Soil Microbiome: Diversity, Benefits and Interactions with Plants
Abstract
:1. Introduction
2. Diversity of Soil Microbes in Rhizosphere
2.1. Plant Growth-Promoting (Rhizo)Bacteria (PGPR or PGPB)
2.2. Fungi
Mycorrhiza
2.3. Plant Growth-Promoting Fungi (PGPF) in Soil
2.4. Endophytes
2.4.1. Fungal Endophytes
2.4.2. Bacterial Endophytes
3. Rhizosphere as the Hotspot for Plant–Microbe Interaction
4. Types of Interactions
4.1. Beneficial Plant–Microbe Interactions
4.1.1. Legume-Rhizobia Symbiosis
4.1.2. Actinobacteria-Actinorhizal Plants Symbiosis
4.1.3. Cyanobacteria–Plant Symbiosis
4.1.4. Mycorrhizal Associations
4.2. Harmful Interactions
4.2.1. Plant–Pathogen Interaction
4.2.2. Antimicrobial Root Exudates
5. Role of Soil Microbes on Plant Growth
6. Suppression of Soil Borne Diseases by Beneficial Soil Microorganisms
7. Role of Soil Microbes in Carbon Sequestration
8. Phytoremediation
9. Nutrient Acquisition
10. Role of Soil Microbes in Combating Abiotic Stress
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brimecombe, M.J.; De Leij, F.A.A.M.; Lynch, J.M. Rhizodeposition and microbial populations. In The Rhizosphere Biochemistry and Organic Susbstances at the Soil-Plant Interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 73–109. [Google Scholar] [CrossRef]
- Shu, W.S.; Huang, L.N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2022, 20, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Rothballer, M.; Schmid, M. Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 2008, 312, 7–14. [Google Scholar] [CrossRef]
- Venturi, V.; Keel, C. Signaling in the rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef]
- Sundin, P. Plant root exudates in interactions between plants and soil micro-organisms. A gnotobiotic approach. Ph.D. Thesis, 1990. Available online: https://lib.ugent.be/catalog/rug01:000202767 (accessed on 13 August 2023).
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Drogue, B.; Combes-Meynet, E.; Moënne-Loccoz, Y.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Control of the cooperation between plant growth-promoting rhizobacteria and crops by rhizosphere signals. In Molecular Microbial Ecology of the Rhizosphere, 1st ed.; de Bruijn, F.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 1, pp. 281–294. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Guo, X.; Zhang, Y.; Ye, B. Comparative analysis of bacterial community structure in the rhizosphere of maize by high-throughput pyrosequencing. PLoS ONE 2017, 12, e0178425. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef]
- Yang, C.H.; Crowley, D.E. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl. Environ. Microbiol. 2000, 66, 345–351. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions and emerging trends in microbial application. J. Adv. Res. 2019, 20, 29–37. [Google Scholar] [CrossRef]
- Badri, D.V.; Weir, T.L.; Van der Lelie, D.; Vivanco, J.M. Rhizosphere chemical dialogues: Plant-microbe interactions. Curr. Res. Biotechnol. 2009, 20, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Mishra, M.; Jain, S.; Singh, J.; Upadhyay, N.; Verma, R.K.; Verma, P.; Tripathi, D.K.; Kumar, V.; Mishra, R.; et al. Exploring the role of plant-microbe interactions in improving soil structure and function through root exudation: A key to sustainable agriculture. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 467–487. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Sharma, S.; Kumar, V.; Upadhyay, N.; Kumar, N.; Mishra, R.; Yadav, G.; Verma, R.K.; Tripathi, D.K. Current scenario of root exudate-mediated plant-microbe interaction and promotion of plant growth. In Probiotics in Agroecosystem; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 349–369. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, D.; Liu, Y. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2014, 374, 689–700. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; Kumar, P.P. Salt tolerance research in date palm tree (Phoenix dactylifera L.), past, present, and future perspectives. Front. Plant Sci. 2015, 6, 348. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Yaish, M.W.; Antony, I.; Glick, B.R. Isolation and characterization of endophytic plant growth-promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie Van Leeuwenhoek 2015, 107, 1519–1532. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Mechanisms used by plant growth-promoting bacteria. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–46. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Varma, A. Unravelling the role of endophytes in micronutrient uptake and enhanced crop productivity. In Symbiotic Soil Microorganisms. Soil Biology; Shrivastava, N., Mahajan, S., Varma, A., Eds.; Springer Nature AG: Cham, Switzerland, 2020; Volume 60, pp. 63–85. [Google Scholar] [CrossRef]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial inoculants for soil quality and plant fitness. In Sustainable Agriculture Review; Lichtfouse, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 181–308. [Google Scholar] [CrossRef]
- Ahmad, M.; Pataczek, L.; Hilger, T.H.; Zahir, Z.A.; Hussain, A.; Rasche, F.; Schafleitner, R.; Solberg, S.Ø. Perspectives of microbial inoculation for sustainable development and environmental management. Front. Microbiol. 2018, 9, 2992. [Google Scholar] [CrossRef]
- Kabir, A.H.; Debnath, T.; Das, U.; Prity, S.A.; Haque, A.; Rahman, M.M.; Parvez, M.S. Arbuscular mycorrhizal fungi alleviate Fe-deficiency symptoms in sunflower by increasing iron uptake and its availability along with antioxidant defense. Plant Physiol. Biochem. 2020, 150, 254–262. [Google Scholar] [CrossRef]
- Kloepper, J.W. Plant growth-promoting rhizobacteria on radishes. In Proceedings of the 4th International Conference on Plant Pathogenic Bacteria, Station de Pathologie Vegetale et Phytobacteriologie, INRA, Angers, France, 27 October–2 September 1978; pp. 879–882. [Google Scholar]
- Martínez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.M.L.M.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Barriuso, J.; Ramos Solano, B.; Lucas, J.A.; Lobo, A.P.; García-Villaraco, A.; Gutiérrez Mañero, F.J. Ecology, genetic diversity and screening strategies of plant growth promoting rhizobacteria (PGPR). In Plant-Bacteria Interactions: Strategies and Techniques to Promote Plant Growth, 1st ed.; Ahmad, I., Pichtel, J., Hayat, S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 1–17. [Google Scholar] [CrossRef]
- Jha, C.K.; Saraf, M. Plant growth promoting rhizobacteria (PGPR): A review. J. Agric. Res. Dev. 2015, 5, 108–119. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer International Publishing: Switzerland, Cham; Berlin/Heidelberg, Germany, 2015; p. 243. [Google Scholar] [CrossRef]
- Agami, R.A.; Medani, R.A.; Abd El-Mola, I.A.; Taha, R.S. Exogenous application with plant growth promoting rhizobacteria (PGPR) or proline induces stress tolerance in basil plants (Ocimum basilicum L.) exposed to water stress. Int. J. Agric. Environ. Res. 2016, 2, 78–92. [Google Scholar]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Soares, H.M.; Soares, E.V. Promising bacterial genera for agricultural practices: An insight on plant growth-promoting properties and microbial safety aspects. Sci. Total Environ. 2019, 682, 779–799. [Google Scholar] [CrossRef]
- Batista, B.D.; Lacava, P.T.; Ferrari, A.; Teixeira-Silva, N.S.; Bonatelli, M.L.; Tsui, S.; Mondin, M.; Kitajima, E.W.; Pereira, J.O.; Azevedo, J.L.; et al. Screening of tropically derived, multi-trait plant growth- promoting rhizobacteria and evaluation of corn and soybean colonization ability. Microbiol. Res. 2018, 206, 33–42. [Google Scholar] [CrossRef]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; de Carlan, C.L.N.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O.; et al. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soil. 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Exp. 2018, 8, 73. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing; Elsevier: Oxford, UK, 2019; pp. 129–157. [Google Scholar] [CrossRef]
- Tariq, M.; Hameed, S.; Yasmeen, T.; Zahid, M.; Zafar, M. Molecular characterization and identification of plant growth promoting endophytic bacteria isolated from the root nodules of pea (Pisum sativum L.). World J. Microbiol. Biotechnol. 2014, 30, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, M.; Johri, B.N. The changing face of rhizobial systematics. Curr. Sci. 2003, 84, 43–48. [Google Scholar]
- OlanSaxena, A.K.; Tilak, K.V.B.R. Free-living nitrogen fixers: Its role in crop production. In Microbes for Health, Wealth and Sustainable Environment; Verma, A.K., Ed.; Malhotra Publ. Co.: New Delhi, India, 1998; pp. 25–64. [Google Scholar]
- Ingold, C.T. The Biology of Fungi; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Harley, J.L.; Smith, S.E. Mycorrhizal Symbiosis; Academic Press: London, UK, 1983; p. 483. [Google Scholar]
- Blasius, D.; Feil, W.; Kottke, I.; Oberwinkler, F. Hartig net structure and formation in fully ensheathed ectomycorrhizas. Nordic J. Bot. 1986, 6, 837–842. [Google Scholar] [CrossRef]
- Dighton, J. Mycorrhizae. In Encyclopedia of Microbiology; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 153–162. [Google Scholar]
- Van Der Heijden, M.G.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Pattnaik, S.S.; Busi, S. Rhizospheric fungi: Diversity and potential biotechnological applications. In Recent Advancement in White Biotechnology through Fungi, Diversity and Enzymes Perspectives; Yadav, A.N., Mishra, S., Singh, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 1, pp. 63–84. [Google Scholar] [CrossRef]
- Murali, M.; Naziya, B.; Ansari, M.A.; Alomary, M.N.; AlYahya, S.; Almatroudi, A.; Thriveni, M.C.; Gowtham, H.G.; Singh, S.B.; Aiyaz, M.; et al. Bioprospecting of rhizosphere-resident fungi: Their role and importance in sustainable agriculture. J. Fungi 2021, 7, 314. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Babalola, O.O. Fungi that promote plant growth in the rhizosphere boost crop growth. J. Fungi 2023, 9, 239. [Google Scholar] [CrossRef]
- Smith, S.A.; Tank, D.C.; Boulanger, L.A.; Bascom-Slack, C.A.; Eisenman, K.; Kingery, D.; Babbs, B.; Fenn, K.; Greene, J.S.; Hann, B.D.; et al. Bioactive endophytes warrant intensified exploration and conservation. PLoS ONE 2008, 3, e3052. [Google Scholar] [CrossRef]
- Partida-Martínez, L.P.; Heil, M. The microbe-free plant: Fact or artifact? Front. Plant Sci. 2011, 2, 100. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ashraf, N.; Mohiuddin, T.; Riyaz-Ul-Hassan, S. Plant-endophyte symbiosis, an ecological perspective. Appl. Microbiol. Biotechnol. 2015, 99, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Papik, J.; Folkmanova, M.; Polivkova-Majorova, M.; Suman, J.; Uhlik, O. The invisible life inside plants: Deciphering the riddles of endophytic bacterial diversity. Biotechnol. Adv. 2020, 44, 107614. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C. Understanding the roles of multifunctional mycorrhizal and endophytic fungi. In Microbial Root Endophytes. Soil Biology; Schulz, B., Boyle, C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 9, pp. 281–298. [Google Scholar] [CrossRef]
- Dubey, A.; Malla, M.A.; Kumar, A.; Dayanandan, S.; Khan, M.L. Plants endophytes: Unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit. Rev. Biotechnol. 2020, 40, 1210–1231. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, M.S.; Kernaghan, G.; Harper, K.A. The effects of fungal root endophytes on plant growth: A meta-analysis. Mycorrhiza 2013, 23, 119–128. [Google Scholar] [CrossRef]
- Badri Suryanarayanan, T.S. Endophyte research: Going beyond isolation and metabolite documentation. Fungal Ecol. 2013, 6, 561–568. [Google Scholar] [CrossRef]
- Mandyam, K.; Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef]
- Pedone-Bonfim, M.V.L.; da Silva, D.K.A.; da Silva-Batista, A.R.; de Oliveira, A.P.; da Silva Almeida, J.R.G.; Yano-Melo, A.M.; Maia, L.C. Mycorrhizal inoculation as an alternative for the sustainable production of Mimosa tenuiflora seedlings with improved growth and secondary compounds content. Fungal Biol. 2018, 122, 918–927. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. What Are Endophytes? Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–13. [Google Scholar]
- Reinhold-Hurek, B.; Hurek, T. Life in grasses: Diazotrophic endophytes. Trends Microbiol. 1998, 6, 139–144. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic actinobacteria of medicinal plants: Diversity and bioactivity. Antonie Van Leeuwenhoek 2015, 108, 267–289. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Miliute, I.; Buzaite, O.; Baniulis, D.; Stanys, V. Bacterial endophytes in agricultural crops and their role in stress tolerance: A review. Zemdirbyste-Agric. 2015, 102, 465–478. [Google Scholar] [CrossRef]
- Pinski, A.; Betekhtin, A.; Hupert-Kocurek, K.; Mur, L.A.; Hasterok, R. Defining the genetic basis of plant–endophytic bacteria interactions. Int. J. Mol. Sci. 2019, 20, 1947. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Pathan, S.I.; Ceccherini, M.T.; Sunseri, F.; Lupini, A. Rhizosphere as hotspot for plant-soil-microbe interaction. In Carbon and Nitrogen Cycling in Soil; Datta, R., Meena, R., Pathan, S., Ceccherini, M., Eds.; Springer Nature: Singapore, 2020; pp. 17–43. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.M.; Thomashow, L.S. Current challenges in introducing beneficial microorganisms into the rhizosphere. In Molecular Ecology of Rhizosphere Microorganisms: Biotechnology and Release of GMOs; O’Gara, F., Dowling, D.N., Boesten, B., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: New York, NY, USA, 1994; pp. 1–18. [Google Scholar]
