Macro- and Micronutrient Contents and Their Relationship with Growth in Six Eucalyptus Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Conducting the Experiment
2.2. Experimental Design
2.3. Variables Assessed
2.4. Statistical Analysis
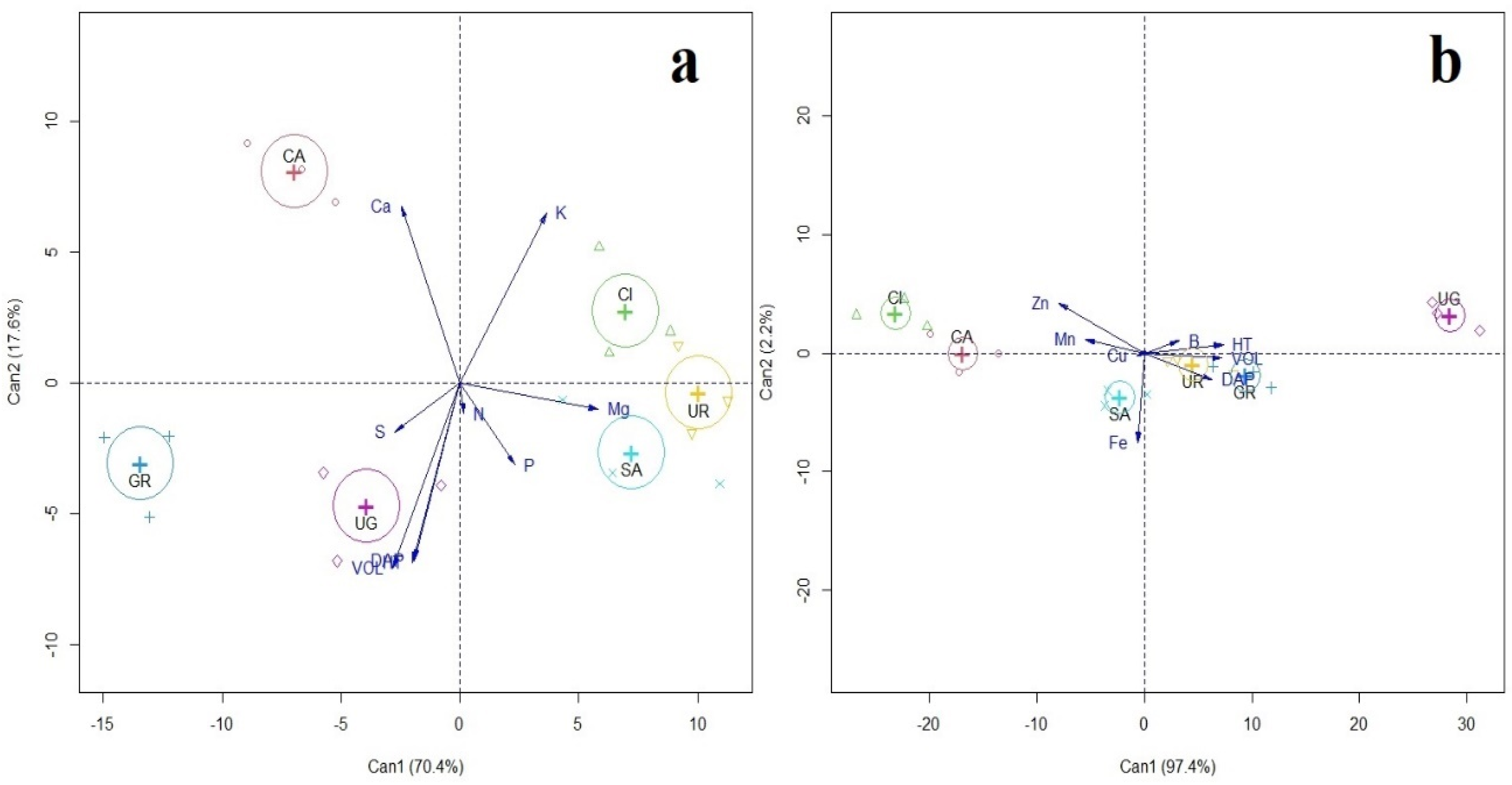
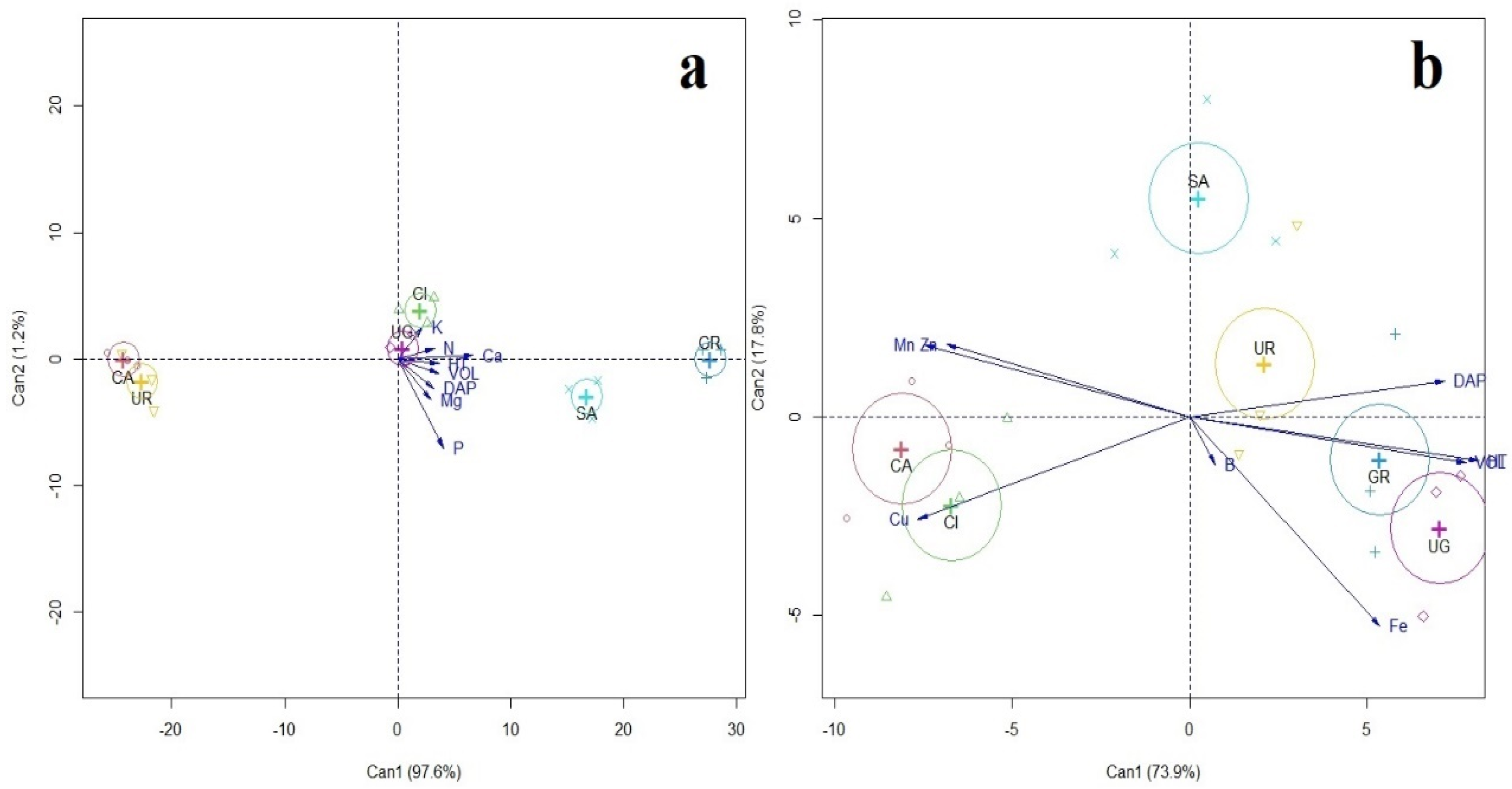
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alongi, D.M. Impact of global change on nutrient dynamics in mangrove forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Veldkamp, E.; Corre, M.D. Tree species diversity effects on productivity, soil nutrient availability and nutrient response efficiency in a temperate deciduous forest. For. Ecol. Manag. 2015, 338, 114–123. [Google Scholar] [CrossRef]
- Chiang, J.M.; Spasojevic, M.J.; Muller-Landau, H.C.; Sun, I.F.; Lin, Y.; Su, S.H.; Chen, Z.S.; Chen, C.T.; Swenson, N.G.; McEwan, R.W. Functional composition drives ecosystem function through multiple mechanisms in a broadleaved subtropical forest. Oecologia 2016, 182, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Niinemets, Ü.; Sheffield, J.; Lichstein, J.W. Shifts in tree functional composition amplify the response of forest biomass to climate. Nature 2018, 556, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Bayle, G.K. Ecological and social impacts of eucalyptus tree plantation on the environment. J. Biodivers. Conserv. Bioresour. Manag. 2019, 5, 93–104. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Alvares, C.A.; Rocha, J.H.; Brandani, C.B.; Hakamada, R. Eucalypt plantation management in regions with water stress. South. For. J. For. Sci. 2017, 79, 169–183. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; de Moraes Gonçalves, J.L.; Gava, J.L.; de Oliveira Godinho, T.; Melo, E.A.; Bazani, J.H.; Hubner, A.; Junior, J.C.A.; Wichert, M.P. Forest residue maintenance increased the wood productivity of a Eucalyptus plantation over two short rotations. For. Ecol. Manag. 