Persistence of Hg-Contaminated Soil Stabilization in Typical Areas of Dehua County, Fujian Province, China
Abstract
:1. Introduction
2. Materials and Methods
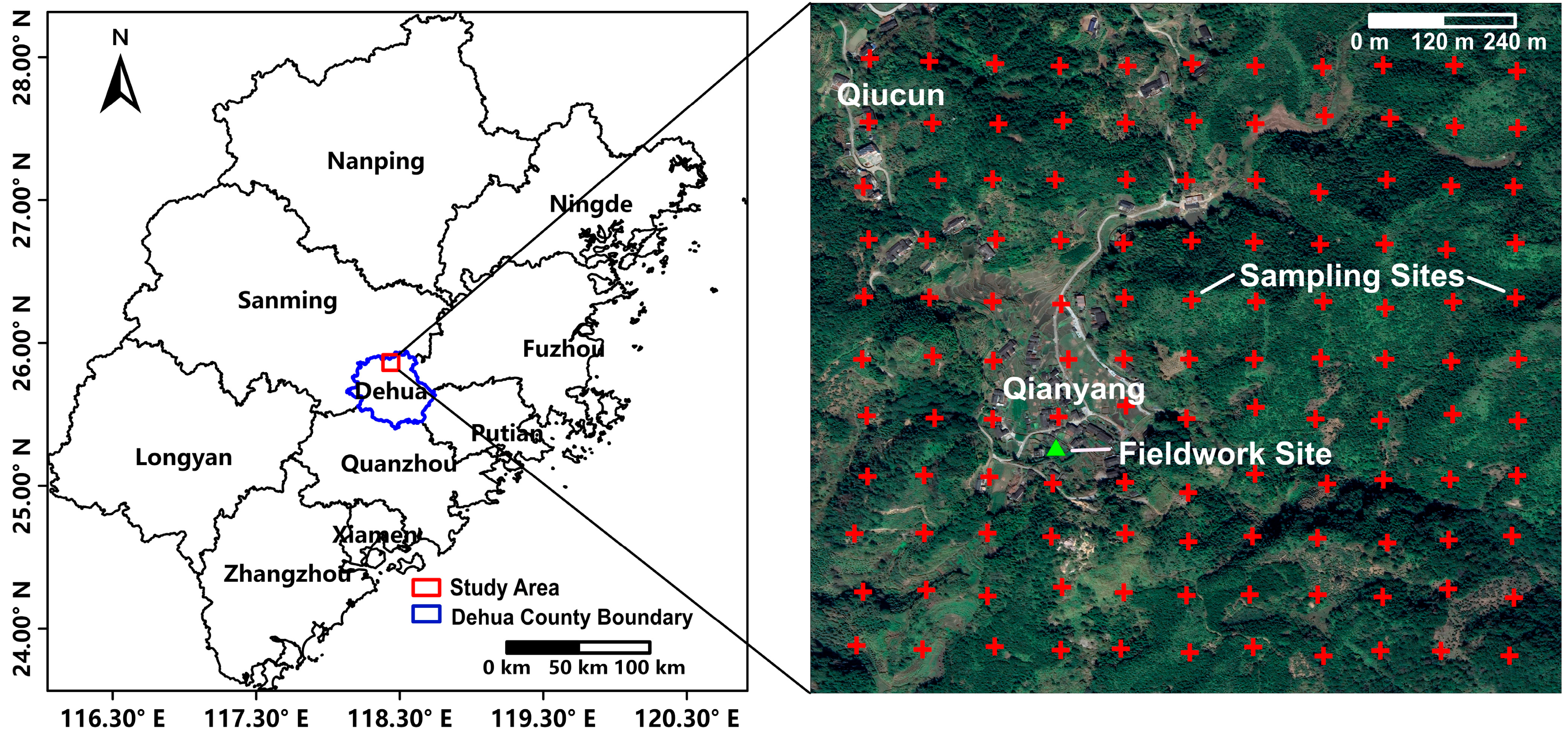
2.1. The Study Area
2.2. Chemicals
2.3. Experimental Design and Processing
2.3.1. Pre-Test
Soil
Amendments
Stabilization Orthogonal Experiment
2.3.2. Pot Experiment
2.3.3. Field Plot Experiment
2.3.4. Sample Pretreatment and Analysis Methods
2.3.5. Assessment of the Stabilization Effect
2.3.6. Data Processing
3. Results
3.1. Results of the Stabilization Orthogonal Experiment
3.2. Results of the Pot Test
3.3. Results of the Field Plot Experiment
3.3.1. Variation in the Available Hg Contents in the Soil
3.3.2. Soil Hg Form Changes
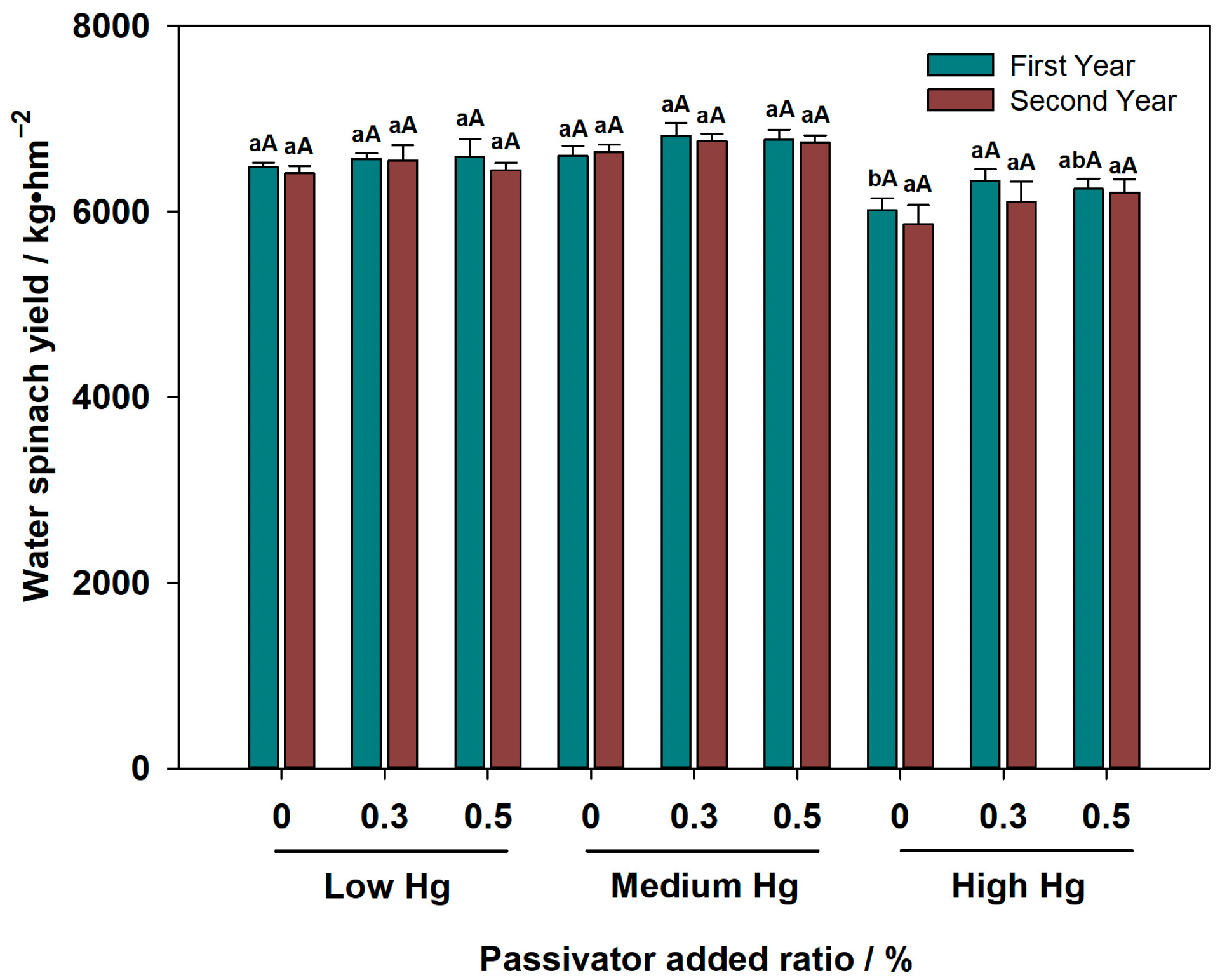
3.3.3. Hg content in the Water Spinach and the Water Spinach Yield
3.3.4. Changes in the Enzyme Activity in the Soil
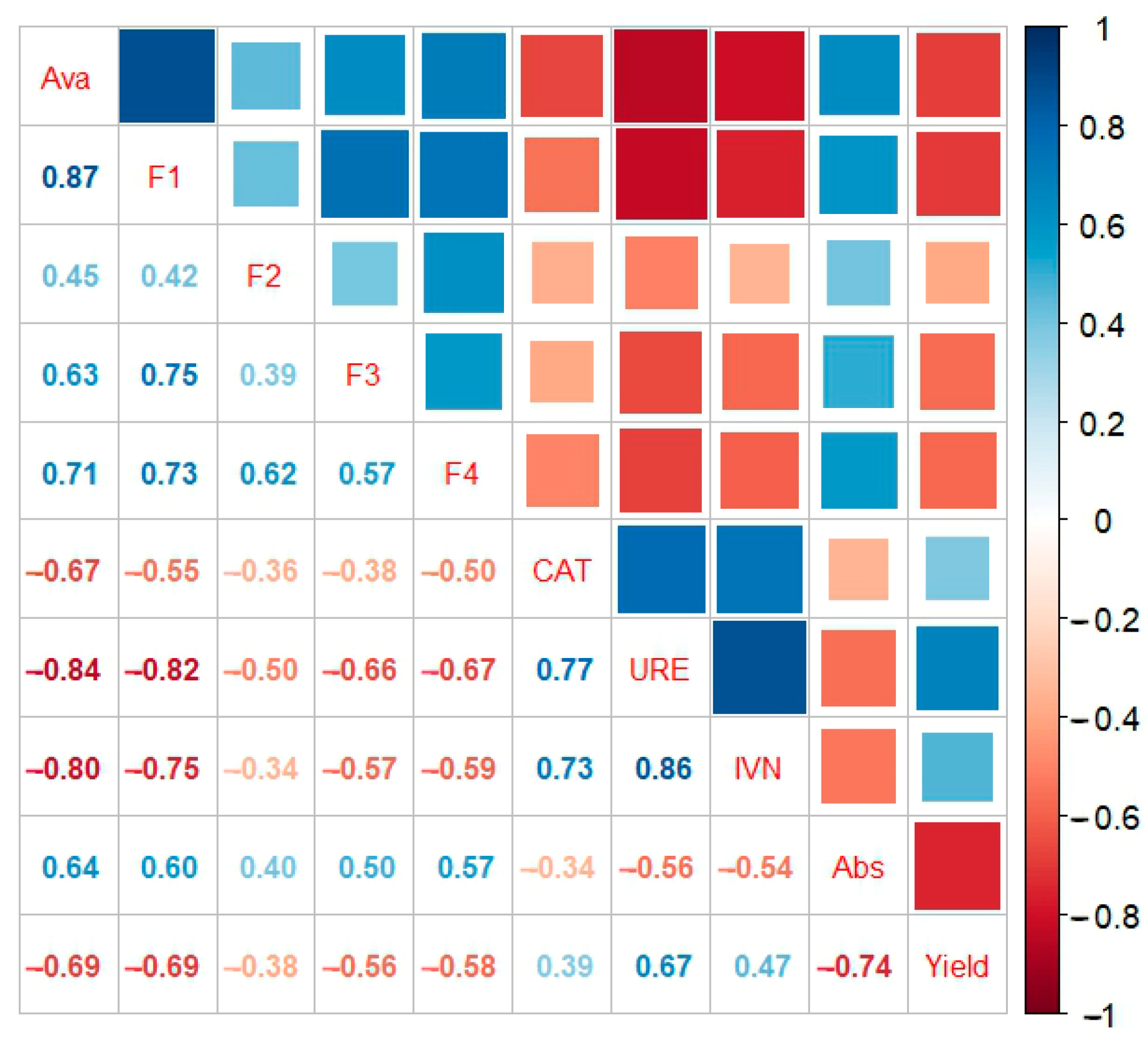
3.3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, G.L.; Feng, X.B.; Wang, S.F.; Shang, L.H. Environmental contamination of mercury from Hg-mining areas in Wuchuan, northeastern Guizhou, China. Environ. Pollut. 2006, 142, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Ju, Y.R.; Lim, Y.C.; Chen, C.W.; Wu, C.H.; Lin, Y.L.; Dong, C.D. Dry and wet seasonal variation of total mercury, inorganic mercury, and methylmercury formation in estuary and harbor sediments. J. Environ. Manag. 2020, 253, 109683. [Google Scholar] [CrossRef] [PubMed]
- Acquavita, A.; Floreani, F.; Covelli, S. Occurrence and speciation of arsenic and mercury in alluvial and coastal sediments. Curr. Opin. Environ. Sci. Health 2021, 22, 100272. [Google Scholar] [CrossRef]
- Uc-Peraza, R.G.; Gutiérrez-Galindo, E.A.; Delgado-Blas, V.H.; Munoz-Barbosa, A. Total mercury content in the California ribbed sea mussel Mytilus californianus from the west coast of Baja California, México: Levels of contamination and human health risk. Mar. Pollut. Bull. 2021, 170, 112585. [Google Scholar] [CrossRef] [PubMed]