- Egamberdieva, D.; Kamilova, F.; Validov, S.; Gafurova, L.; Kucharova, Z.; Lugtenberg, B. High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ. Microbiol. 2008, 10, 1–9. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; De Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Shi, S.; Nuccio, E.E.; Shi, Z.J.; He, Z.; Zhou, J.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon flow in the rhizosphere: Carbon trading at the soil–root interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Kopriva, S. Metabolic niches in the rhizosphere microbiome: New tools and approaches to analyse metabolic mechanisms of plant–microbe nutrient exchange. J. Exp. Bot. 2019, 70, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Doornbos, R.F.; van Loon, L.C.; Bakker, P.A. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Desbrosses, G.J.; Stougaard, J. Root nodulation: A paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host Microbe 2011, 10, 348–358. [Google Scholar] [CrossRef]
- Graham, P.H. Ecology of the root-nodule bacteria of legumes. In Nitrogen-Fixing Leguminous Symbioses; Dilworth, M.J., James, E.K., Sprent, J.I., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 7, pp. 23–58. [Google Scholar] [CrossRef]
- Provorov, N.A.; Vorobyov, N.I. Host plant as an organizer of microbial evolution in the beneficial symbioses. Phytochem. Rev. 2009, 8, 519–534. [Google Scholar] [CrossRef]
- Murray, J.D. Invasion by invitation: Rhizobial infection in legumes. Mol. Plant-Microbe Interact. 2011, 24, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Lindström, K.; Mousavi, S.A. Effectiveness of nitrogen fixation in rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335. [Google Scholar] [CrossRef]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current progress in nitrogen fixing plants and microbiome research. Plants 2020, 9, 97. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, L.; Gao, Z.; Tian, B.; Chen, T.; Xie, J.; Cheng, J.; Fu, Y.; Li, Y.; Xiao, S.; et al. Exploring a rhizobium to fix nitrogen in non-leguminous plants by using a tumor-formation root pathogen. Phytopathol. Res. 2022, 4, 48. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef]
- Coleman, D.C.; Callaham, M.A.; Crossley, D.A., Jr. Fundamentals of Soil Ecology; Academic Press, Elsevier: London, UK, 2017. [Google Scholar]
- Svistoonoff, S.; Hocher, V.; Gherbi, H. Actinorhizal root nodule symbioses: What is signalling telling on the origins of nodulation? Curr. Opin. Plant Biol. 2014, 20, 11–18. [Google Scholar] [CrossRef]
- Ktari, A.; Gueddou, A.; Nouioui, I.; Miotello, G.; Sarkar, I.; Ghodhbane-Gtari, F.; Sen, A.; Armengaud, J.; Gtari, M. Host plant compatibility shapes the proteogenome of Frankia coriariae. Front. Microbiol. 2017, 8, 720. [Google Scholar] [CrossRef] [PubMed]
- Ardley, J.; Sprent, J. Evolution and biogeography of actinorhizal plants and legumes: A comparison. J. Ecol. 2021, 109, 1098–1121. [Google Scholar] [CrossRef]
- Rikkinen, J. Cyanobacteria in terrestrial symbiotic systems. In Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects, 1st ed.; Hallenbeck, P.C., Ed.; Springer: Cham, Switzerland, 2017; pp. 243–294. [Google Scholar] [CrossRef]
- Bergman, B.; Rai, A.N.; Rasmussen, U. Cyanobacterial associations. In Associative and Endophytic Nitrogen-Fixing Bacteria and Cyanobacterial Associations; Elmerich, C., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 5, pp. 257–301. [Google Scholar] [CrossRef]
- Gentili, F.; Nilsson, M.C.; Zackrisson, O.; DeLuca, T.H.; Sellstedt, A. Physiological and molecular diversity of feather moss associative N2-fixing cyanobacteria. J. Exp. Bot. 2005, 56, 3121–3127. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, J.C.; Renzaglia, K.S. The hornworts: Important advancements in early land plant evolution. J. Bryol. 2015, 37, 157–170. [Google Scholar] [CrossRef]
- Adams, D.G.; Duggan, P.S. Cyanobacteria–bryophyte symbioses. J. Exp. Bot. 2008, 59, 1047–1058. [Google Scholar] [CrossRef]
- Adams, D.G.; Bergman, B.; Nierzwicki-Bauer, S.A.; Rai, A.N.; Schüßler, A. Cyanobacterial-plant symbioses. In The Prokaryotes: Symbiotic Associations, Biotechnology, Applied Microbiology; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 1, pp. 331–363. [Google Scholar] [CrossRef]
- Thajuddin, N.; Muralitharan, G.; Sundaramoorthy, M.; Ramamoorthy, R.; Ramachandran, S.; Akbarsha, M.A.; Gunasekaran, M. Morphological and genetic diversity of symbiotic cyanobacteria from cycads. J. Basic Microbiol. 2010, 50, 254–265. [Google Scholar] [CrossRef]
- Lindblad, P. Cyanobacteria in symbiosis with cycads. In Prokaryotic Symbionts in Plants; Pawlowski, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 225–233. [Google Scholar] [CrossRef]
- Bergman, B. The Nostoc-Gunnera symbiosis. In Cyanobacteria in Symbiosis; Rai, A.N., Bergman, B., Rasmussen, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 207–232. [Google Scholar]
- Tooley, P.W.; Kyde, K.L.; Englander, L. Susceptibility of selected ericaceous ornamental host species to Phytophthora ramorum. Plant Dis. 2004, 88, 993–999. [Google Scholar] [CrossRef]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and their synergistic interactions to counteract the negative effects of saline soil on agriculture: Key macromolecules and mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef]
- Bagyaraj, D.J.; Sridhar, K.R.; Revanna, A. Arbuscular mycorrhizal fungi influence crop productivity, plant diversity, and ecosystem services. In Fungal Diversity, Ecology and Control Management, 1st ed.; Rajpal, V.N., Singh, I., Navi, S.S., Eds.; Springer: Singapore, 2022; pp. 345–362. [Google Scholar]
- Marks, G.C. (Ed.) Ectomycorrhizae: Their Ecology and Physiology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Agrios, G.N. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- García-Guzmán, G.; Heil, M. Life histories of hosts and pathogens predict patterns in tropical fungal plant diseases. New Phytol. 2014, 201, 1106–1120. [Google Scholar] [CrossRef]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. Microbiol. Spectr. 2017, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Musheer, N.; Ashraf, S.; Choudhary, A.; Kumar, M.; Saeed, S. Role of microbiotic factors against the soil-borne phytopathogens. In Phytobiomes: Current Insights and Future Vistas; Solanki, M.K., Kashyap, P.L., Kumari, B., Eds.; Springer: Singapore, 2020; pp. 251–280. [Google Scholar] [CrossRef]
- Rodriguez-Moreno, L.; Ebert, M.K.; Bolton, M.D.; Thomma, B.P. Tools of the crook-infection strategies of fungal plant pathogens. Plant J. 2018, 93, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Root exudates and their molecular interactions with rhizospheric microbes. In Plant, Soil and Microbes: Mechanisms and Molecular Interactions; Springer International Publishing: Cham, Switzerland, 2016; Volume 2, pp. 59–77. [Google Scholar]
- Haichar, F.E.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Ann. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Baetz, U.; Martinoia, E. Root exudates: The hidden part of plant defense. Trends Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Lareen, A.; Burton, F.; Schäfer, P. Plant root-microbe communication in shaping root microbiomes. Plant Mol. Biol. 2016, 90, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.G.; Kingery, W.L. Changes in soil microbial community structure in relation to plant succession and soil properties during 4000 years of pedogenesis. Eur. J. Soil Biol. 2018, 88, 80–88. [Google Scholar] [CrossRef]
- Nanda, M.; Kumar, V.; Sharma, D.K. Multimetal tolerance mechanisms in bacteria: The resistance strategies acquired by bacteria that can be exploited to “clean-up” heavy metal contaminants from water. Aquat. Toxicol. 2019, 212, 1–10. [Google Scholar] [CrossRef]
- Lopes, M.J.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful plant growth-promoting microbes: Inoculation methods and abiotic factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Prakash, J.; Mishra, S. Role of beneficial soil microbes in alleviating climatic stresses in plants. In Microbiome under Changing Climate; Kumar, A., Singh, J., Romanholo-Ferreira, L.F., Eds.; Academic Press, Elsevier; Woodhead Publishing: Delhi, India, 2022; pp. 29–68. [Google Scholar] [CrossRef]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Cornforth, D.M.; Foster, K.R. Competition sensing: The social side of bacterial stress responses. Nat. Rev. Microbiol. 2013, 11, 285–293. [Google Scholar] [CrossRef]
- Egamberdiyeva, D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl. Soil Ecol. 2007, 36, 184–189. [Google Scholar] [CrossRef]
- Cataldi, M.P.; Heuer, S.; Mauchline, T.H.; Wilkinson, M.D.; Masters-Clark, E.; Di Benedetto, N.A.; Corbo, M.R.; Flagella, Z. Effect of plant growth promoting bacteria on the growth of wheat seedlings subjected to phosphate starvation. Agronomy 2020, 10, 978. [Google Scholar] [CrossRef]
- Nuncio-Orta, G.; Mendoza-Villarreal, R.; Robledo-Torres, V.; VazquezBadillo, M.; Almaraz Suarez, J.J. Influence of rhizobacteria on seed germination and vigor of seeds chili jalapeno (Capsicum annuum L. ‘var. Grande’). ITEA Inf. Tec. Econ. Agrar. 2015, 111, 18–33. [Google Scholar]
- Kumar, V.; Singh, P.; Jorquera, M.A.; Sangwan, P.; Kumar, P.; Verma, A.K.; Agrawal, S. Isolation of phytase-producing bacteria from Himalayan soils and their effect on growth and phosphorus uptake of Indian mustard (Brassica juncea). World J. Microbiol. Biotechnol. 2013, 29, 1361–1369. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, V.; Agrawal, S. Evaluation of phytase producing bacteria for their plant growth promoting activities. Int. J. Microbiol. 2014, 2014, 426483. [Google Scholar] [CrossRef]
- Meena, V.; Maurya, B.; Bahadur, I. Potassium solubilization by bacterial strain in waste mica. Bangladesh J. Bot. 2014, 43, 235–237. [Google Scholar] [CrossRef]
- Masood, S.; Bano, A. Mechanism of potassium solubilization in the agricultural soils by the help of soil microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 137–147. [Google Scholar] [CrossRef]
- Singh, S.K.; Wu, X.; Shao, C.; Zhang, H. Microbial enhancement of plant nutrient acquisition. Stress Biol. 2022, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Ahmad, I.; Aqil, F.; Khan, M.S.; Hayat, S. Diversity and potential of nonsymbiotic diazotrophic bacteria in promoting plant growth. In Plant-Bacteria Interactions: Strategies and Techniques to Promote Plant Growth; Ahmad, I., Pichtel, J., Hayat, S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 81–109. [Google Scholar] [CrossRef]
- Perrig, D.; Boiero, M.L.; Masciarelli, O.A.; Penna, C.; Ruiz, O.A.; Cassán, F.D.; Luna, M.V. Plant-growth promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl. Microbiol. Biotechnol. 2007, 75, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Hasnain, S. Cytokinin production by some bacteria: Its impact on cell division in cucumber cotyledons. Afr. J. Microbiol. Res. 2009, 3, 704–712. [Google Scholar]
- Nieto, K.F.; Frankenberger, W.T., Jr. Biosynthesis of cytokinins by Azotobacter chroococcum. Soil Biol. Biochem. 1989, 21, 967–972. [Google Scholar] [CrossRef]
- Sturtevant, D.B.; Taller, B.J. Cytokinin production by Bradyrhizobium japonicum. Plant Physiol. 1989, 89, 1247–1252. [Google Scholar] [CrossRef]
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D.W.; Kannepalli, A.; Kumar, S. Significance of plant growth promoting rhizobacteria in grain legumes: Growth promotion and crop production. Plants 2020, 9, 1596. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P. Regulation of WRKY genes in plant defense with beneficial fungus Trichoderma: Current perspectives and future prospects. Arch. Phytopathol. Plant Protect. 2019, 52, 1–17. [Google Scholar] [CrossRef]
- Ma, Y.; Vosátka, M.; Freitas, H. Beneficial microbes alleviate climatic stresses in plants. Front. Plant Sci. 2019, 10, 595. [Google Scholar] [CrossRef]
- Noumavo, P.A.; Agbodjato, N.A.; Baba-Moussa, F.; Adjanohoun, A.; Baba-Moussa, L. Plant growth promoting rhizobacteria: Beneficial effects for healthy and sustainable agriculture. Afr. J. Biotechnol. 2016, 15, 1452–1463. [Google Scholar]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight into the role of PGPR in sustainable agriculture and environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.D. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: An overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Kashikar, M.S.; Lakshmi, S.G.; Madempudi, R. Identification and characterization of antimicrobial peptide produced by indigenously isolated Bacillus paralicheniformis UBBLi30 strain. 3 Biotech 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Mareque, C.; da Silva, T.F.; Vollú, R.E.; Beracochea, M.; Seldin, L.; Battistoni, F. The endophytic bacterial microbiota associated with sweet sorghum (Sorghum bicolor) is modulated by the application of chemical N fertilizer to the field. Int. J. Genom. 2018, 7403670. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Prasad, R.; Varma, A.; Sharma, A.K. Glycoprotein associated with Funneliformis coronatum, Gigaspora margarita and Acaulospora scrobiculata suppress the plant pathogens in vitro. Asian J. Plant Pathol. 2017, 11, 192–202. [Google Scholar] [CrossRef]
