis-analysis-volume-2-spatial-measurements-and-statistics-second-edition (accessed on 10 February 2022).
- Manore, C.A.; Hickmann, K.S.; Hyman, J.M.; Foppa, I.M.; Davis, J.K.; Wesson, D.M.; Mores, C.N. A network-patch methodology for adapting agent-based models for directly transmitted disease to mosquito-borne disease. J. Biol. Dyn. 2015, 9, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lin, Q.; Ran, J.; Musa, S.S.; Yang, G.; Wang, W.; Lou, Y.; Gao, D.; Yang, L.; He, D.; et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020, 92, 214–217. [Google Scholar] [CrossRef]
- Zhang, S.; Diao, M.; Yu, W.; Pei, L.; Lin, Z.; Chen, D. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: A data-driven analysis. Int. J. Infect. Dis. 2020, 93, 201–204. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Alagoz, O.; Sethi, A.K.; Patterson, B.W.; Churpek, M.; Safdar, N. Effect of timing of and adherence to social distancing measures on COVID-19 burden in the United States: A simulation modeling approach. Ann. Intern. Med. 2021, 174, 50–57. [Google Scholar] [CrossRef]
- Sah, P.; Vilches, T.N.; Moghadas, S.M.; Fitzpatrick, M.C.; Singer, B.H.; Hotez, J.; Galvani, A. Accelerated vaccine rollout is imperative to mitigate highly transmissible COVID-19 variants. eClinicalMedicine 2021, 35, 100865. [Google Scholar] [CrossRef]
- Xu, J.; Deng, Y.; Yang, J.; Huang, W.; Yan, Y.; Xie, Y.; Li, Y.; Jing, W. Effect of Population Migration and Socioeconomic Factors on the COVID-19 Epidemic at County Level in Guangdong, China. Front. Environ. Sci. 2022, 10, 841996. [Google Scholar] [CrossRef]
- Razavi-Termeh, S.V.; Sadeghi-Niaraki, A.; Farhangi, F.; Choi, S.M. COVID-19 risk mapping with considering socio-economic criteria using machine learning algorithms. Int. J. Environ. Res. Public Health 2021, 18, 9657. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.N.; Chew, L.Y. Modelling Singapore COVID-19 pandemic with a SEIR multiplex network model. Sci. Rep. 2021, 11, 10122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, L.; Ghasemi, Y.; Kallagudde, R.; Goyal, K.; Thakur, D. An agent-based model for simulating COVID-19 transmissions on university campus and its implications on mitigation interventions: A case study. Inf. Discov. Deliv. 2021, 49, 216–224. [Google Scholar] [CrossRef]
- Alsaeed, N.I.; Alqaissi, E.Y.; Siddiqui, M.A. An Agent-based Simulation of the SIRD model of COVID-19 Spread. Int. J. Biol. Biomed. Eng. 2020, 14, 210–217. [Google Scholar]
- Ying, F.; O’Clery, N. Modelling COVID-19 transmission in supermarkets using an agent-based model. PLoS ONE 2021, 16, e0249821. [Google Scholar] [CrossRef]
- Bahl, R.; Eikmeier, N.; Fraser, A.; Junge, M.; Keesing, F.; Nakahata, K.; Reeves, L. Modeling COVID-19 spread in small colleges. PLoS ONE 2021, 16, e0255654. [Google Scholar] [CrossRef]
- Scott, N.; Palmer, A.; Delport, D.; Abeysuriya, R.; Stuart, R.M.; Kerr, C.C.; Mistry, D.; Klein, D.J.; Sacks-Davis, R.; Heath, K.; et al. Modelling the impact of relaxing COVID-19 control measures during a period of low viral transmission. Med. J. Aust. 2021, 214, 79–83. [Google Scholar] [CrossRef]
- Gressman, T.; Peck, J.R. Simulating COVID-19 in a university environment. Math. Biosci. 2020, 328, 108436. [Google Scholar] [CrossRef]