2016, 379, 1–10. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Leite, F.P.; Silva, I.R.; Novais, R.F.; Barros, N.F.D.; Neves, J.C.L.; Villani, E.M.D.A. Nutrient relations during an eucalyptus cycle at different population densities. Rev. Bras. Cien. Solo 2011, 35, 949–959. [Google Scholar] [CrossRef]
- Grove, T.S.; Thomson, B.D.; Malajczuk, N. Nutritional physiology of eucalypts: Uptake, distribution and utilization. In Nutrition of Eucalypts; Attiwill, P.M., Adams, M.A., Eds.; CSIRO Publishing: Collingwood, Australia, 1996; pp. 77–108. [Google Scholar]
- Akmakjian, G.Z.; Riaz, N.; Guerinot, M.L. Photoprotection during iron deficiency is mediated by the bHLH transcription factors PYE and ILR3. Proc. Natl. Acad. Sci. USA 2021, 118, e2024918118. [Google Scholar] [CrossRef] [PubMed]
- Khodour, Y.; Kaguni, L.S.; Stiban, J. Iron–sulfur clusters in nucleic acid metabolism: Varying roles of ancient cofactors. Enzymes 2019, 45, 225–256. [Google Scholar] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Thomine, S.; Buckhout, T.J. Iron nutrition and interactions in plants. Front. Plant Sci. 2020, 10, 1670. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.S.; Mincato, R.L.; Santos, B.R. Heavy metals availability and soil fertility after land application of sewage sludge on dystroferric red latosol. Cienc. Agrotec. 2013, 37, 512–520. [Google Scholar] [CrossRef]
- Grassein, F.; Till-Bottraud, I.; Lavorel, S. Plant resource-use strategies: The importance of phenotypic plasticity in response to a productivity gradient for two subalpine species. Ann. Bot. 2010, 106, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Lambert, M.J. Nutrient cycling in age sequences of two Eucalyptus plantation species. For. Ecol. Manag. 2008, 255, 1701–1712. [Google Scholar] [CrossRef]