- Fehrer, J.; Réblová, M.; Bambasová, V.; Vohník, M. The root-symbiotic Rhizoscyphus ericae aggregate and Hyaloscypha (Leotiomycetes) are congeneric: Phylogenetic and experimental evidence. Stud. Mycol. 2019, 92, 195–225. [Google Scholar] [CrossRef]
- Sathiyadash, K.; Muthukumar, T.; Karthikeyan, V.; Rajendran, K. Orchid mycorrhizal fungi: Structure, function, and diversity. In Orchid Biology: Recent Trends & Challenges; Khasim, S., Hegde, S., González-Arnao, M., Thammasiri, K., Eds.; Springer: Singapore, 2020; pp. 239–280. [Google Scholar] [CrossRef]
- Mejía, L.C.; Herre, E.A.; Sparks, J.P.; Winter, K.; García, M.N.; Van Bael, S.A.; Stitt, J.; Shi, Z.; Zhang, Y.; Guiltinan, M.J.; et al. Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front. Microbiol. 2014, 5, 479. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.N.; Valero, J.R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef]
- Zou, Y.N.; Srivastava, A.K.; Wu, Q.S. Glomalin: A potential soil conditioner for perennial fruits. Int. J. Agric. Biol. 2016, 18, 293–297. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef]
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci. Total Environ. 2016, 566–567, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M. Arbuscular mycorrhizal fungi act as bio-stimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Alotaibi, M.O.; Saleh, A.M.; Sobrinho, R.L.; Sheteiwy, M.S.; El-Sawah, A.M.; Mohammed, A.E.; AbdElgawad, H. Arbuscular mycorrhizae mitigate aluminum toxicity and regulate proline metabolism in plants grown in acidic soil. J. Fungi 2021, 7, 531. [Google Scholar] [CrossRef]
- Hu, Y.; Pandey, A.K.; Wu, X.; Fang, P.; Xu, P. The role of arbuscular mycorrhiza fungi in drought tolerance in legume crops: A review. Legume Res. Int. J. 2022, 45, 1–9. [Google Scholar] [CrossRef]
- AbdElgawad, H.; El-Sawagh, A.M.; Mohammed, A.E.; Alotaibi, M.O.; Yehia, R.S.; Selim, S.; Saleh, A.M.; Beemster, G.T.S.; Sheteiwy, M.S. Increasing atmospheric CO2 differentially supports arsenite stress mitigating impact of arbuscular mycorrhizal fungi in wheat and soyabean plants. Chemosphere 2022, 296, 134044. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Swapnil, P.; Harish; Marwal, A.; Kumar, S. Multifarious responses of forest soil microbial community toward climate change. Microb. Ecol. 2022, 86, 49–74. [Google Scholar] [CrossRef]
- Pvaithra, D.; Yapa, N. Arbuscular mycorrhizal fungi inoculation enhances drought stress tolerance of plants. Groundw. Sustain. Dev. 2018, 7, 490–494. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Xu, G.; Zhou, L.; Li, Y. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2019, 50, 593–604. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.-C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef]
- Wang, H.; An, T.; Huang, D.; Liu, R.; Xu, B.; Zhang, S.; Deng, X.; Siddique, K.H.M.; Chen, Y. Arbuscular mycorrhizal symbioses alleviating salt stress in maize is associated with a decline in root-to-leaf gradient of Na+/K+ ratio. BMC Plant Biol. 2021, 21, 457. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.J.; Yan, H.Y.; Zou, B.Y.; Guo, R.Z.; Ci, D.W.; Tang, Z.H.; Zou, X.X.; Zhang, X.J.; Yu, X.N.; Wang, Y.F. Arbuscular mycorrhizal fungi alleviate salinity stress in peanut: Evidence from pot-grown and field experiments. Food Energy Secur. 2021, 10, e314. [Google Scholar] [CrossRef]
- Huang, S.; Gill, S.; Ramzan, M.; Ahmad, M.Z.; Danish, S.; Huang, P.; Obaid, S.A.; Alharbi, S.A. Uncovering the impact of AM fungi on wheat nutrient uptake, ion homeostasis, oxidative stress, and antioxidant defense under salinity stress. Sci. Rep. 2023, 13, 8249. [Google Scholar] [CrossRef] [PubMed]
- Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Torequl Islam, M.; Verma, R. Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy 2020, 10, 815. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; El-Sawah, A.M.; Korany, S.M.; Alsherif, E.A.; Mowafy, A.M.; Chen, J.; Jośko, I.; Selim, S.; AbdElgawad, H. Arbuscular mycorrhizal fungus “Rhizophagus irregularis” impacts on physiological and biochemical responses of ryegrass and chickpea plants under beryllium stress. Environ. Pollut. 2022, 315, 120356. [Google Scholar] [CrossRef]
- Boorboori, M.R.; Zhang, H.Y. Arbuscular Mycorrhizal fungi are an influential factor in improving the phytoremediation of arsenic, cadmium, lead, and chromium. J. Fungi 2022, 8, 176. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, M.; Sulpice, R.; Chen, H.; Tian, S.; Ban, Y. Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J. Plant Growth Regul. 2014, 33, 612–625. [Google Scholar] [CrossRef]
- Abd-Allah, W.H.; Khater, R.M. Effect of rock phosphate and mycorrhiza on vegetative growth and productivity of Ricinus communis var Red Arish under North Sinai Conditions. Middle East J. Agric. 2016, 5, 412–421. [Google Scholar]
- Yan, Q.; Li, X.; Xiao, X.; Chen, J.; Liu, J.; Lin, C.; Guan, R.; Wang, D. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Cinnamomum migao by enhancing physio-biochemical responses. Ecol. Evol. 2022, 12, e9091. [Google Scholar] [CrossRef]
- Xiao, X.; Liao, X.; Yan, Q.; Xie, Y.; Chen, J.; Liang, G.; Chen, M.; Xiao, S.; Chen, Y.; Liu, J. Arbuscular mycorrhizal fungi improve the growth, water status, and nutrient uptake of Cinnamomum migao and the soil nutrient stoichiometry under drought stress and recovery. J. Fungi 2023, 9, 321. [Google Scholar] [CrossRef]
- Adedeji, A.A.; Babalola, O.O. Secondary metabolites as plant defensive strategy: A large role for small molecules in the near root region. Planta 2020, 252, 61. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Gharagoz, S.F.; Pourakbar, L.; Moghaddam, S.S.; Jamalomidi, M. Arbuscular mycorrhizal fungi alleviate salinity stress and alter phenolic compounds of Moldavian balm. Rhizosphere 2021, 19, 100417. [Google Scholar] [CrossRef]
- El-Sawah, A.M.; Abdel-Fattah, G.G.; Holford, P.; Korany, S.M.; Alsherif, E.A.; AbdElgawad, H.; Ulhassan, Z.; Jośko, I.; Ali, B.; Sheteiwy, M.S. Funneliformis constrictum modulates polyamine metabolism to enhance tolerance of Zea mays L. to salinity. Microbiol. Res. 2023, 266, 127254. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Gill, R.A. Heavy metal toxicity in plants: Recent insights on physiological and molecular aspects, Volume II. Front. Plant Sci. 2022, 13, 1016257. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Tirry, N.; Kouchou, A.; El Omari, B.; Ferioun, M.; El Ghachtouli, N. Improved chromium tolerance of Medicago sativa by plant growth-promoting rhizobacteria (PGPR). J. Genet. Eng. Biotechnol. 2021, 19, 149. [Google Scholar] [CrossRef]
- Adeyemi, N.O.; Atayese, M.O.; Sakariyawo, O.S.; Azeez, J.O.; Sobowale, S.P.A.; Olubode, A.; Mudathir, R.; Adebayo, R.; Adeoye, S. Alleviation of heavy metal stress by arbuscular mycorrhizal symbiosis in Glycine max (L.) grown in copper, lead and zinc contaminated soils. Rhizosphere 2021, 18, 100325. [Google Scholar] [CrossRef]
- García-Latorre, C.; Rodrigo, S.; Santamaria, O. Effect of fungal endophytes on plant growth and nutrient uptake in Trifolium subterraneum and Poa pratensis as affected by plant host specificity. Mycol. Prog. 2021, 20, 1217–1231. [Google Scholar] [CrossRef]
- Ye, B.; Wu, Y.; Zhai, X.; Zhang, R.; Wu, J.; Zhang, C.; Rahman, K.; Qin, L.; Han, T.; Zheng, C. Beneficial effects of endophytic fungi from the Anoectochilus and Ludisia species on the growth and secondary metabolism of Anoectochilus roxburghii. ACS Omega 2020, 5, 3487–3497. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Swapnil, P.; Harish; Marwal, A.; Yadav, G.; Sonigra, P. Endophytic nanotechnology: An approach to study scope and potential applications. Front. Chem. 2021, 9, 613343. [Google Scholar] [CrossRef]
- Hou, L.; Yu, J.; Zhao, L.; He, X. Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Front. Microbiol. 2020, 10, 3061. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Meena, M. Bioprospecting of endophytes in medicinal plants of Thar Desert: An attractive resource for biopharmaceuticals. Biotechnol. Rep. 2021, 30, e00629. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Meena, M. Biological control of plant diseases by endophytes. In Endophytic Association: What, Why and How: Volume: Developments in Applied Microbiology and Biotechnology; Shah, M.P., Deka, D., Eds.; Academic Press, Elsevier: London, UK, 2022; pp. 119–135. [Google Scholar] [CrossRef]
- Goutam, J.; Singh, R.; Vijayaraman, R.S.; Meena, M. Endophytic Fungi: Carrier of Potential Antioxidants. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; pp. 539–551. [Google Scholar] [CrossRef]
- Adesalu, T.A.; Olugbemi, O.M. Soil algae: A case study of two vegetable farmlands in Lagos and Ogun states, southwest Nigeria. IFE J. Sci. 2015, 17, 765–772. [Google Scholar]
- De Corato, U. Soil microbiota manipulation and its role in suppressing soil-borne plant pathogens in organic farming systems under the light of microbiome-assisted strategies. Chem. Biol. Technol. Agric. 2020, 7, 17. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 2007, 39, 1–23. [Google Scholar] [CrossRef]
- Lyu, D.; Backer, R.; Subramanian, S.; Smith, D.L. Phytomicrobiome coordination signals hold potential for climate change-resilient agriculture. Front. Plant Sci. 2020, 11, 634. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen biocontrol using plant growth-promoting bacteria (PGPR): Role of bacterial diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Punja, Z.K.; Rodriguez, G.; Tirajoh, A. Effects of Bacillus subtilis strain qst 713 and storage temperatures on post-harvest disease development on greenhouse tomatoes. Crop Protect. 2016, 84, 98–104. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Hariprasad, P.; Nayak, S.C.; Niranjana, S.R. Application of rhizobacteria antagonistic to Fusarium oxysporum f. sp. lycopersici for the management of Fusarium wilt in tomato. Rhizosphere 2016, 2, 72–74. [Google Scholar] [CrossRef]
- Jiao, X.; Takishita, Y.; Zhou, G.; Smith, D.L. Plant associated rhizobacteria for biocontrol and plant growth enhancement. Front. Plant Sci. 2021, 12, 634796. [Google Scholar] [CrossRef]
- Raupach, G.S.; Kloepper, J.W. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 1998, 88, 1158–1164. [Google Scholar] [CrossRef]
- Xiang, N.; Lawrence, K.S.; Kloepper, J.W.; Donald, P.A.; McInroy, J.A. Biological control of Heterodera glycines by spore-forming plant growth-promoting rhizobacteria (PGPR) on soybean. PLoS ONE 2017, 12, e0181201. [Google Scholar] [CrossRef] [PubMed]
- Pangesti, N.; Reichelt, M.; van de Mortel, J.E.; Kapsomenou, E.; Gershenzon, J.; van Loon, J.J.A.; Dicke, M.; Pineda, A. Jasmonic acid and ethylene signaling pathways regulate glucosinolate levels in plants during rhizobacteria-induced systemic resistance against a leaf-chewing herbivore. J. Chem. Ecol. 2016, 42, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Larbi-Koranteng, S.; Kankam, F.; Adomako, J.; Abdulai, M. Role of Mycorrhizae in Crop Protection; Intech Open: London, UK, 2023. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Antagonistic assessment of Trichoderma spp. by producing volatile and non-volatile compounds against different fungal pathogens. Arch. Phytopathol. Plant Prot. 2017, 50, 629–648. [Google Scholar] [CrossRef]
- Zehra, A.; Meena, M.; Dubey, M.K.; Aamir, M.; Upadhyay, R.S. Activation of defense response in tomato against Fusarium wilt disease triggered by Trichoderma harzianum supplemented with exogenous chemical inducers (SA and MeJA). Braz. J. Bot. 2017, 21, 1–14. [Google Scholar] [CrossRef]
- Zehra, A.; Meena, M.; Dubey, M.K.; Aamir, M.; Upadhyay, R.S. Synergistic effects of plant defense elicitors and Trichoderma harzianum on enhanced induction of antioxidant defense system in tomato against Fusarium wilt disease. Bot. Stud. 2017, 58, 44. [Google Scholar] [CrossRef]
- Aseel, D.G.; Rashad, Y.M.; Hammad, S.M. Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against tomato mosaic virus. Sci. Rep. 2019, 9, 9692. [Google Scholar] [CrossRef]
- Whipps, J.M. Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can. J. Bot. 2004, 82, 1198–1227. [Google Scholar] [CrossRef]
- Barea, J.M.; Pozo, M.J.; Azcón, R.; Azcón-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Verhage, A.; García-Andrade, J.; García, J.M.; Azcón-Aguilar, C. Priming plant defenses against pathogens by arbuscular mycorrhizal fungi. In Mycorrhizas: Functional Processes and Ecological Impact; Azcón-Aguilar, C., Barea, J.M., Gianinazzi, S., Gianinazzi-Pearson, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 137–149. [Google Scholar] [CrossRef]
- Pozo, M.J.; Jung, S.C.; López-Ráez, J.A.; Azcón-Aguilar, C. Impact of arbuscular mycorrhizal symbiosis on plant response to biotic stress: The role of plant defense mechanisms. In Arbuscular Mycorrhizas: Physiology and Function, 2nd ed.; Koltai, H., Kapulnik, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 193–207. [Google Scholar] [CrossRef]
- Dreischhoff, S.; Das, I.S.; Jakobi, M.; Kasper, K.; Polle, A. Local responses and systemic induced resistance mediated by ectomycorrhizal fungi. Front. Plant Sci. 2020, 11, 590063. [Google Scholar] [CrossRef] [PubMed]
- Gianinazzi, S.; Gollotte, A.; Binet, M.N.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Eo, J.K.; Ka, K.H.; Eom, A.H. Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology 2013, 41, 121–125. [Google Scholar] [CrossRef]