- Jarvis, K.F.; Kelley, J.B. Temporal dynamics of viral load and false negative rate influence the levels of testing necessary to combat COVID-19 spread. Sci. Rep. 2021, 11, 9221. [Google Scholar] [CrossRef]
- Alvarez Castro, D.; Ford, A. 3D agent-based model of pedestrian movements for simulating COVID-19 transmission in university students. ISPRS Int. J. Geo-Inf. 2021, 10, 509. [Google Scholar] [CrossRef]
- Al-Shaery, A.M.; Hejase, B.; Tridane, A.; Farooqi, N.S.; Jassmi, H.A. Agent-based modeling of the Hajj Rituals with the possible spread of COVID-19. Sustainability 2021, 13, 6923. [Google Scholar] [CrossRef]
- Filatova, T.; Verburg, P.H.; Parker, D.C.; Stannard, C.A. Spatial agent-based models for socio-ecological systems: Challenges and prospects. Environ. Model. Softw. 2013, 45, 1–7. [Google Scholar] [CrossRef]
| Variable | Description | Values | Source |
|---|---|---|---|
| Contagion days (TC) | No. of days it takes for infected people to be contagious, and the virus can spread from them to others | 4 days | [2] |
| Contagion distance (DC) | Maximum distance for possible contagion | 2 m | [28] |
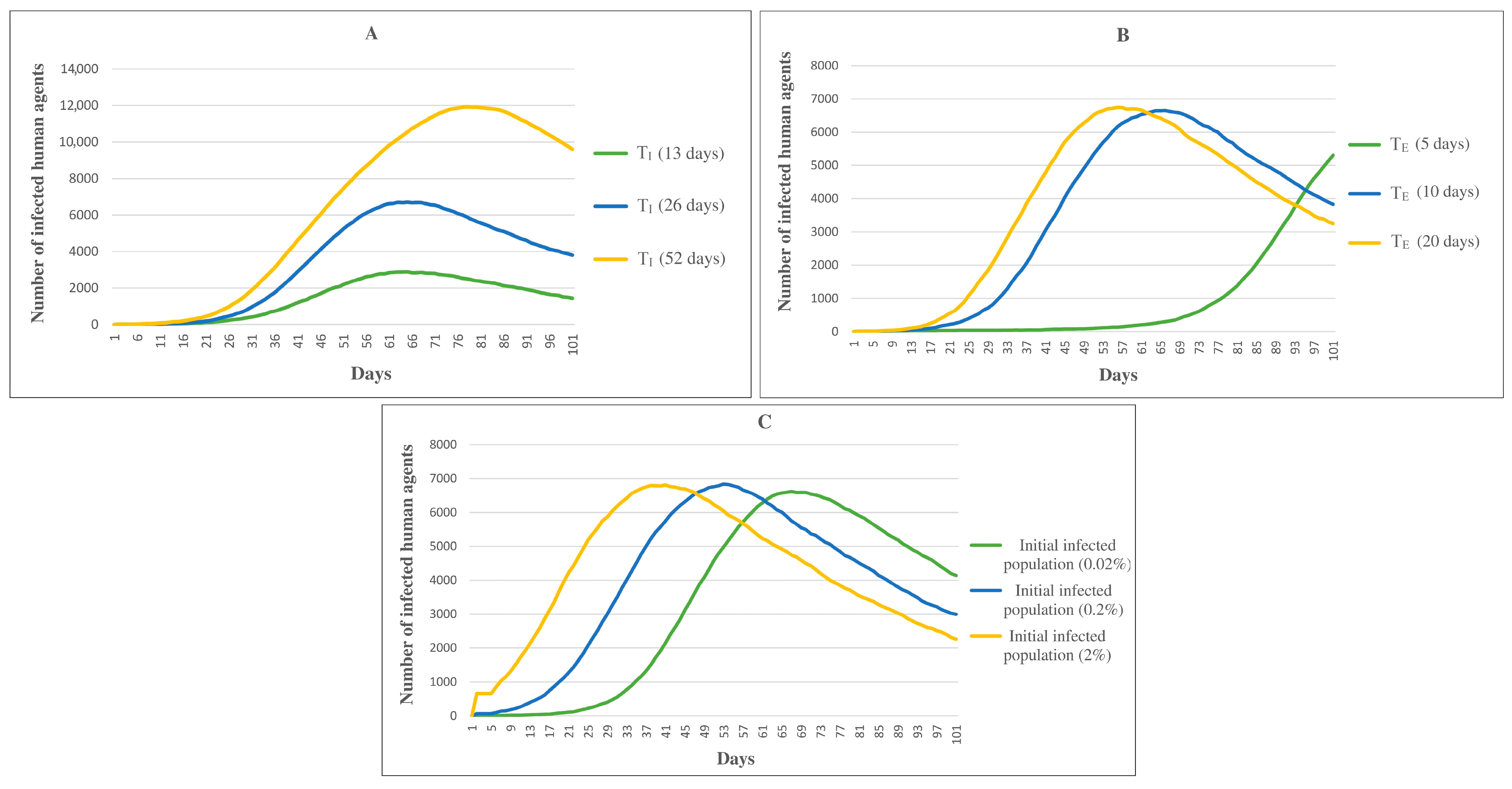
| Infection period (TI) | The time interval between exposure to the virus and immunity to the virus | N(26,5) a days | [29] |
| Incubation period (TE) | The time interval between exposure to the virus and symptom onset | N(10,4) a days | [2] |
| Diagnostic delay (TD) | The time interval between the onset of symptoms and confirmed diagnosis of the disease | R(0,24) b hours | [21] |
| Aggravated symptoms days (TA) | The time interval between exposure to the virus and the onset of aggravated symptoms | TI divided by 2.5 | [21] |
| Chance of aggravated infection (CA) | The probability that the acute stages of the disease will be seen in the infected person | 18.5% | [21] |
| Mortality multiplier | The multiplying factor for the chance of death in the infected person | 1 or 2 (1: when the aggravated symptoms are not seen in the infected person, 2: when the infected person has entered the aggravated infection stage) | [21] |
| Medical care capacity | The capacity of hospital beds in the study area | 24.7 beds per 10 thousand people | [22] |
| Chance of infection (CI) | The probability of disease exposure for each age group | Minimum 0.07% (age group 5–9) and maximum 6.44% (age group 75 and above) | [21] |
| Chance of death (CD) | The probability of death for each age group | Minimum 1.18% (age group 25–29) and maximum 30.91% (age group 75 and above) | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabasi, M.; Alesheikh, A.A.; Kalantari, M.; Mollalo, A.; Hatamiafkoueieh, J. Spatio-Temporal Modeling of COVID-19 Spread in Relation to Urban Land Uses: An Agent-Based Approach. Sustainability 2023, 15, 13827. https://doi.org/10.3390/su151813827
Tabasi M, Alesheikh AA, Kalantari M, Mollalo A, Hatamiafkoueieh J. Spatio-Temporal Modeling of COVID-19 Spread in Relation to Urban Land Uses: An Agent-Based Approach. Sustainability. 2023; 15(18):13827. https://doi.org/10.3390/su151813827
Chicago/Turabian StyleTabasi, Mohammad, Ali Asghar Alesheikh, Mohsen Kalantari, Abolfazl Mollalo, and Javad Hatamiafkoueieh. 2023. "Spatio-Temporal Modeling of COVID-19 Spread in Relation to Urban Land Uses: An Agent-Based Approach" Sustainability 15, no. 18: 13827. https://doi.org/10.3390/su151813827