- Windham-Myers, L.W.; Marvin-DiPasquale, M.; Kakouros, E.; Agee, J.L.; Kieu, L.H.; Stricker, C.A.; Fleck, J.A.; Ackerman, J.T. Mercury cycling in agricultural and managed wetlands of California, USA: Seasonal influences of vegetation on mercury methylation, storage, and transport. Sci. Total Environ. 2014, 484, 308–318. [Google Scholar] [CrossRef]
- Elumalai, V.; Sujitha, S.B.; Jonathan, M.P. Mercury pollution on tourist beaches in Durban, South Africa: A chemometric analysis of exposure and human health. Mar. Pollut. Bull. 2022, 180, 113742. [Google Scholar] [CrossRef]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Phar. 2005, 20, 351–360. [Google Scholar] [CrossRef]
- Sun, G.Y.; Feng, X.B.; Yang, C.M.; Zhang, L.M.; Yin, R.S.; Li, Z.G.; Bi, X.Y.; Wu, Y.J. Levels, sources, isotope signatures, and health risks of mercury in street dust across China. J. Hazard. Mater. 2020, 392, 122276. [Google Scholar] [CrossRef]
- Jung, S.; Kwon, S.Y.; Hong, Y.; Yin, R.; Motta, L.C. Isotope investigation of mercury sources in a creek impacted by multiple anthropogenic activities. Chemosphere 2021, 282, 130947. [Google Scholar] [CrossRef]
- Martinez, G.; Restrepo-Baena, O.J.; Veiga, M.M. The myth of gravity concentration to eliminate mercury use in artisanal gold mining. Extract. Ind. Soc. 2021, 8, 477–485. [Google Scholar] [CrossRef]
- Cordy, P.; Veiga, M.M.; Salih, I.; Al-Saadi, S.; Console, S.; Garcia, O.; Mesa, L.A.; Velásquez-López, P.C.; Roeser, M. Mercury contamination from artisanal gold mining in Antioquia, Colombia: The world’s highest per capita mercury pollution. Sci. Total Environ. 2011, 410–411, 154–160. [Google Scholar] [CrossRef]
- Yevugah, L.L.; Darko, G.; Bak, J. Does mercury emission from small-scale gold mining cause widespread soil pollution in Ghana? Environ. Pollut. 2021, 284, 116945. [Google Scholar] [CrossRef]