- Li, F.; Guo, Y.; Christensen, M.J.; Gao, P.; Li, Y.; Duan, T. An arbuscular mycorrhizal fungus and Epichloë festucae var. lolii reduce Bipolaris sorokiniana disease incidence and improve perennial ryegrass growth. Mycorrhiza 2018, 28, 159–169. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Razak, N.A.; Gange, A.C. Multitrophic interactions between arbuscular mycorrhizal fungi, foliar endophytic fungi and aphids. Microb. Ecol. 2021, 85, 146–156. [Google Scholar] [CrossRef]
- Allsup, C.M.; Lankau, R.A.; Paige, K.N. Herbivory and soil water availability induce changes in arbuscular mycorrhizal fungal abundance and composition. Microb. Ecol. 2021, 84, 141–152. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Miransari, M. Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl. Microbiol. Biotechnol. 2011, 89, 917–930. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, M.; Wang, M.; Li, Y.; Liu, J.; Chen, Y. Inoculation with arbuscular mycorrhizal fungus modulates defense-related genes expression in banana seedlings susceptible to wilt disease. Plant Signal. Behav. 2021, 16, 1884782. [Google Scholar] [CrossRef]
- St-Arnaud, M.; Vimard, B.; Fortin, J.A.; Hamel, C.; Caron, M. Inhibition of Fusarium oxysporum f. sp. dianthi inthe non-VAM species Dianthus caryophyllus by co-culture with Tagetes patula companion plants colonized by Glomus intraradices. Can. J. Bot. 1997, 75, 998–1005. [Google Scholar] [CrossRef]
- Declerck, S.; Risede, J.M.; Rufyikiri, G.; Delvaux, B. Effects of arbuscular mycorrhizal fungi on severity of root rot of bananas caused by Cylindrocladium spathiphylli. Plant Pathol. 2002, 51, 109–115. [Google Scholar] [CrossRef]
- Ortas, İ.; Rafique, M.; Akpinar, C.; Kacar, Y.A. Growth media and mycorrhizal species effect on acclimatization and nutrient uptake of banana plantlets. Sci. Hortic. 2017, 217, 55–60. [Google Scholar] [CrossRef]
- Ramírez-Flores, M.R.; Bello-Bello, E.; Rellán-Álvarez, R.; Sawers, R.J.H.; Olalde-Portugal, V. Inoculation with the mycorrhizal fungus Rhizophagus irregularis modulates the relationship between root growth and nutrient content in maize (Zea mays ssp. mays L.). Plant Direct. 2019, 3, e00192. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.M. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Ann. Rev. Phytopathol. 1988, 26, 379–407. [Google Scholar] [CrossRef]
- Emmert, E.A.B.; Handelsman, J. Biocontrol of plant disease: A (Gram-) positive perspective. FEMS Microbiol. Lett. 1999, 171, 1–9. [Google Scholar] [CrossRef]
- Kurze, S.; Dahl, R.; Bahl, H.; Berg, G. Biological control of fungal strawberry diseases by Serratia HRO-C48. Plant Dis. 2001, 85, 529–534. [Google Scholar] [CrossRef]
- Mohamad, O.A.A.; Li, L.; Ma, J.B.; Hatab, S.; Xu, L.; Guo, J.W.; Rasulov, B.A.; Liu, Y.H.; Hedlund, B.P.; Li, W.J. Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front. Microbiol. 2018, 9, 924. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, G.J.; Lee, H.B.; Kim, K.M.; Jung, H.S.; Lee, S.W.; Jang, K.S.; Cho, K.Y.; Jin-Cheol, K. Griseofulvin from Xylaria sp. strain F0010, an endophytic fungus of Abies holophylla and its antifungal activity against plant pathogenic fungi. J. Microbiol. Biotechnol. 2005, 15, 112–117. [Google Scholar]
- Qin, J.C.; Zhang, Y.M.; Gao, J.M.; Bai, M.S.; Yang, S.X.; Laatsch, H.; Zhang, A.L. Bioactive metabolites produced by Chaetomium globosum, an endophytic fungus isolated from Ginkgo biloba. Bioorg. Med. Chem. Lett. 2009, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Huang, R.; Miao, C.P.; Chen, Y.W. Two new steroids from an endophytic fungus Phomopsis sp. Chem. Biodiver. 2013, 10, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, I.P.D.; da Silva, L.C.N.D.; da Silva, M.V.D.; de Araújo, J.M.D.; Cavalcanti, M.D.S.; Lima, V.L. Antibacterial activity of endophytic fungi from leaves of Indigofera suffruticosa Miller (Fabaceae). Front. Microbiol. 2015, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Gos, F.M.W.R.; Savi, D.C.; Shaaban, K.A.; Thorson, J.S.; Aluizio, R.; Possiede, Y.M.; Rohr, J.; Glienke, C. Antibacterial activity of endophytic actinomycetes isolated from the medicinal plant Vochysia divergens (Pantanal, Brazil). Front. Microbiol. 2017, 8, 1642. [Google Scholar] [CrossRef]
- Singh, R.; Dubey, A.K. Diversity and applications of endophytic Actinobacteria of plants in special and other ecological niches. Front. Microbiol. 2018, 9, 1767. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Tan, W.F.; Zhang, R.; Cao, H.; Huang, C.; Yang, Q.; Wang, M.; Koopal, L.K. Soil inorganic carbon stock under different soil types and land uses on the loess plateau region of China. CATENA 2014, 121, 22–30. [Google Scholar] [CrossRef]
- Ahmed, A.A.Q.; Odelade, K.A.; Babalola, O.O. Microbial inoculants for improving carbon sequestration in agroecosystems to mitigate climate change. In Handbook of Climate Change Resilience, 1st ed.; Filho, W.L., Ed.; Springer: Cham, Switzerland, 2019; pp. 381–401. [Google Scholar] [CrossRef]
- Chan, Y. Increasing soil organic carbon of agricultural land. Nsw Dep. Prim. Ind. 2008, 735, 1–5. [Google Scholar]
- Hoorman, J.J.; Islam, R. Understanding Soil Microbes and Nutrient Recycling: SAG-16, Agriculture and Natural Resources; Ohio State University: Columbus, OH, USA, 2010. [Google Scholar]
- Trumbore, S. Carbon respired by terrestrial ecosystems–Recent progress and challenges. Global Chang. Biol. 2006, 12, 141–153. [Google Scholar] [CrossRef]
- Fang, H.; Cheng, S.; Wang, Y.; Yu, G.; Xu, M.; Dang, X.; Li, L.; Wang, L. Changes in soil heterotrophic respiration, carbon availability, and microbial function in seven forests along a climate gradient. Ecol. Res. 2014, 29, 1077–1086. [Google Scholar] [CrossRef]
- Gougoulias, C.; Clark, J.M.; Shaw, L.J. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Buragohain, P.; Nath, D.J.; Phonglosa, A. Role of microbes on carbon sequestration. Int. J. Microbiol. Res. 2019, 11, 1464–1468. [Google Scholar]
- Barupal, T.; Meena, M.; Sharma, K. Comparative analysis of bioformulations against Curvularia lunata (Wakker) Boedijn causing leaf spot disease of maize. Arch. Phytopathol. Plant Protect. 2020, 54, 261–272. [Google Scholar] [CrossRef]
- Barupal, T.; Meena, M.; Sharma, K. A study on preventive effects of Lawsonia inermis L. bioformulations against leaf spot disease of maize. Biocatal. Agric. Biotechnol. 2020, 23, 101473. [Google Scholar] [CrossRef]
- Song, W.; Tong, X.; Liu, Y.; Li, W. Microbial community, newly sequestered soil organic carbon, and δ15N variations driven by tree roots. Front. Microbiol. 2020, 11, 314. [Google Scholar] [CrossRef]
- Treseder, K.K.; Holden, S.R. Fungal carbon sequestration. Science 2013, 339, 1528–1529. [Google Scholar] [CrossRef]
- Cheng, W.X.; Parton, W.J.; Gonzalez-Meler, M.A.; Phillips, R.; Asao, S.; McNickle, G.G.; Brzostek, E.; Jastrow, J.D. Synthesis and modeling perspectives of rhizosphere priming. New Phytol. 2014, 201, 31–44. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buée, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef] [PubMed]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Han, J.; Lian, B.; Ling, H. Induction of calcium carbonate by Bacillus cereus. Geomicrobiol. J. 2013, 30, 682–689. [Google Scholar] [CrossRef]
- Komala, T.; Khun, T. Biological carbon dioxide sequestration potential of Bacillus pumilus. Sains Malays. 2014, 43, 1149–1156. [Google Scholar]
- Nie, M.; Bell, C.; Wallenstein, M.D.; Pendall, E. Increased plant productivity and decreased microbial respiratory C loss by plant growth-promoting rhizobacteria under elevated CO2. Sci. Rep. 2015, 5, 9212. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Li, N.; Xu, Y.; Han, X.; He, H.; Zhang, X.; Zhang, B. Fungi contribute more than bacteria to soil organic matter through necromass accumulation under different agricultural practices during the early pedogenesis of a mollisol. Eur. J. Soil Biol. 2015, 67, 51–58. [Google Scholar] [CrossRef]
- Fernandez, C.W.; Koide, R.T. The role of chitin in the decomposition of ectomycorrhizal fungal litter. Ecology 2012, 93, 24–28. [Google Scholar] [CrossRef]
- Ekblad, A.; Wallander, H.; Godbold, D.L.; Cruz, C.; Johnson, D.; Baldrian, P.; Björk, R.G.; Epron, D.; Kieliszewska-Rokicka, B.; Kjøller, R.; et al. The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: Role in carbon cycling. Plant Soil 2013, 366, 1–27. [Google Scholar] [CrossRef]
- Leake, J.; Johnson, D.; Donnelly, D.; Muckle, G.; Boddy, L.; Read, D. Networks of power and influence: The role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can. J. Bot. 2004, 82, 1016–1045. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Miller, R.M. Carbon cycling by arbuscular mycorrhizal fungi in soil-plant systems. Trends Plant Sci. 2003, 8, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Driver, J.D.; Holben, W.E.; Rillig, M.C. Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 101–106. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Miranda, A.M.; Hernandez-Tenorio, F.; Ocampo, D.; Vargas, G.J.; Sáez, A.A. Trends on CO2 capture with microalgae: A bibliometric analysis. Molecules 2022, 27, 4669. [Google Scholar] [CrossRef]
- Andersen, R.A. The microalgal cell. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 3–20. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 1–48. [Google Scholar]
- Elster, J. Ecological classification of terrestrial algal communities in polar environments. In Geoecology of Antarctic Ice-Free Coastal Landscapes; Beyer, L., Bölter, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 303–326. [Google Scholar] [CrossRef]
- Lee, S.M.; Ryu, C.M. Algae as new kids in the beneficial plant microbiome. Front. Plant Sci. 2021, 12, 599742. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Rai, A.K. Molecular interaction of nitrate transporter proteins with recombinant glycinebetaine results in efficient nitrate uptake in the cyanobacterium Anabaena PCC 7120. PLoS ONE 2021, 16, e0257870. [Google Scholar] [CrossRef]
- Gobler, C.J.; Norman, C.; Panzeca, C.; Taylor, G.T.; Sañudo-Wilhelmy, S.A. Effect of B-vitamins (B1, B12) and inorganic nutrients on algal bloom dynamics in a coastal ecosystem. Aquat. Microb. Ecol. 2007, 49, 181–194. [Google Scholar] [CrossRef]
- Kazamia, E.; Czesnick, H.; Nguyen, T.T.V.; Croft, M.T.; Sherwood, E.; Sasso, S.; Hodson, S.J.; Warren, M.J.; Smith, A.G. Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ. Microbiol. 2012, 14, 1466–1476. [Google Scholar] [CrossRef]
- Belnap, J. The world at your feet: Desert biological soil crusts. Front. Ecol. Environ. 2003, 1, 181–189. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E. Microbial populations of arid lands and their potential for restoration of deserts. In Soil Biology and Agriculture in the Tropics, 1st ed.; Dion, P., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2010; pp. 109–137. [Google Scholar] [CrossRef]
- Malam Issa, O.M.; Stal, L.J.; Défarge, C.; Couté, A.; Trichet, J. Nitrogen fixation by microbial crusts from desiccated Sahelian soils (Niger). Soil Biol. Biochem. 2001, 33, 1425–1428. [Google Scholar] [CrossRef]
- Pointing, S.B.; Belnap, J. Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 2012, 10, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jin, G.; Liu, Q.; Pan, K.; Zhu, B.; Li, Y. Tolerance comparison among selected Spirulina strains cultured under high carbon dioxide and coal power plant flue gas supplements. J. Ocean Univ. China 2021, 20, 1567–1577. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Steiner, C.; Glaser, B.; Geraldes Teixeira, W.; Lehmann, J.; Blum, W.E.H.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 2010, 171, 893–899. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Zhao, Y.; Shi, H.; Liu, G. Effect of biochar on rhizosphere soil microbial diversity and metabolism in tobacco-growing soil. Ecologies 2022, 3, 539–556. [Google Scholar] [CrossRef]
- Wu, S.; He, H.; Inthapanya, X.; Yang, C.; Lu, L.; Zeng, G.; Han, Z. Role of biochar on composting of organic wastes and remediation of contaminated soils-a review. Environ. Sci. Pollut. Res. 2017, 24, 16560–16577. [Google Scholar] [CrossRef]
- Chen, W.F.; Zhang, W.M.; Meng, J. Advances and prospects in research of biochar utilization in agriculture. Sci. Agric. Sin. 2013, 46, 3324–3333. [Google Scholar]
- Zhang, J.; Shen, J.L. Effects of biochar on soil microbial diversity and community structure in clay soil. Ann. Microbiol. 2022, 72, 35. [Google Scholar] [CrossRef]
- Deshoux, M.; Sadet-Bourgeteau, S.; Gentil, S.; Prévost-Bouré, N.C. Effects of biochar on soil microbial communities: A meta-analysis. Sci. Total Environ. 2023, 902, 166079. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Abbott, L.K.; Murphy, D.V. Biochar phosphorus concentration dictates mycorrhizal colonisation, plant growth and soil phosphorus cycling. Sci. Rep. 2019, 9, 5062. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Tang, C.; Xu, J. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Dai, Z.; Enders, A.; Rodrigues, J.L.M.; Hanley, K.L.; Brookes, P.C.; Xu, J.; Lehmann, J. Soil fungal taxonomic and functional community composition as affected by biochar properties. Soil Biol. Biochem. 2018, 126, 159–167. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA; Routledge: New York, NY, USA, 2015. [Google Scholar]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’–does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Das, K.C.; Kanagaratnam, J.; de Neve, S. Biochar amendment to soils with contrasting organic matter level: Effects on N mineralization and biological soil properties. GCB Bioenergy 2015, 7, 135–144. [Google Scholar] [CrossRef]
- Vanek, S.J.; Lehmann, J. Phosphorus availability to beans via interactions between mycorrhizas and biochar. Plant Soil 2015, 395, 105–123. [Google Scholar] [CrossRef]
- Mickan, B.S.; Abbott, L.K.; Stefanova, K.; Solaiman, Z.M. Interactions between biochar and mycorrhizal fungi in a water-stressed agricultural soil. Mycorrhiza 2016, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Jabborova, D.; Annapurna, K.; Paul, S.; Kumar, S.; Saad, H.A.; Desouky, S.; Ibrahim, M.F.M.; Elkelish, A. Beneficial features of biochar and arbuscular mycorrhiza for improving spinach plant growth, root morphological traits, physiological properties, and soil enzymatic activities. J. Fungi 2021, 7, 571. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Li, Y.; Pang, H.D.; He, L.Y.; Wang, Q.; Sheng, X.F. Cd immobilization and reduced tissue Cd accumulation of rice (Oryza sativa wuyun-23) in the presence of heavy metal resistant bacteria. Ecotoxicol. Environ. Saf. 2017, 138, 56–63. [Google Scholar] [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Dharni, S.; Srivastava, A.K.; Samad, A.; Patra, D.D. Impact of plant growth promoting Pseudomonas monteilii PsF84 and Pseudomonas plecoglossicida PsF610 on metal uptake and production of secondary metabolite (monoterpenes) by rose-scented geranium (Pelargonium graveolens cv. bourbon) grown on tannery sludge amended soil. Chemosphere 2014, 117, 433–439. [Google Scholar]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- McComb, R.B.; Bowers, G.N.; Posen, S., Jr. Alkaline Phosphatase; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2010; pp. 79–436. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Neilands, J.B. Iron absorption and transport in microorganisms. Ann. Rev. Nutr. 1981, 1, 27–46. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; Navarro, J.A.; Molina-Heredia, F.P.; Mariscal, V. Endophytic colonization of rice (Oryza sativa L.) by the symbiotic strain Nostoc punctiforme PCC 73102. Mol. Plant. Microbe Interact. 2020, 33, 1040–1045. [Google Scholar] [CrossRef]
- Whitelaw, M.; Harden, T.; Helyar, K. Phosphate solubilisation in solution culture by the soil fungus Penicillium radicum. Soil. Biol. Biochem. 1999, 31, 655–665. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–243. [Google Scholar] [CrossRef]
- Mitchell, D.B.; Vogel, K.; Weimann, B.J.; Pasamontes, L.; Van Loon, A.P. The phytase subfamily of histidine acid phosphatases: Isolation of genes for two novel phytases from the fungi aspergillus terreus and Myceliophthora thermophila. Microbiology 1997, 143, 245–252. [Google Scholar] [CrossRef]
- Lim, B.L.; Yeung, P.; Cheng, C.; Hill, J.E. Distribution and diversity of phytatemineralizing bacteria. ISME J. 2007, 1, 321–330. [Google Scholar] [CrossRef]
- Vílchez, J.I.; Yang, Y.; He, D.; Zi, H.; Peng, L.; Lv, S.; Kaushal, R.; Wang, W.; Huang, W.; Liu, R.; et al. DNA demethylases are required for myo-inositol-mediated mutualism between plants and beneficial rhizobacteria. Nat. Plants 2020, 6, 983–995. [Google Scholar] [CrossRef]
- Kong, Z.; Glick, B.R. The role of plant growth-promoting bacteria in metal phytoremediation. Adv. Microb. Physiol. 2017, 71, 97–132. [Google Scholar] [CrossRef]
- Kumar, R.; Swapnil, P.; Meena, M.; Selpair, S.; Yadav, B.G. Plant growth-promoting rhizobacteria (PGPR): Approaches to alleviate abiotic stresses for enhancement of growth and development of medicinal plants. Sustainability 2022, 14, 15514. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, M.; Gupta, P.; Dubey, M.K.; Nath, G.; Upadhyay, R.S. Plant growth promoting rhizobacteria and their biopriming for growth promotion in mung bean (Vigna radiata (L.) R. Wilczek). Biocatal. Agric. Biotechnol. 2018, 16, 163–171. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Meena, M.; Dubey, M.K.; Swapnil, P.; Zehra, A.; Singh, S.; Kumari, P.; Upadhyay, R.S. The rhizosphere microbial community and methods of its analysis. In Advances in PGPR Research; Singh, H.B., Sarma, B.K., Keswani, C., Eds.; CAB International: Wallingford, UK, 2017; pp. 275–295. [Google Scholar]
- Seth, K.; Vyas, P.; Deora, S.; Gupta, A.K.; Meena, M.; Swapnil, P.; Harish. Understanding plant-plant growth-promoting rhizobacteria (PGPR) interactions for inducing plant defense. In Plant-Microbe Interaction—Recent Advances in Molecular and Biochemical Approaches; Swapnil, P., Meena, M., Harish, Marwal, A., Vijayalakshmi, S., Zehra, A., Eds.; Academic Press, Elsevier: London, UK, 2023; Volume 2, pp. 201–226. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, M.P. Plant-microbe interactions in alleviating abiotic stress—A mini review. Front. Agronomy 2021, 3, 667903. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, J.S.; Meena, V.S.; Srivastava, R. Recent advances of PGPR based approaches for stress tolerance in plants for sustainable agriculture. Biocatal. Agric. Biotechnol. 2019, 20, 101271. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Swapnil, P. Plant growth-promoting rhizobacteria as a green alternative for sustainable agriculture. Sustainability 2021, 13, 10986. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, M.; Upadhyay, R.S. Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean). Biocatal. Agric. Biotechnol. 2018, 16, 155–162. [Google Scholar] [CrossRef]
- Meena, M.; Mehta, T.; Nagda, A.; Yadav, G.; Sonigra, P. PGPR-mediated synthesis and alteration of different secondary metabolites during plant-microbe interactions. In Plant-Microbe Interaction—Recent Advances in Molecular and Biochemical Approaches; Swapnil, P., Meena, M., Harish, Marwal, A., Vijayalakshmi, S., Zehra, A., Eds.; Academic Press, Elsevier: London, UK, 2023; Volume 1, pp. 229–255. [Google Scholar] [CrossRef]
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and strategies of plant microbiome interactions to mitigate abiotic stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Zehra, A.; Aamir, M.; Dubey, M.K.; Upadhyay, R.S. Beneficial microbes for disease suppression and plant growth promotion. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D., Singh, H., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 395–432. [Google Scholar]
- Aloo, B.N.; Dessureault-Rompre, J.; Tripathi, V.; Nyongesa, B.O.; Were, B.A. Signaling and crosstalk of rhizobacterial and plant hormones that mediate abiotic stress tolerance in plants. Front Microbiol. 2023, 14, 1171104. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Rhizobacteria AK1 remediates the toxic effects of salinity stress via regulation of endogenous phytohormones and gene expression in soybean. Biochem. J. 2019, 476, 2393–2409. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.M.; Kim, K.M.; Lee, I.J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef]
- Ali, S.; Xie, L. Plant growth promoting and stress mitigating abilities of soil born microorganisms. Recent Pat. Food Nutr. Agric. 2020, 11, 96–104. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, P.K.; Pramanik, K.; Mitra, S.; Soren, T.; Pandey, S.; Mondal, M.H.; Maiti, T.K. A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res. Microbiol. 2018, 169, 20–32. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Al-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Bhise, K.K.; Dandge, P.B. Mitigation of salinity stress in plants using plant growth promoting bacteria. Symbiosis 2019, 79, 191–204. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.M.; Lee, I.J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Meena, M.; Nagda, A.; Mehta, T.; Yadav, G.; Sonigra, P. Mechanistic basis of the symbiotic signaling pathway between the host and the pathogen. In Plant-Microbe Interaction—Recent Advances in Molecular and Biochemical Approaches; Swapnil, P., Meena, M., Harish, Marwal, A., Vijayalakshmi, S., Zehra, A., Eds.; Academic Press, Elsevier: London, UK, 2023; Volume 1, pp. 375–387. [Google Scholar] [CrossRef]
- Zehra, A.; Meena, M.; Swapnil, P. Immune signaling networks in plant-pathogen interactions. In Plant-Microbe Interaction—Recent Advances in Molecular and Biochemical Approaches; Swapnil, P., Meena, M., Harish, Marwal, A., Vijayalakshmi, S., Zehra, A., Eds.; Academic Press, Elsevier: London, UK, 2023; Volume 2, pp. 137–147. [Google Scholar] [CrossRef]
- Patel, T.; Saraf, M. Biosynthesis of phytohormones from novel rhizobacterial isolates and their in vitro plant growth-promoting efficacy. J. Plant Interact. 2017, 12, 480–487. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Davranov, K.; Wirth, S.; Hashem, A.; AbdAllah, E.F. Impact of soil salinity on the plant-growth-promoting and biological control abilities of root associated bacteria. Saudi J. Biol. Sci. 2017, 24, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Bhise, K.K.; Dandge, P.B. Alleviation of salinity stress in rice plant by encapsulated salt tolerant plant growth promoting bacteria Pantoea agglomerans strain KL and its root colonization ability. Arch. Agron. Soil Sci. 2019, 65, 1955–1968. [Google Scholar] [CrossRef]
- Bhise, K.K.; Bhagwat, P.K.; Dandge, P.B. Synergistic effect of Chryseobacterium gleum sp. SUK with ACC deaminase activity in alleviation of salt stress and plant growth promotion in Triticum aestivum L. 3 Biotech 2017, 7, 105. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.; Jha, P.N. Bio-inoculation of plant growth-promoting rhizobacterium Enterobacter cloacae ZNP-3 increased resistance against salt and temperature stresses in wheat plant (Triticum aestivum L.). J. Plant Growth Regul. 2017, 36, 783–798. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Reg. 2016, 80, 23–36. [Google Scholar]
- Nadeem, S.M.; Ahmad, M.; Naveed, M.; Imran, M.; Zahir, Z.A.; Crowley, D.E. Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch. Microbiol. 2016, 198, 379–387. [Google Scholar] [CrossRef]
- Zerrouk, I.Z.; Benchabane, M.; Khelifi, L.; Yokawa, K.; Ludwig-Müller, J.; Baluska, F. A Pseudomonas strain isolated from date-palm rhizospheres improves root growth and promotes root formation in maize exposed to salt and aluminum stress. J. Plant Physiol. 2016, 191, 111–119. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. Alleviation of salinity-induced damage on wheat plant by an ACC deaminase-producing halophilic bacterium Serratia sp. SL-12 isolated from a salt lake. Symbiosis 2016, 69, 101–111. [Google Scholar] [CrossRef]
- Yoolong, S.; Kruasuwan, W.; Thanh Phạm, H.T.; Jaemsaeng, R.; Jantasuriyarat, C.; Thamchaipenet, A. Modulation of salt tolerance in Thai jasmine rice (Oryza sativa L. cv. KDML105) by Streptomyces venezuelae ATCC 10712 expressing ACC deaminase. Sci. Rep. 2019, 9, 1275. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wu, D.; Liang, Y.; Li, L.; Guo, Y. Disruption of acdS gene reduces plant growth promotion activity and maize saline stress resistance by Rahnella aquatilis HX2. J. Basic Microbiol. 2019, 59, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Khan, M.S. Fungicide tolerant Bradyrhizobium japonicum mitigate toxicity and enhance green gram production under hexaconazole stress. J. Environ. Sci. 2019, 78, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, S.; Chatterjee, P.; Choudhury, A.R.; Ahmed, S.; Sa, T. Interactions between Pseudomonas spp. and their role in improving the red pepper plant growth under salinity stress. Microbiol. Res. 2019, 219, 66–73. [Google Scholar] [CrossRef]
- Liu, J.; Tang, L.; Gao, H.; Zhang, M.; Guo, C. Enhancement of alfalfa yield and quality by plant growth-promoting rhizobacteria under saline-alkali conditions. J. Sci. Food Agric. 2019, 99, 281–289. [Google Scholar] [CrossRef]
- Chatterjee, P.; Kanagendran, A.; Samaddar, S.; Pazouki, L.; Sa, T.M.; Niinemets, Ü. Inoculation of Brevibacterium linens RS16 in Oryza sativa genotypes enhanced salinity resistance: Impacts on photosynthetic traits and foliar volatile emissions. Sci. Total Environ. 2019, 645, 721–732. [Google Scholar] [CrossRef]
- Cui, L.; Liu, Y.; Yang, Y.; Ye, S.; Luo, H.; Qiu, B.; Gao, X. The drnf1 gene from the drought-adapted cyanobacterium Nostoc flagelliforme improved salt tolerance in transgenic Synechocystis and Arabidopsis plant. Genes 2018, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Vimal, S.R.; Patel, V.K.; Singh, J.S. Plant growth promoting Curtobacterium albidum strain SRV4: An agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indic. 2019, 105, 553–562. [Google Scholar] [CrossRef]
- Kasotia, A.; Varma, A.; Tuteja, N.; Choudhary, D.K. Amelioration of soybean plant from saline-induced condition by exopolysaccharide producing Pseudomonas-mediated expression of high affinity K+−transporter (HKT1) gene. Curr. Sci. 2016, 111, 1961–1967. [Google Scholar] [CrossRef]
- Zhou, C.; Li, F.; Xie, Y.; Zhu, L.; Xiao, X.; Ma, Z.; Wang, J. Involvement of abscisic acid in microbe-induced saline-alkaline resistance in plants. Plant Signal. Behav. 2017, 12, 1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Y.; Liu, C.; Chen, F.; Ge, H.; Tian, F.; Zhang, Y. Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Eco. Environ. Saf. 2019, 170, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Jinal, H.N.; Amaresan, N. Biocatalysis and agricultural biotechnology isolation and characterization of drought resistance bacteria for plant growth promoting properties and their effect on chilli (Capsicum annuum) seedling under salt stress. Biocatal. Agric. Biotechnol. 2017, 12, 85–89. [Google Scholar] [CrossRef]