- Gyamfi, O.; Sørensen, P.B.; Darko, G.; Ansah, E.; Vorkamp, K.; Bak, J.L. Contamination, exposure and risk assessment of mercury in the soils of an artisanal gold mining community in Ghana. Chemosphere 2021, 267, 128910. [Google Scholar] [CrossRef]
- Eckley, C.S.; Gilmour, C.C.; Janssen, S.; Luxton, T.P.; Randall, P.M.; Whalin, L.; Austin, C. The assessment and remediation of mercury contaminated sites: A review of current approaches. Sci. Total Environ. 2020, 707, 136031. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.S.; Muñawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2021, 287, 132369. [Google Scholar] [CrossRef]
- Xu, D.M.; Fu, R.B.; Wang, J.X.; Shi, Y.X.; Guo, X.P. Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods—A critical review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Makarova, A.S.; Yarovaya, O.V.; Fedoseev, A.N.; Yakubovich, L.M. Development of a technology for immobilizing mercury in solid mercury-containing wastes. Clean. Eng. Technol. 2020, 1, 100030. [Google Scholar] [CrossRef]
- Aghaei, E.; Alorro, R.D.; Tadesse, B.; Browner, R. A review on current practices and emerging technologies for sustainable management, sequestration and stabilization of mercury from gold processing streams. J. Environ. Manag. 2019, 249, 109367. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhu, Y.; Cheng, L.R.; Andserson, B.; Zhao, X.H.; Wang, D.Y.; Ding, A.Z. Review on utilization of biochar for metal-contaminated soil and sediment remediation. J. Environ. Sci. 2018, 63, 156–173. [Google Scholar] [CrossRef]
- Xie, X.F.; Zhang, Z.H.; Chen, Z.K.; Wu, J.Y.; Li, Z.L.; Zhong, S.P.; Liu, H.; Xu, Z.F.; Liu, Z.L. In-situ preparation of zinc sulfide adsorbent using local materials for elemental mercury immobilization and recovery from zinc smelting flue gas. Chem. Eng. J. 2022, 429, 132115. [Google Scholar] [CrossRef]