- Kruasuwan, W.; Thamchaipenet, A. 1-Aminocyclopropane-1- carboxylate (ACC) deaminase-producing endophytic diazotrophic Enterobacter sp. EN-21 modulates salt–stress response in sugarcane. J. Plant Growth Regul. 2018, 37, 849–858. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Iqbal, S.; Amjad, M.; Naveed, M.; Zahir, Z.A.; Jacobsen, S.E. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 2016, 43, 632–642. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Plant growth-promoting rhizobacteria ameliorates salinity stress in pea (Pisum sativum). J. Plant Growth Regul. 2022, 41, 647–656. [Google Scholar] [CrossRef]
- Mellidou, I.; Ainalidou, A.; Papadopoulou, A.; Leontidou, K.; Genitsaris, S.; Karagiannis, E.; Van de Poel, B.; Karamanoli, K. Comparative transcriptomics and metabolomics reveal an intricate priming mechanism involved in PGPR-mediated salt tolerance in tomato. Front. Plant Sci. 2021, 12, 713984. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Singh, V.K.; Mishra, A. Halotolerant PGPR Stenotrophomonas maltophilia BJ01 induces salt tolerance by modulating physiology and biochemical activities of Arachis hypogaea. Front. Microbiol. 2020, 11, 568289. [Google Scholar] [CrossRef] [PubMed]
- Hahm, M.S.; Son, J.S.; Hwang, Y.J.; Kwon, D.K.; Ghim, S.Y. Alleviation of salt stress in pepper (Capsicum annum L.) plants by plant growth-promoting rhizobacteria. J. Microbiol. Biotechnol. 2017, 27, 1790–1797. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, H.; Leng, J.; Niu, H.; Chen, X.; Liu, D.; Ying, H. Isolation and characterization of plant growth-promoting rhizobacteria and their effects on the growth of Medicago sativa L. under salinity conditions. Antonie Van Leeuwenhoek 2020, 113, 1263–1278. [Google Scholar] [PubMed]
- Vaishnav, A.; Choudhary, D.K. Regulation of drought-responsive gene expression in Glycine max L. Merrill is mediated through Pseudomonas simiae strain AU. J. Plant Growth Regul. 2018, 38, 333–342. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2015, 97, 30–39. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Danish, S.; Abbas, M.; Ahmad, M.; Munir, T.M. ACC deaminase producing PGPR Bacillus amyloliquefaciens and Agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy 2019, 9, 343. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M.; Hussain, M.; Shaaban, M.; Nunez-Delgado, A.; Hussain, S.; Qayyum, M.F. Rhizobacteria with ACC-deaminase activity improve nutrient uptake, chlorophyll contents and early seedling growth of wheat under PEG-induced osmotic stress. Int. J. Agric. Biol. 2019, 21, 1212–1220. [Google Scholar]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in asemi-arid agroecosystem and their potential in alleviating drought stress. Front. Micrbiol. 2017, 8, 2580. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Sharma, A.K. Influence of IAA and ACC deaminase producing fluorescent Pseudomonads in alleviating drought stress in wheat (Triticum aestivum). Agric. Res. 2018, 7, 290–299. [Google Scholar] [CrossRef]
- Ghosh, D.; Gupta, A.; Mohapatra, S. A comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana. World J. Microbiol. Biotechnol. 2019, 35, 90. [Google Scholar] [CrossRef]
- Li, H.; Guo, Q.; Jing, Y.; Liu, Z.; Zheng, Z.; Sun, Y.; Xue, Q.; Lai, H. Application of Streptomyces pactum Act12 enhances drought resistance in wheat. J. Plant Growth Regul. 2020, 39, 122–132. [Google Scholar] [CrossRef]
- Behrooz, A.; Vahdati, K.; Rejali, F.; Lotfi, M.; Sarikhani, S.; Leslie, C. Arbuscular mycorrhiza and plant growth-promoting bacteria alleviate drought stress in walnut. Hort. Sci. 2019, 54, 1087–1092. [Google Scholar] [CrossRef]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.D.R.; Sosa Alderete, L.G.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2018, 64, 574–587. [Google Scholar] [CrossRef]
- Martins, S.J.; Rocha, G.A.; de Melo, H.C.; de Castro Georg, R.; Ulhôa, C.J.; de Campos Dianese, É.; Oshiquiri, L.H.; da Cunha, M.G.; da Rocha, M.R.; de Araújo, L.G.; et al. Plant-associated bacteria mitigate drought stress in soybean. Environ. Sci. Pollut. Res. 2018, 25, 13676–13686. [Google Scholar] [CrossRef]
- Bruno, L.B.; Karthik, C.; Ma, Y.; Kadirvelu, K.; Freitas, H.; Rajkumar, M. Amelioration of chromium and heat stresses in Sorghum bicolor by Cr6+ reducing-thermotolerant plant growth promoting bacteria. Chemosphere 2020, 244, 125521. [Google Scholar] [CrossRef]
- Ibort, P.; Molina, S.; Ruiz-Lozano, J.M.; Aroca, R. Molecular insights into the involvement of a never ripe receptor in the interaction between two beneficial soil bacteria and tomato plants under well-watered and drought conditions. Mol. Plant Microbe Interact. 2018, 31, 633–650. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Babar, M.A. Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PLoS ONE 2019, 14, e0213040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xie, Z.; Zhang, X.; Lang, D.; Zhang, X. Growth-promoting bacteria alleviates drought stress of G. uralensis through improving photosynthesis characteristics and water status. J. Plant Interact. 2019, 14, 580–589. [Google Scholar] [CrossRef]
- Saleem, A.R.; Brunetti, C.; Khalid, A.; Della Rocca, G.; Raio, A.; Emiliani, G.; De Carlo, A.; Mahmood, T.; Centritto, M. Drought response of Mucuna pruriens (L.) DC. inoculated with ACC deaminase and IAA producing rhizobacteria. PLoS ONE 2018, 13, e0191218. [Google Scholar] [CrossRef] [PubMed]
- Jochum, M.D.; McWilliams, K.L.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.K. Bioprospecting plant growth-promoting rhizobacteria that mitigate drought stress in grasses. Front. Microbiol. 2019, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Santana, S.R.A.; Voltolini, T.V.; Antunes, G.D.R.; da Silva, V.M.; Simões, W.L.; Morgante, C.V.; de Freitas, A.D.S.; Chaves, A.R.D.M.; Aidar, S.D.T.; Fernandes-Júnior, P.I. Inoculation of plant growth-promoting bacteria attenuates the negative effects of drought on sorghum. Arch. Microbiol. 2020, 202, 1015–1024. [Google Scholar] [CrossRef]
- Moreno-Galván, A.; Cortés-Patiño, S.; Romero-Perdomo, F.; Uribe-Vélez, D.; Bashan, Y.; Bonilla, R.R. Proline accumulation and glutathione reductase activity induced by drought-tolerant rhizobacteria as potential mechanisms to alleviate drought stress in Guinea grass. Appl. Soil Ecol. 2020, 147, 103367. [Google Scholar] [CrossRef]
- Vigani, G.; Rolli, E.; Marasco, R.; Dell’Orto, M.; Michoud, G.; Soussi, A.; Raddadi, N.; Borin, S.; Sorlini, C.; Zocchi, G.; et al. Root bacterial endophytes confer drought resistance and enhance expression and activity of a vacuolar H+-pumping pyrophosphatase in pepper plants. Environ. Microbiol. 2019, 21, 3212–3228. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Bacillus velezensis 5113 induced metabolic and molecular reprogramming during abiotic stress tolerance in wheat. Sci. Rep. 2019, 9, 16282. [Google Scholar] [CrossRef]
- Silva, E.R.; Zoz, J.; Oliveira, C.E.S.; Zuffo, A.M.; Steiner, F.; Zoz, T.; Vendruscolo, E.P. Can co-inoculation of Bradyrhizobium and Azospirillum alleviate adverse effects of drought stress on soybean (Glycine max L. Merrill.)? Arch. Microbiol. 2019, 201, 325–335. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Valentine, A.; Cornejo, P. Role of Curtobacterium herbarum strain CAH5 on aluminum bioaccumulation and enhancement of Lactuca sativa growth under aluminum and drought stresses. Ecotoxicol. Environ. Saf. 2019, 183, 109573. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, L.; Silva, R.; Almeida, I.; Vidal, M.; Baldani, J.I.; Meneses, C.H.S.G. Gluconacetobacter diazotrophicus mitigates drought stress in Oryza sativa L. Plant Soil 2020, 451, 57–73. [Google Scholar] [CrossRef]
- Kim, Y.N.; Khan, M.A.; Kang, S.M.; Hamayun, M.; Lee, I.J. Enhancement of drought-stress tolerance of Brassica oleracea var. italica L. by newly isolated Variovorax sp. YNA59. J. Microbiol. Biotechnol. 2020, 30, 1500–1509. [Google Scholar] [CrossRef]
- Batool, T.; Ali, S.; Seleiman, M.F.; Naveed, N.H.; Ali, A.; Ahmed, K.; Abid, M.; Rizwan, M.; Shahid, M.R.; Alotaibi, M. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020, 10, 16975. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb. Ecol. 2022, 83, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Vahid, J.; Bihamta, M.R.; Islam, M.; Farrokh, D. The effect of endophytic fungi in drought resistance of Lolium perenne in Iran (Isfahan) condition. Adv. Stud. Biol. 2015, 7, 245–257. [Google Scholar] [CrossRef]
- Choo, J.; Sabri, N.B.M.; Tan, D.; Mujahid, A.; Müller, M. Heavy metal resistant endophytic fungi isolated from Nypa fruticans in Kuching Wetland National Park. Ocean Sci. J. 2015, 50, 445–453. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, T.; Liu, G.Y.; Smith, J.M.; Zhao, Z.W. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: Physiological, cytological and genic aspects. Sci. Rep. 2016, 6, 22028. [Google Scholar] [CrossRef]
- Khan, A.L.; Lee, I.J. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013, 13, 86. [Google Scholar] [CrossRef]
- Mukherjee, G.; Saha, C.; Naskar, N.; Mukherjee, A.; Mukherjee, A.; Lahiri, S.; Majumdar, A.L.; Seal, A. An endophytic bacterial consortium modulates multiple strategies to improve arsenic phytoremediation efficacy in Solanum nigrum. Sci. Rep. 2018, 8, 6979. [Google Scholar] [CrossRef]
- Vivas, A.; Biro, B.; Ruiz-Lozano, J.; Barea, J.; Azcon, R. Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn-toxicity. Chemosphere 2006, 62, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Yasmeen, T.; Ali, Q.; Ali, S.; Arif, M.S.; Hussain, S.; Rizvi, H. Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol. Environ. Saf. 2014, 104, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Gajdos, E.; Levai, L.; Veres, S.; Kovacs, B. Effects of biofertilizers on maize and sunflower seedlings under cadmium stress. Commun. Soil Sci. Plant Anal. 2012, 43, 272–279. [Google Scholar] [CrossRef]
- Shah, A.A.; Yasin, N.A.; Akram, K.; Ahmad, A.; Khan, W.U.; Akram, W.; Akbar, M. Ameliorative role of Bacillus subtilis FBL-10 and silicon against lead induced stress in Solanum melongena. Plant Physiol. Biochem. 2021, 158, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhu, L.; Guo, J.; Xiao, X.; Ma, Z.; Wang, J. Bacillus subtilis STU6 ameliorates iron deficiency in tomato by enhancement of polyamine-mediated iron remobilization. J. Agric. Food Chem. 2018, 67, 320–330. [Google Scholar] [CrossRef]
- El-Nahrawy, S.; Elhawat, N.; Alshaal, T. Biochemical traits of Bacillus subtilis MF497446: Its implications on the development of cowpea under cadmium stress and ensuring food safety. Ecotoxicol. Environ. Saf. 2019, 180, 384–395. [Google Scholar] [CrossRef]
- Ndeddy Aka, R.J.; Babalola, O.O. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int. J. Phytoremediation 2016, 18, 200–209. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Effects of exogenously applied salicylic acid and putrescine alone and in combination with rhizobacteria on the phytoremediation of heavy metals and chickpea growth in sandy soil. Int. J. Phytoremediation 2018, 20, 405–414. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Sharma, K. Microbial biodiversity and bioremediation assessment through omics approaches. Front. Environ. Chem. 2020, 1, 570326. [Google Scholar] [CrossRef]
- Arif, M.S.; Yasmeen, T.; Shahzad, S.M.; Riaz, M.; Rizwan, M.; Iqbal, S.; Asif, M.; Soliman, M.H.; Ali, S. Lead toxicity induced phytotoxic effects on mung bean can be relegated by lead tolerant Bacillus subtilis (PbRB3). Chemosphere 2019, 234, 70–80. [Google Scholar] [CrossRef]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance generated by plant/fungal symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Bano, A.; Ali, S.A. Characterisation of plant growth-promoting rhizobacteria from rhizosphere soil of heat stressed and unstressed wheat and their use as bio-inoculant. Plant Biol. 2019, 21, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Khan, A.L.; Waqas, M.; Asaf, S.; Lee, K.E.; Park, Y.G.; Kim, A.Y.; Khan, M.A.; You, Y.H.; Lee, I.J. Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 2019, 14, 416–423. [Google Scholar] [CrossRef]
- Ali, A.H.; Radwan, U.; El-Zayat, S.; El Sayed, M.A. The role of the endophytic fungus, Thermomyces lanuginosus, on mitigation of heat stress to its host desert plant Cullen plicata. Biol. Futura 2019, 70, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular mechanisms of the 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing Trichoderma asperellum MAP1 in enhancing wheat tolerance to waterlogging stress. Front. Plant Sci. 2021, 11, 614971. [Google Scholar] [CrossRef]
- Bisht, N.; Kumar Mishra, S.; Singh Chauhan, P. Bacillus amyloliquefaciens inoculation alters physiology of rice (Oryza sativa L. var. IR-36) through modulating carbohydrate metabolism to mitigate stress induced by nutrient starvation. Int. J. Biol. Macromol. 2019, 143, 937–951. [Google Scholar] [CrossRef]
- Park, Y.G.; Mun, B.G.; Kang, S.M.; Hussain, A.; Shahzad, R.; Seo, C.W.; Kim, A.Y.; Lee, S.U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef]
- Subramanian, P.; Kim, K.; Krishnamoorthy, R.; Mageswari, A.; Selvakumar, G.; Sa, T. Cold stress tolerance in psychrotolerant soil bacteria and their conferred chilling resistance in tomato (Solanum lycopersicum Mill.) under low temperatures. PLoS ONE 2016, 11, e0161592. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Swapnil, P.; Marwal, A.; Zehra, A. Advantageous features of plant growth-promoting microorganisms to improve plant growth in difficult condition. In Plant-Microbe Interaction—Recent Advances in Molecular and Biochemical Approaches; Swapnil, P., Meena, M., Harish, Marwal, A., Vijayalakshmi, S., Zehra, A., Eds.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 279–296. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Srinivasan, R.; Mageswari, A.; Subramanian, P.; Maurya, V.K.; Sugnathi, C.; Amballa, C.; Sa, T.; Gothandam, K.M. Exogenous expression of ACC deaminase gene in psychrotolerant bacteria alleviates chilling stress and promotes plant growth in millets under chilling conditions. Indian J. Agric. Sci. 2017, 55, 463–468. [Google Scholar]
- Zubair, M.; Hanif, A.; Farzand, A.; Sheikh, T.M.M.; Khan, A.R.; Suleman, M.; Ayaz, M.; Gao, X. Genetic screening and expression analysis of psychrophilic Bacillus spp. reveal their potential to alleviate cold stress and modulate phytohormones in wheat. Microorganisms 2019, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Tripathi, A.; Chanotiya, C.S.; Barnawal, D.; Singh, P.; Patel, V.K.; Kalra, A. Cold stress alleviation using individual and combined inoculation of ACC deaminase producing microbes in Ocimum sanctum. Envir. Sustain. 2020, 3, 289–301. [Google Scholar] [CrossRef]
- Meena, M.; Singh, S.K.; Swapnil, P. The role of PGPRs in medicinal plants under abiotic stress. In Medicinal Plants: Their Response to Abiotic Stress; Husen, A., Iqbal, M., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2023; pp. 267–285. [Google Scholar] [CrossRef]
- De Souza, E.M.; Lamb, T.I.; Lamb, T.A.; dos Santos Silva, A.; da Fré de Carvalho, S.; Nyland, V.; Lopes, M.C.B.; Grohs, M.; Marconatto, L.; dos Anjos Borges, L.G.; et al. Rhizospheric soil from rice paddy presents isolable bacteria able to induce cold tolerance in rice plants. J. Soil Sci. Plant Nutr. 2021, 21, 1993–2006. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Cui, L.; Tang, Z.; Ci, D.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, T. The multifaceted roles of Arbuscular Mycorrhizal Fungi in peanut responses to salt, drought, and cold stress. BMC Plant Biol. 2023, 23, 36. [Google Scholar] [CrossRef]
- Jaemsaeng, R.; Jantasuriyarat, C.; Thamchaipenet, A. Positive role of 1-aminocyclopropane-1-carboxylate deaminase-producing endophytic Streptomyces sp. GMKU 336 on flooding resistance of mung bean. Agric. Nat. Resour. 2018, 52, 330–334. [Google Scholar] [CrossRef]
- Bal, H.B.; Adhya, T.K. Alleviation of submergence stress in rice seedlings by plant growth-promoting rhizobacteria with ACC deaminase activity. Front. Sustain. Food Syst. 2021, 5, 606158. [Google Scholar] [CrossRef]
- Kim, A.Y.; Shahzad, R.; Kang, S.M.; Seo, C.W.; Park, Y.G.; Park, H.J.; Lee, I.J. IAA-producing Klebsiella variicola AY13 reprograms soybean growth during flooding stress. J. Crop Sci. Biotechnol. 2017, 20, 235–242. [Google Scholar] [CrossRef]
- Carreiras, J.; Cruz-Silva, A.; Fonseca, B.; Carvalho, R.C.; Cunha, J.P.; Proença Pereira, J.; Paiva-Silva, C.A.; Santos, S.; Janeiro Sequeira, R.; Mateos-Naranjo, E.; et al. Improving grapevine heat stress resilience with marine plant growth-promoting rhizobacteria consortia. Microorganisms 2023, 11, 856. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Zehra, A.; Swapnil, P. Role of microbial bioagents as elicitors in plant defense regulation. In Transcription Factors for Biotic Stress Tolerance in Plants; Wani, S.H., Nataraj, V., Singh, G.P., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 103–128. [Google Scholar] [CrossRef]
- Mukhtar, T.; Rehman, S.U.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.; et al. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Issa, A.; Esmaeel, Q.; Sanchez, L.; Courteaux, B.; Guise, J.F.; Gibon, Y.; Ballias, P.; Clément, C.; Jacquard, C.; Vaillant-Gaveau, N.; et al. Impacts of Paraburkholderia phytofirmans strain PsJN on tomato (Lycopersicon esculentum L.) under high temperature. Front. Plant Sci. 2018, 9, 1397. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Vangnai, A.S.; Kamaraj, B.; Cornejo, P. Plant growth-promoting actinobacterial inoculant assisted phytoremediation increases cadmium uptake in Sorghum bicolor under drought and heat stresses. Environ. Pollut. 2022, 307, 119489. [Google Scholar] [CrossRef] [PubMed]
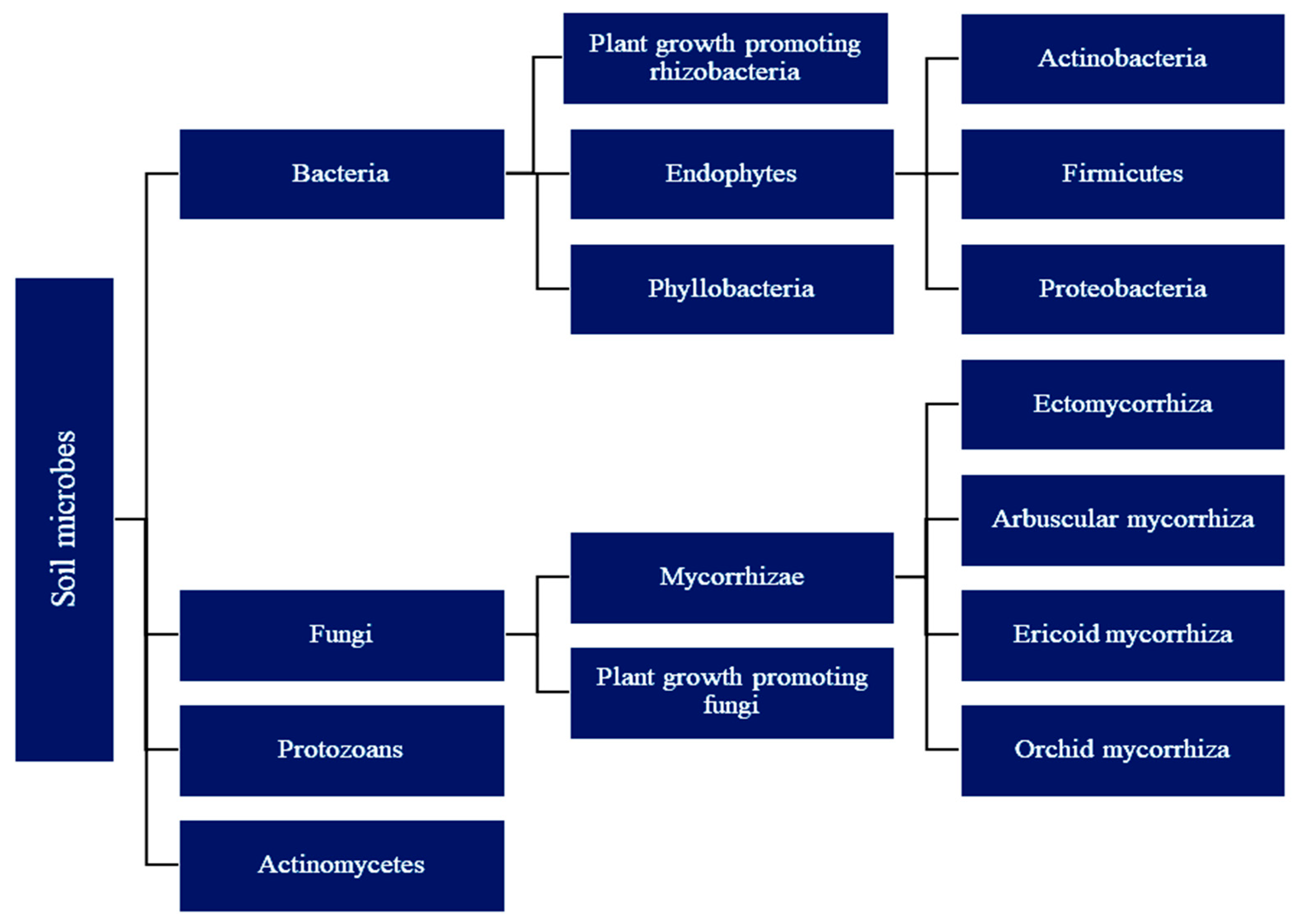
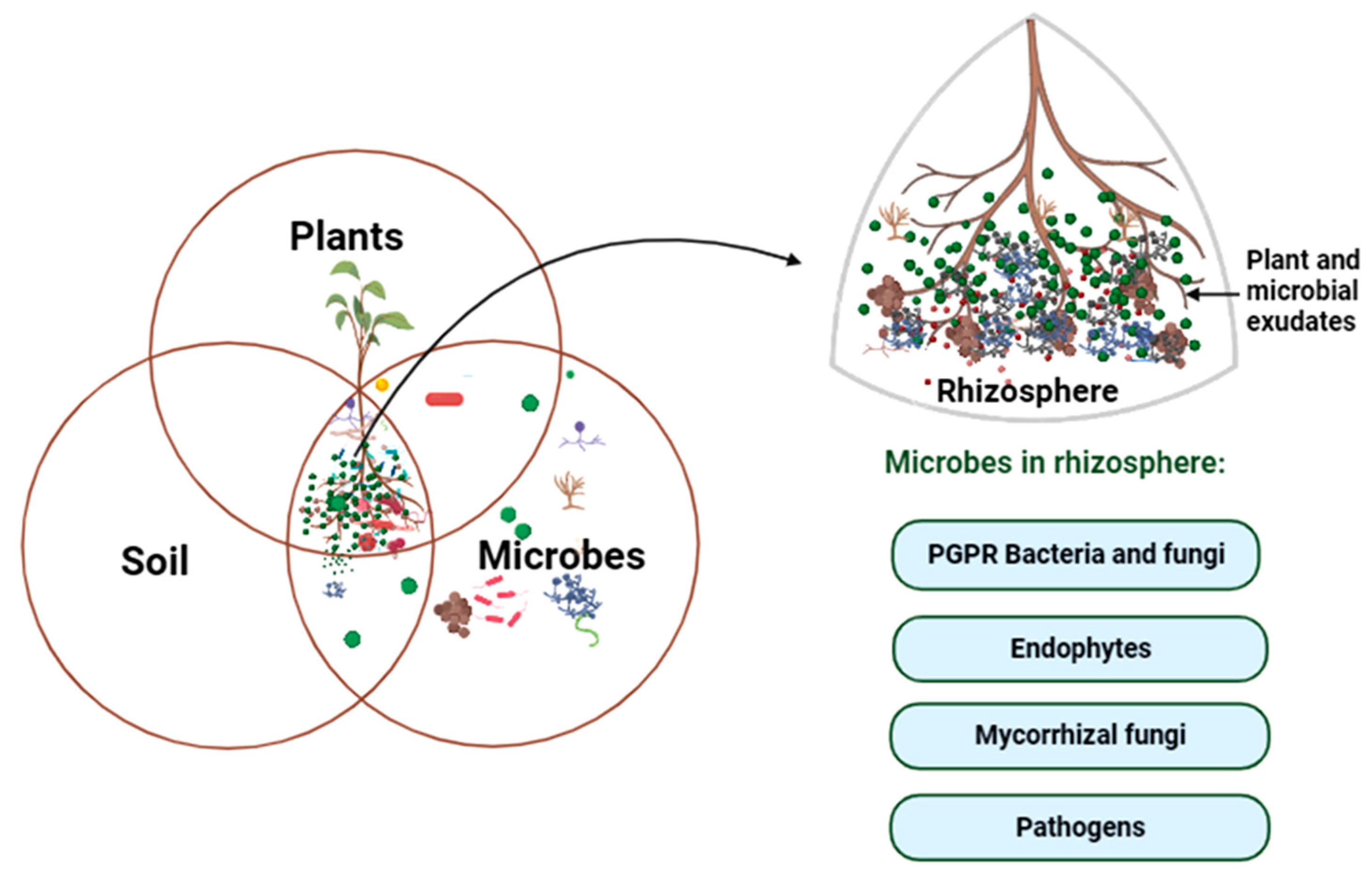

| Types of Microbes | Subtype | Examples | Function | References |
|---|---|---|---|---|
| Plant growth-promoting bacteria (PGPB) | Plant growth-promoting rhizobacteria (PGPR) | Azotobacter, Azospirillum, Acetobacter, Bacillus, Diazotrophicus, Herbaspirillum, Burkholderia, Rhizobium Paenibacillus, and Bradyrhizobium | Auxin production, increase root length and number of lateral roots | [138] |
| Achromobacter xylosoxidans, Gluconobacter diazotrophicus, Acinetobacter calcoaceticus, Rhizobia, Azotobacter spp., Bacillus spp., Herbaspirillum seropedicae, Azospirillum spp., Azotobacter, Pseudomonas, Rhizobium, Arthrobacter, Bacillus pumilus Agrobacterium, Clostridium, Flavobacteirum, and Bacillus licheniformis | Production of GAs (gibberellins) | [139,140] | ||
| Pseudomonas, Azospirillum, Rhizobium, Azotobacter, Bacillus, and Arthrobacter | Cytokinins production | [141] | ||
| Frankia casuarinae, Frankia irregularis, Frankia inefficax, and Frankia saprophytica | [142] | |||
| Bacillus cereus | Bioremediation, production of amylase, remediation of industrial waste | [143] | ||
| Bacillus subtilis | Bioremediation (Degrading xenobiotics and allelochemicals) | [143] | ||
| Pseudomonas aeruginosa | Bioremediation (Cellulase production, heavy metals uptake | [143,144] | ||
| Bacillus thuringiensis, and Pseudomonas sp. Lk9 | Bioremoval of, Cu, Zn, Cd in metal amended media | [145] | ||
| Acinetobacter, Achromobacter, Enterobacter, Ralstonia, Agrobacterium, Burkholderia, Alcaligenes, Azospirillum, Bacillus, Pseudomonas, Serratia, and Rhizobium | Produce ACC-deaminase, which controls ethylene levels and lessens growth inhibition induced on by elevated ethylene levels | [146] | ||
| Pseudomonas putida, Escherichia coli, and Agrobacterium tumefaciens | Deamination of ACC deaminase that regulates ethylene level | [147] | ||
| Endophytes | Proteobacteria | Pantoea, Pseudomonas spp., Duganella, Kosakonia, Klebsiella, Massilia, Bordetella, Salmonella, and Serratia | IAA, Siderophores, ACC deaminase production, nitrogen fixation phosphorus/potassium solubilization, and trace metal tolerance | [138,148,149] |
| Firmicutes | Bacillus paralicheniformis, Bacillus, Micrococcus, Staphylococcus, and Exiguobacterium | Nitrogen fixation, ACC deaminase, amylase, IAA, lipase cellulase, protease production, phosphate solubilization | [150] | |
| Actinobacterium | Curtobacterium, Microbacterium, Nocardia, Sediminihabitans, and Frankia | IAA production, nitrogen fixation fungicidal and bactericidal activities | [138,149] | |
| Fungi | Ectomycorrhiza | Amanita, Boletus, and Ramaria | Soil aggregation, carbon cycle and plant productivity, nitrogen and phosphorus acquisition, decomposition, seedling survival regulation of plant diversity (reduction and stimulation) | [51] |
| Arbuscular mycorrhiza | Glomus, Funneliformis, and Gigaspora | Carbon cycle plant productivity, nitrogen acquisition, plant phosphorus uptake, seedling survival | [151] | |
| Ericoid mycorrhiza | Rhizoscyphus ericae | [152] | ||
| Orchid mycorrhiza | Armillaria mellea, Epulorrhiza, and Rhizoctonia solani | Carbon cycle plant productivity, Plant nitrogen phosphorus uptake acquisition, seedling survival | [153] | |
| PGPF | Aspergillus, and Penicillium spp. | Helps in phosphate mobilization | [154] | |
| Endophytes | Colletotrichum tropicale | Increase plant immunity | [155] | |
| Antagonists | Trichoderma sp., and Gliocladium | Production of antibiotics, phytotoxins and lytic enzymes | [156] |
| Type of Nutrient | Microbes | Function | References |
|---|---|---|---|
| Nitrogen | Allorhizobium, Rhizobium, Frankia, Azorhizobium, Mesorhizobium, Sinorhizobium, Bradyrhizobium, Azoarcus, Achromobacter, Burkholderia, and Herbaspirillum, Sinorhizobium, Neorhizobium, and Pararhizobium | Symbiotic nitrogen fixation (conversion of nitrogen into ammonia) | [41,322,323,324] |
| Azotobacter and Azospirillum | Non-symbiont nitrogen fixation and can also solubilize phytate | ||
| Phosphate | Bacillus, Rhizobium, and Pseudomonas aeruginosa | Predominantly manufacturs acid phosphatases that aid in the mineralization of organic phosphorus in soil, phosphate-solubilizing bacteria play a crucial role in the solubilization of insoluble forms of phosphorus | [312] |
| Achromobacter, Flavobacterium, Agrobacterium, Mycobacterium, Gluconacetobacter, Bacillus, Enterobacter, Erwinia, Pseudomonas, and Serratia | [325] | ||
| Siderophores | Chryseobacterium spp., Pseudomonas sp., Serratia marcescens, and Streptomyces sp. | It binds to iron, phytopathogens are prevented from growing on the host plant. The iron is subsequently released from the siderophore either by reduction to the ferrous state (Fe2+) or by cleavage of the siderophore molecule once the now-soluble iron-siderophore complex has been bound and imported by receptor molecules on the surfaces of bacteria or plants | [326,327,328] |
| Stress Type | Microorganisms | Plant | Effect | References |
|---|---|---|---|---|
| Salt stress | Enterobacter sp. P23 | Rice seed | IAA-production | [344] |
| Ensifer meliloti | Medicago truncatula | Improved IAA production | [345] | |
| Azospirillum brasilense NH | Durum wheat | Enhanced the IAA production | [346] | |
| Acinetobacter calcoaceticus SE370, Promicromonospora sp. SE188, and Burkholderia cepacia SE4 | Cucumber | Increase proteins, reduced sugars, antioxidant enzymes and ribonuclease production | [347] | |
| Dietzia natronolimnaea STR1 and Bacillus amyloliquefaciens RWL-1 | Wheat and rice | Changes ABA signaling and auxin cascades | [348,349,350,351] | |
| Pseuodomonas putida, Pseuodomonas stutzeri, and Stenotrophomonas maltophilia | Coleus | Enhances production of gibberellins and IAA | [352] | |
| Pseudomonas extremorientalis TSAU20 | Tomato | Enhances level of proline | [353] | |
| Bacillus subtilis | Acacia gerrardii | Increases proline levels and sustain balance of water in plant tissues | [354] | |
| Bacillus subtilis | Arabidopsis | Reduce the activity of high-affinity potassium ion transporters (HKT1) to avoid excessive Na+ ion absorption by plant tissues and to keep ions in balance | [355] | |
| Pantoea agglomerans strain KL | Rice | Increases length roots and shoots, increases fresh and dry weight, chlorophyll content, and reduces proline and melondialdehyde (MDA); It also improves ACC deaminase activity and increases the synthesis of ammonia, phosphate, and exopolysaccharide | [356] | |
| Chryseobacterium gleum sp. SUK | Wheat | By increasing ACC deaminase activity, IAA, siderophore, ammonia, and hydrogen cyanide production, the root and shoot length, fresh and dry weight, chlorophyll, proteins, amino acids, phenolics, flavonoids, and potassium ion content improved | [20] | |
| Enterobacter cloacae ZNP-3 | Wheat | By promoting the formation of ACC deaminase, phosphate solubilization, IAA, and HCN (hydrogen cyanide), the root and shoot length, fresh and dry weight, chlorophyll content, and potassium ion uptake are all increased. MDA, sodium ion uptake, H2O2 and O2 are decreased | [357] | |
| Enterobacter cloacae, and Paenibacillus xylanexedens | Canola | By increasing ACC deaminase activity and IAA production, root length increase | [358] | |
| Bacillus cereus Pb25 | Mung beans | Increase in fresh and dry weight of root, shoot and nodule, potassium, chlorophyll content, phosphorous, nitrogen level, enhance ACC deaminase activity, phosphate solubilisation, IAA and siderophore production | [359] | |
| Bacillus megaterium, Pseudomonas fluorescens, and Variovorax paradoxus | Cucumber | [360] | ||
| Pseudomonas fluorescens 002 | Maize | [361] | ||
| Serratia sp. SL-12 | Wheat | [362] | ||
| Streptomyces venezuelae ATCC 10712 | Rice | Reduction in Na+ ion contents, ethylene and accumulation of proline, relative water content, chlorophyll content, malondialdehyde and K+ ions | [363] | |
| Rahnella aquatilis HX2 | Maize | ACC-deaminase production | [364] | |
| Bradyrhizobium japonicum (KY940048) | Greengram | Production of 2,3-dihydroxybenzoic acid (2,3-DHBA) and ACC deaminase | [365] | |
| Pseudomonas frederiksbergensis OB139, and Pseudomonas vancouverensis OB155 | Red pepper | Decrease in ethylene and increased the plant growth | [366] | |
| Enterobacter aerogenes LJL-5, and Pseudomonas aeruginosa LJL-13 | Alfalfa | Phosphate solubilisation, ACC deaminase and siderophore production, plant biomass increase | [367] | |
| Methylobacterium oryzae CBMB20 | Rice | Improved photosynthesis and ACC deaminase production | [368] | |
| Nostoc flagelliforme | Arabidopsis | Enhancement in seed germination and shoot growth of transgenic plants | [369] | |
| Curtobacterium albidum strain SRV4 | Rice | Production of IAA, hydrogen cyanide (HCN) and nitrogen (N2) fixation, Increase in plant height, carotenoid content, antioxidant enzyme activity, dry weight, chlorophyll content, potassium ion uptake and decrease in sodium ion uptake | [370] | |
| Pseudomonas sp. strain AK-1 | Soybean | Upregulation of different genes in shoots and down-regulation in roots | [371] | |