- Donatello, S.; Fernández-Jiménez, A.; Palomo, A. An assessment of mercury immobilisation in alkali activated fly ash (AAFA) cements. J. Haz. Mat. 2012, 213–214, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; He, F.; Zhao, D.Y.; Barnett, M.O. Immobilization of mercury in sediment using stabilized iron sulfide nanoparticles. Water Res. 2009, 43, 5171–5179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Chen, H.Q.; Zhu, N.L.; Zhang, J.; Li, Y.F.; Xu, D.D.; Gao, Y.X.; Zhao, J.T. Detection and remediation of mercury contaminated environment by nanotechnology: Progress and challenges. Environ. Pollut. 2022, 293, 118557. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-S.; Kim, S.-H.; Chon, C.-M.; Kwon, S.; Kim, J.G.; Choi, H.W.; Ahn, J.S. Effect of FeS on mercury behavior in mercury-contaminated stream sediment: A case study of Pohang Gumu Creek in South Korea. J. Hazard. Mater. 2020, 393, 122373. [Google Scholar] [CrossRef] [PubMed]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.R.; Yin, D.L.; Han, Y.X.; Zhou, X.; Zhang, G.; Tian, X. Modified clay mineral: A method for the remediation of the mercury-polluted paddy soil. Ecotoxicol. Environ. Saf. 2020, 204, 111121. [Google Scholar] [CrossRef]
- Wang, Z.X.; Zhang, Z.J.; Xia, L.; Farías, M.E.; Sánchez, R.M.T.; Belfiore, C.; Montes, M.L.; Tian, X.; Chen, J.H.; Song, S.X. Sulfate induced surface modification of Chlorella for enhanced mercury immobilization. J. Environ. Chem. Eng. 2022, 10, 108156. [Google Scholar] [CrossRef]
- Huang, P.C.; Yang, W.C.; Johnson, V.E.; Si, M.Y.; Zhao, F.P.; Liao, Q.; Su, C.Q.; Yang, Z.H. Selenium-sulfur functionalized biochar as amendment for mercury-contaminated soil: High effective immobilization and inhibition of mercury re-activation. Chemosphere 2022, 306, 135552. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Shen, Z.T.; Tsang, D.C.W.; Rinklebe, J.; Hou, D.Y. Sulfur-modified biochar as a soil amendment to stabilize mercury pollution: An accelerated simulation of long-term aging effects. Environ. Pollut. 2020, 264, 114687. [Google Scholar] [CrossRef]
- Cho, J.H.; Eom, Y.; Lee, T.G. Pilot-test of the calcium sodium phosphate (CNP) process for the stabilization/solidification of various mercury-contaminated wastes. Chemosphere 2014, 117, 374–381. [Google Scholar] [CrossRef]
- Wang, J.X.; Xing, Y.; Xie, Y.Y.; Meng, Y.; Xia, J.C.; Feng, X.B. The use of calcium carbonate-enriched clay minerals and diammonium phosphate as novel immobilization agents for mercury remediation: Spectral investigations and field applications. Sci. Total Environ. 2019, 646, 1615–1623. [Google Scholar] [CrossRef]
- He, L.Z.; Meng, J.; Wang, Y.; Tang, X.J.; Liu, X.M.; Tang, C.X.; Ma, L.Q.; Xu, J.M. Attapulgite and processed oyster shell powder effectively reduce cadmium accumulation in grains of rice growing in a contaminated acidic paddy field. Ecotoxicol. Environ. Saf. 2021, 209, 111840. [Google Scholar] [CrossRef]
- Fernández-Ondoño, E.; Bacchetta, G.; Lallena, A.M.; Navarro, F.B.; Ortiz, I.; Jiménez, M.N. Use of BCR sequential extraction procedures for soils and plant metal transfer predictions in contaminated mine tailings in Sardinia. J. Geochem. Explor. 2017, 172, 133–141. [Google Scholar] [CrossRef]
- Pérez-Moreno, S.M.; Gázquez, M.J.; Pérez-López, R.; Bolivar, J.P. Validation of the BCR sequential extraction procedure for natural radionuclides. Chemosphere 2018, 198, 397–408. [Google Scholar] [CrossRef]
- Ji, Y.N.; Wang, N.; Xu, Y.M.; Wang, R.L.; Sun, Y.B. Effect of inorganic-organic compound mixtures on the immobilization remediation of Cd contaminated soil. Environ. Chem. 2017, 36, 2333–2340. [Google Scholar]
- Wang, Y.G.; Liu, J.J.; Li, Y.X.; Liang, M.A. Effective factors of urease activities in soil by using the phenol-sodium hypochlorite colorimetric method. Chinese. J. Soil Sci. 2019, 50, 1166–1170. [Google Scholar]
- Gu, Y.; Wang, P.; Kong, C.H. Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur. J. Soil Biol. 2009, 45, 436–441. [Google Scholar] [CrossRef]
- GB 2762-2017; National Food Safety Standard—Limit of Pollutants in Food. National Medical Products Administration: Beijing, China, 2017.