| Bacillus licheniformis SA03 | Chrysanthemum | Help in mediation of ABA levels in plants | [372] | |
| Trichoderma harzianum | Cucumber | Increased ROS scavenging activity and stabilized osmotic stress | [373] | |
| Bacillus spp., Alcaligenes spp., Proteus spp., and Aneurinibacillus aneurinilyticus | Chili | Significant increase in root and shoot length | [374] | |
| Enterobacter sp. EN-21 | Sugarcane | Plant length, fresh and dry weight, chlorophyll content, potassium ion absorption, and proline level increases | [375] | |
| Enterobacter sp. MN17, and Bacillus sp. MN54 | Chenopodium quinoa | Improve plant water condition | [376] | |
| Novosphingobium sp. HR1a and Pseudomonas putida KT2440 | Citrus macrophylla | Decreased stomatal conductance and transpiration rate in plants with significant decrease in the levels of abscisic acid (ABA), salicylic acid (SA) and increase in growth of plant | [377] | |
| Acinetobacter bereziniae IG2, Alcaligenes faecalis IG27, and Enterobacter ludwigii IG10 | Pisum sativum | Lower the levels of electrolyte leakage and H2O2 contents, improvement in chlorophyll, proline content and total soluble sugar | [378] | |
| Pseudomonas oryzihabitans AXSa06 | Solanum lycopersicum | Regulation of plant growth, activate antioxidant metabolism | [379] | |
| Stenotrophomonas maltophilia BJ01 | Arachis hypogaea | Stimulated the growth and development, auxin production, lowers the levels of electrolyte leakage, lipid peroxidation, proline contents, total amino acids were enhanced | [380] | |
| Brevibacterium iodinum KNUC7183, Microbacterium oleivorans KNUC7074, and Rhizobium massiliae KNUC7586 | Pepper | Increased in height of plant, dry and fresh weight, chlorophyll content, total sugar and proline contents | [381] | |
| Bacillus subtilis NRCB003 | Medicago sativa | Enhance ACC deaminase activity | [382] | |
| Drought stress | PGPB strain and Pseudomonas simiae AU | Soybean | Total soluble sugar concentration and gene expression associated with induced proline | [383] |
| Enterobacter sp. FD17 and Burkholderia phytofirmans PsJN | Maize | Improved plant development by modifying photosynthetic activity and glucose metabolism | [384] | |
| Agrobacterium fabrum and Bacillus amyloliquifaciens | Wheat | Production of ACC deaminase resulting in increased grain yield and biomass | [385] | |
| Leclercia decarboxylata and Agrobacterium fabrum | Wheat | Increase nutrients uptake and chlorophyll contents | [386] | |
| Ochrobactrum pseudogrignonens eRJ12, Pseudomonas sp. RJ15, and Bacillus subtilis RJ46 | Pea | Lowers ACC accumulation | [387] | |
| Pseudomonas fluorescens, Enterobacter hormaechei, and Pseudomonas migulae | Foxtail millet | Improvement in seedling growth and seed germination | [388] | |
| Psuedomonas flourescens DPB15 and Pseudomonas palleroniana DPB16 | Wheat | Enhancement in root and shoot growth | [389] | |
| Pseudomonas aeruginosa PM389, Pseudomonas aeruginosa ZNP1, Bacillus endophyticus J13, and B. tequilensis J12 | Arabidopsis thaliana | Increased phytohormones production | [390] | |
| Streptomyces pactum | Wheat | Accumulation of ABA and upregulation of drought resistant genes | [391] | |
| Azospirillum lipoferum | Juglans regia | Increase in secondary metabolites and activity of peroxidase enzyme which helps in plant growth | [392] | |
| Azotobacter chroococcum | Mentha pulegium | Stimulate biosynthesis of secondary metabolites | [393] | |
| Bacillus amyloliquefaciens | Mentha piperita | Decrease in the membrane lipid peroxidation, higher total phenolic content and enzymatic activities | [394] | |
| Bacillus amyloliquefaciens and Moraxella pluranimalium | Triticum aestivum | Modulation of auxin | [395] | |
| Bacillus cereus | Glycine max | Increase in the stomatal conductance | [396] | |
| Bacillus cereus and Myroides odoratimimus | Sorghum bicolor | Regulation of gene expression, production of IAA and Phosphate solubilization | [397] | |
| Bacillus megaterium | Solanum lycopersicum | Decrease in the expression of genes related to ethylene | [398] | |
| Bacillus megaterium | Cicer arietinum | Modulation of phytohormones | [399] | |
| Bacillus pumilus | Glycyrrhiza uralensis | Protection of chloroplast, increase in chlorophyll content, improvement in water state | [400] | |
| Bacillus sp. | Mucuna pruriens | Regulation of aminocyclopropane-1-carboxylate (ACC) deaminase | [401] | |
| Bacillus sp. | Triticum aestivum, Zea mays | Regulation of salicylic acid and IAA acid, alterations in root system architecture | [402] | |
| Bacillus sp. ESA 402 | Sorghum bicolor | Nitrogen accumulation | [403] | |
| Bacillus spp. | Megathyrsus maximus | Reduced glutathione reductase activity, increased proline accumulation | [404] | |
| Bacillus subtilis Paenibacillus illinoisensis | Capsicuum annuum | Enhancement in the expression and activity of vacuolar H+-pumping pyrophosphatase | [405] | |
| Bacillus subtilis | Cicer arietinum | Regulation of phytohormones production | [406] | |
| Bacillus velezensis | Triticum aestivum | Reprogramming of metabolites | [407] | |
| Bradyrhizobium japonicum | Glycine max | Improvement in root nodulation and reduction in pod abortion rate | [408] | |
| Curtobacterium herbarum | Lactuca sativa | Reduction in lipid peroxidation and oxidative stress | [409] | |
| Gluconacetobacter diazotrophicus | Oryza sativa | Positive regulation of defence genes, increased plant biomass | [410] | |
| Variovorax sp. YNA59 | Brassica oleracea | Sugar production, ABA production and tolerate oxidative stress like H2O2 | [411] | |
| Bacillus subtilis HAS31 | Solanum tuberosum | Significant increase in the growth attributes along with tuber weight and yield | [412] | |
| Arbuscular mychorrhizal fungi and PGPR | Nicotiana tabacum | Increase in chlorophyll, enhancement in phenol and flavonoids content | [413] | |
| Neotyphodium spp. | Lolium perenne | Increase in biomass, plant height and tiller numbers | [414] | |
| Heavy metal stress | Pestalotiopsis spp. | Nypa fruticans | Enhancement in heavy metals tolerance including zinc (Zn), copper (Cu), lead (Pb) and chromium (Cr) | [415] |
| Exophiala pisciphila | Maize | Provide tolerance against soil cadmium (Cd) toxicity | [416] | |
| Penicillium funiculosum LHL06 | Soybean | Secretion of gibberellin | [417] | |
| Enterobacter spp. and Kocuria spp. | Solanum nigrum | Enhanced phytoremediation arsenic (As) tolerance | [418] | |
| Glomusmosseae, Rhizobium trifolii, and Brevibacillus brevis | Clover | Increase in plant biomass and P and N contents under nickel stress | [419] | |
| Pseudomonas aeruginosa ZN3 | Wheat | Improvement in nutrient uptake, increase in leaf chlorophyll and total soluble protein under Zn stress condition | [420] | |
| Azotobacter chroococcum and Bacillus megaterium | Maize and Sunflower | Increase nutrient uptake in Cd stress condition | [421] | |
| Bacillus subtilis FBL-10 | Solanum melongena | Activation of antioxidant system in plants and decrease the level of MDA and peroxide under Pb toxicity | [422] | |
| Bacillus subtilis STU-6 | Tomato | Enhanced polyamine mediated iron remobilization under Fe (iron) deficiency | [423] | |
| Bacillus subtilis MF497446 | Cow pea | Produce phytohormones and siderophores to induce plant growth under Cd stress | [424,425] | |
| Bacillus subtilis KP717559 | Brassica juncea | Enhance phytoextraction by plants while producing phytohormones, HCN for plant growth under Ni, Cd and Cr stress | [426] | |
| Bacillus subtilis | Chick pea | Higher proline content and decrease in lipid peroxidation under Ni, Pb, Cd stress conditions | [427] | |
| Bacillus subtilis PBRB3 | Mung bean | Increase in antioxidative enzyme activity in lead toxicity | [428] | |
| Thermal stress | Curvularia protuberate | Dichanthelium lanuginosum | Increase tolerance at high soil temperatures | [429] |
| Bacillus subtilis | Wheat | Enhanced activity of proline and antioxidants | [430] | |
| Bacillus tequilensis SSB07 | Glycine max | Production of phytohormones, increase in length of shoot, photosynthetic pigments, leaf development and endogenous jasmonic acid, salicylic acid content | [431] | |
| Thermomyces lanuginosus | Cullen plicata | Change in the concentration of different antioxidants | [432] | |
| Water logging stress | Trichoderma asperellum MAP1 | Triticum aestivum | Improvement in morphological attributes of seedlings | [433] |
| Nutrient deprivation | Paenibacillus lentimorbus B-30488 (B-30488), Bacillus amyloliquefaciens SN13 (SN13) | Oryza sativa | Enhanced the growth of seedlings; improved concentrations, uptake, and distribution nutrient elements | [434,435] |
| Oxidative and Nitrosative stress | Bacillus aryabhattai SRB02 | Soyabean | Enhanced the levels of IAA, jasmonic acid (JA), GA12, GA4, and GA7, resulting in longer roots and shoots | [436] |
| Cold stress | Pseudomonas frederiksbergensis OS211, Flavobacterium glaciei OB146, Pseudomonas vancouverensis OB155, and Pseudomonas frederiksbergensis OS261 | Solanum lycopersicum Mill. | Reduction in membrane damage and activation of antioxidant enzymes and proline synthesis in the leaves | [437] |
| Trichoderma harzianum | Solanum lycopersicum Mill. | Reduction in the rate of the lipid peroxidation and electrolyte leakage and enhancement in leaf water content and proline accumulation. | [438] | |
| Sphingomonas faeni | Finger millet and foxtail millet | Improved root and shoot growth, as well as increased biomass; enhanced antioxidant activity | [439,440] | |
| Bacillus spp. CJCL2, RJGP41 and Bacillus velezensis FZB42 | Wheat | Regulation of abscisic acid, lipid peroxidation and proline accumulation pathways in a beneficial manner, positive regulation of phytohormones expression leading to improved plant growth | [441] | |
| Brevibacterium halotolerans (Sd-6), Bacillus subtilis (Ldr-2), Achromobacter xylosoxidans (Fd-2) and Burkholderia cepacia (Art-7), Trichoderma harzianum (Th) | Ocimum sanctum | Improved photosynthetic efficiency, enhanced fresh weight, improved nutrient uptake, increased accumulation of proline, starch, total phenolics, and reduced accumulation of ACC | [442] | |
| Kosakonia sp. CIR2 and Staphylococcus sp. CSR1T2 | Rice | Higher survival rates, increased fertiility, improvement in grain length, seed weight per plant, and seed yield | [443] | |
| Salt, drought, and cold stress | Rhizophagus irregularis SA, Rhizophagus clarus BEG142, Glomus lamellosum ON393, and Funneliformis mosseae BEG95 | Arachis hypogaea L. | Higher plant growth, leaf relative water content, net photosynthetic rate, photosystem II efficiency, antioxidant enzymes activities, and K+: Na+ ratio while lower leaf relative electrolyte conductivity, malondialdehyde concentration, and the accumulation of reactive oxygen species, reduction in the damage to thylakoids and mitochondria | [444] |
| Flooding stress | Streptomyces sp. GMKU 336 | Vigna radiata (L.) Wilczek | Enhanced plant growth and biomass, chlorophyll content, leaf area, leaf color, stimulated adventitious roots formation, and reduced ethylene levels | [445] |
| Microbacterium sp. (AR-ACC2), Methylophaga sp. (AR-ACC3), and Paenibacillus sp. (ANR-ACC3) | Rice | Enhanced germination, seedling vigor index, root and shoot length and total chlorophyll contents, reduced ethylene production | [446] | |
| Klebsiella variicola AY13 | Glycine max | Improvement in the plant growth, chlorophyll content, and the quantum efficiency of chlorophyll fluorescence | [446,447] | |
| Heat stress | Bacillus cereus SA1 | Soybean | Improved the biomass, chlorophyll content and fluorescence, increased the ascorbic acid peroxidase, superoxide dismutase, and glutathione contents, enhanced potassium gradients | [448] |
| Pseudomonas composti SDT3, Aeromonas aquariorum SDT13, Bacillus methylotrophicus SMT38, Bacillus aryabhattai SMT48, Bacillus zhangzhouensis HPJ40, and Pseudarthrobacter oxydans SRT15 | Vitis vinifera | Enhanced photoprotection capability and higher thermo-stability, exhibiting a significantly lower dissipation energy flux than the non-inoculated plants, Improved antioxidant mechanisms and membrane stability | [449] | |
| Bacillus cereus | Solanum lycopersicum | Promoted shoot, root length, leaf surface area, fresh and dry weight | [450] | |
| Paraburkholderia phytofirmans strain PsJN | Lycopersicon esculentum Mill. | Enhanced growth parameters, such as increased chlorophyll content and improved gas exchange, at both Normal (25 °C) and elevated (32 °C) temperatures | [451] | |
| Drought and heat stresses | Streptomyces sp. RA04, and Nocardiopsis sp. RA07 | Sorghum-bicolor | Increased growth and photosynthetic pigments, reduced reduced oxidative damage, enhanced cadmium accumulation | [452] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil Microbiome: Diversity, Benefits and Interactions with Plants. Sustainability 2023, 15, 14643. https://doi.org/10.3390/su151914643
Chauhan P, Sharma N, Tapwal A, Kumar A, Verma GS, Meena M, Seth CS, Swapnil P. Soil Microbiome: Diversity, Benefits and Interactions with Plants. Sustainability. 2023; 15(19):14643. https://doi.org/10.3390/su151914643
Chicago/Turabian StyleChauhan, Poonam, Neha Sharma, Ashwani Tapwal, Ajay Kumar, Gaurav Swaroop Verma, Mukesh Meena, Chandra Shekhar Seth, and Prashant Swapnil. 2023. "Soil Microbiome: Diversity, Benefits and Interactions with Plants" Sustainability 15, no. 19: 14643. https://doi.org/10.3390/su151914643