- Zuber, S.M.; Villamil, M.B. Meta-analysis approach to assess effect of tillage on microbial biomass and enzyme activities. Soil Biol. Biochem. 2016, 97, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Y.; Dong, W.Y.; Dai, X.Q.; Schaeffer, S.; Yang, F.T.; Radosevich, M.; Xu, L.L.; Liu, X.Y.; Sun, X.M. Response of absolute and specific soil enzyme activities to long term additions of organic and mineral fertilizer. Sci. Total Environ. 2015, 536, 59–67. [Google Scholar] [CrossRef]
- Natasha; Shahid, M.; Khalid, S.; Bibi, I.; Bundschuh, J.; Niazi, N.K.; Dumat, C. A critical review of mercury speciation, bioavailability, toxicity and detoxification in soil-plant environment: Ecotoxicology and health risk assessment. Sci. Total Environ. 2020, 711, 134749. [Google Scholar] [CrossRef]
- Khan, Z.; Fan, X.T.; Khan, M.N.; Khan, M.A.; Zhang, K.K.; Fu, Y.Q.; Shen, H. The toxicity of heavy metals and plant signaling facilitated by biochar application: Implications for stress mitigation and crop production. Chemosphere 2022, 308, 136466. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Guo, D.; Jeyasundar, P.G.S.A.; Li, Y.; Xiao, R.; Du, J.; Li, R.H.; Zhang, Z.Q. Application of wood biochar in polluted soils stabilized the toxic metals and enhanced wheat (Triticum aestivum) growth and soil enzymatic activity. Ecotoxicol. Environ. Saf. 2019, 184, 109635. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.Y.; Li, G.H.; Wang, S.X.; Duan, L.; Mulder, J.; Cornelissen, G.; Cheng, Z.L.; Yang, S.H.; Hou, D.Y. Sulfur-modified rice husk biochar: A green method for the remediation of mercury contaminated soil. Sci. Total Environ. 2018, 621, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Moreroa-Monyelo, M.; Falayi, T.; Ntuli, F.; Magwa, N. Studies towards the adsorption of sulphate ions from acid mine drainage by modified attapulgite clays. S. Afr. J. Chem. Eng. 2022, 42, 241–254. [Google Scholar] [CrossRef]
- Xu, C.B.; Qi, J.; Yang, W.J.; Chen, Y.; Yang, C.; He, Y.L.; Wang, J.; Lin, A.J. Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay. Sci. Total Environ. 2019, 686, 476–483. [Google Scholar] [CrossRef]
- Yang, J.; Gao, X.H.; Li, J.; Zuo, R.; Wang, J.S.; Song, L.T.; Wang, G.Q. The stabilization process in the remediation of vanadium-contaminated soil by attapulgite, zeolite and hydroxyapatite. Ecol. Eng. 2020, 156, 105975. [Google Scholar] [CrossRef]
- Gong, H.B.; Zhao, L.; Rui, X.; Hu, J.W.; Zhu, N.W. A review of pristine and modified biochar immobilizing typical heavy metals in soil: Applications and challenges. J. Haz. Mat. 2022, 432, 128668. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, Y.T.; Sun, X.P.; Wang, H.Y.; Teng, X.H. Corn stover biochar increased edible safety of spinach by reducing the migration of mercury from soil to spinach. Sci. Total Environ. 2021, 758, 143883. [Google Scholar] [CrossRef]
- Li, X.X.; Zhang, X.; Wang, X.L.; Cui, Z.J. Phytoremediation of multi-metal contaminated mine tailings with Solanum nigrum L. and biochar/attapulgite amendments. Ecotoxicol. Environ. Saf. 2019, 180, 517–525. [Google Scholar] [CrossRef]





| Level | Factors | |||
|---|---|---|---|---|
| A (Amendment Type) | B (Amendment Added Ratio/%) | C (Initial Concentration of the Hg in the Soil/mg·kg−1) | D (Aging Time/d) | |
| 1 | Modified biochar | 0.5 | 3.85 | 30 |
| 2 | Modified Attapulgite | 1 | 8.97 | 60 |
| 3 | Biochar + Attapulgite | 2 | 18.4 | 90 |
| 4 | Modified biochar + Modified attapulgite | 3 | 28.7 | 120 |
| Test Number | Factors | Test Group | ||||
|---|---|---|---|---|---|---|
| A (Amendment Type) | B (Amendment Added Ratio/%) | C (Initial Concentration of the Hg in the Soil/mg·kg−1) | D (Aging Time/d) | E (Null Columns) | ||
| T1 | 1 | 1 | 1 | 1 | 1 | A1B1C1D1 |
| T2 | 1 | 2 | 2 | 2 | 2 | A1B2C2D2 |
| T3 | 1 | 3 | 3 | 3 | 3 | A1B3C3D3 |
| T4 | 1 | 4 | 4 | 4 | 4 | A1B4C4D4 |
| T5 | 2 | 1 | 2 | 3 | 4 | A2B1C2D3 |
| T6 | 2 | 2 | 1 | 4 | 3 | A2B2C1D4 |
| T7 | 2 | 3 | 4 | 1 | 2 | A2B3C4D1 |
| T8 | 2 | 4 | 3 | 2 | 1 | A2B2C1D3 |
| T9 | 3 | 1 | 3 | 4 | 2 | A3B1C3D4 |
| T10 | 3 | 2 | 4 | 3 | 1 | A3B2C4D3 |
| T11 | 3 | 3 | 1 | 2 | 4 | A3B3C1D2 |
| T12 | 3 | 4 | 2 | 1 | 3 | A3B4C2D1 |
| T13 | 4 | 1 | 4 | 2 | 3 | A4B1C4D2 |
| T14 | 4 | 2 | 3 | 1 | 4 | A4B2C3D1 |
| T15 | 4 | 3 | 2 | 4 | 1 | A4B3C2D4 |
| T16 | 4 | 4 | 1 | 3 | 2 | A4B4C1D3 |
| Test Number | Factor | SE1/% | ||||
|---|---|---|---|---|---|---|
| A (Amendment Type) | B (Amendment Added Ratio/%) | C (Initial Concentration of the Hg in the Soil/mg·kg−1) | D (Aging Time/d) | E (Null Columns) | ||
| T1 | 1 | 1 | 1 | 1 | 1 | 30.7 ± 1.61 |
| T2 | 1 | 2 | 2 | 2 | 2 | 34.8 ± 1.93 |
| T3 | 1 | 3 | 3 | 3 | 3 | 27.6 ± 2.62 |
| T4 | 1 | 4 | 4 | 4 | 4 | 20.5 ± 2.52 |
| T5 | 2 | 1 | 2 | 3 | 4 | 42.5 ± 3.25 |
| T6 | 2 | 2 | 1 | 4 | 3 | 33.2 ± 2.40 |
| T7 | 2 | 3 | 4 | 1 | 2 | 24.3 ± 2.26 |
| T8 | 2 | 4 | 3 | 2 | 1 | 24.2 ± 0.95 |
| T9 | 3 | 1 | 3 | 4 | 2 | 35.7 ± 1.61 |
| T10 | 3 | 2 | 4 | 3 | 1 | 29.1 ± 2.07 |
| T11 | 3 | 3 | 1 | 2 | 4 | 26.3 ± 1.45 |
| T12 | 3 | 4 | 2 | 1 | 3 | 29.7 ± 2.70 |
| T13 | 4 | 1 | 4 | 2 | 3 | 32.5 ± 1.92 |
| T14 | 4 | 2 | 3 | 1 | 4 | 33.4 ± 1.57 |
| T15 | 4 | 3 | 2 | 4 | 1 | 37.6 ± 2.46 |
| T16 | 4 | 4 | 1 | 3 | 2 | 35.5 ± 3.06 |
| k1 | 28.4 | 35.4 | 31.4 | 29.5 | 30.4 | |
| k2 | 31.1 | 32.6 | 36.2 | 29.4 | 32.6 | |
| k3 | 30.2 | 29.0 | 30.2 | 33.7 | 30.8 | |
| k4 | 34.8 | 27.5 | 26.6 | 31.2 | 30.7 | |
| R | 6.35 | 7.88 | 9.55 | 4.23 | 2.18 | |
| Order of factors | C > B > A > D | |||||
| Superior level | A4 | B1 | C2 | D3 | ||
| Factor | Deviation Sum of Squares | Degree of Freedom | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| A | 85.700 | 3 | 28.567 | 7.217 | 0.069 | * |
| B | 152.605 | 3 | 50.868 | 12.851 | 0.032 | ** |
| C | 186.495 | 3 | 62.165 | 15.705 | 0.024 | ** |
| D | 49.025 | 3 | 16.342 | 4.128 | 0.137 | |
| Error | 11.875 | 3 | 3.958 |
| Initial Concentration of Hg in Soil/mg·kg−1 | Amendment Added Ratio/% | CAT/mL·g−1 | URE/mg·g−1 | INV/mg·g−1 | |||
|---|---|---|---|---|---|---|---|
| First Year | Second Year | First Year | Second Year | First Year | Second Year | ||
| L | CK | 1.24 ± 0.10 bA | 1.20 ± 0.07 bA | 1.34 ± 0.05 bA | 1.21 ± 0.21 bA | 7.87 ± 1.56 bA | 7.54 ± 1.23 bA |
| 0.3 | 1.74 ± 0.23 aA | 1.54 ± 0.14 aA | 1.91 ± 0.08 aA | 1.61 ± 0.14 aA | 11.4 ± 0.96 aA | 9.92 ± 1.22 aA | |
| 0.5 | 1.63 ± 0.15 aA | 1.37 ± 0.14 abA | 1.87 ± 0.09 aA | 1.47 ± 0.10 abA | 10.7 ± 0.56 aA | 9.43 ± 0.81 abA | |
| M | CK | 1.08 ± 0.13 bA | 1.02 ± 0.30 aA | 1.14 ± 0.09 bB | 1.26 ± 0.13 bA | 6.27 ± 0.54 bA | 6.34 ± 1.03 aA |
| 0.3 | 1.54 ± 0.21 aA | 1.33 ± 0.12 aA | 1.76 ± 0.11 aA | 1.73 ± 0.11 aA | 9.32 ± 0.78 aA | 8.37 ± 1.32 aA | |
| 0.5 | 1.48 ± 0.20 aA | 1.25 ± 0.14 aA | 1.69 ± 0.28 aA | 1.54 ± 0.15 aB | 9.04 ± 0.26 aA | 7.83 ± 0.83 aA | |
| H | CK | 0.95 ± 0.11 bA | 0.87 ± 0.15 aA | 0.66 ± 0.09 aA | 0.54 ± 0.12 aB | 4.61 ± 1.34 aA | 4.43 ± 0.68 aA |
| 0.3 | 1.22 ± 0.15 aA | 1.06 ± 0.11 aA | 0.85 ± 0.07 aA | 0.68 ± 0.08 aA | 5.80 ± 0.88 aA | 5.11 ± 0.69 aA | |
| 0.5 | 1.20 ± 0.14a bA | 0.97 ± 0.12 aA | 0.88 ± 0.27 aA | 0.63 ± 0.15 aA | 6.00 ± 0.90 aA | 5.40 ± 0.49 aA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Chen, N.; Liu, G.; Ding, J.; Chen, M.; Zhang, J. Persistence of Hg-Contaminated Soil Stabilization in Typical Areas of Dehua County, Fujian Province, China. Sustainability 2023, 15, 1018. https://doi.org/10.3390/su15021018
Wang R, Chen N, Liu G, Ding J, Chen M, Zhang J. Persistence of Hg-Contaminated Soil Stabilization in Typical Areas of Dehua County, Fujian Province, China. Sustainability. 2023; 15(2):1018. https://doi.org/10.3390/su15021018
Chicago/Turabian StyleWang, Rui, Nan Chen, Guannan Liu, Jianhua Ding, Ming Chen, and Jiawen Zhang. 2023. "Persistence of Hg-Contaminated Soil Stabilization in Typical Areas of Dehua County, Fujian Province, China" Sustainability 15, no. 2: 1018. https://doi.org/10.3390/su15021018