- De Souza Kulmann, M.S.; de Jesus Eufrade-Junior, H.; Dick, G.; Schumacher, M.V.; de Azevedo, G.B.; Azevedo, G.T.D.O.S.; Guerra, S.P.S. Belowground biomass harvest influences biomass production, stock, export and nutrient use efficiency of second rotation Eucalyptus plantations. Biomass Bioenergy 2022, 161, 106476. [Google Scholar]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015; 761p. [Google Scholar]
- Campos, J.C.C.; Leite, H.G. Mensuração Florestal: Perguntas e Respostas; UFV: Viçosa, Brazil, 2013; 605p. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A.D. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Potafos: Piracicaba, Brazil, 1997; 319p. [Google Scholar]
- Miyazawa, M.; Pavan, M.A.; Muraoka, T.; do Carmo, C.A.F.S.; de Melo, W.J. Análise química de tecido vegetal. In Manual de Análises Químicas de Solos, Planta e Fertilizantes, 2nd ed.; Silva, F.C., Ed.; Embrapa Informação Tecnológica: Brasilia, Brazil, 2009; 627p. [Google Scholar]
- Cruz, C.D.; Carneiro, P.C.S.; Regazzi, A.J. Modelos Biométricos Aplicados AO Melhoramento Genético, 4th ed.; UFV: Viçosa, Brazil, 2013; Volume 1, 514p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 April 2023).
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A role for zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Hörger, A.C.; Fones, H.N.; Preston, G. The current status of the elemental defense hypothesis in relation to pathogens. Front. Plant Sci. 2013, 4, 395. [Google Scholar] [CrossRef] [PubMed]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Ackerly, D. Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol. Monogr. 2004, 74, 25–44. [Google Scholar] [CrossRef]
- Kraft, N.J.; Valencia, R.; Ackerly, D.D. Functional traits and niche-based tree community assembly in an Amazonian forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Ji, H.; Liu, S.; Kang, H.; Yin, S.; Liu, C. Nutrient resorption strategies of three oak tree species in response to interannual climate variability. For. Ecosyst. 2021, 8, 70. [Google Scholar] [CrossRef]
- Raulino, W.N.C.; Freire, F.J.; Assunção, E.A.D.A.; Ataide, K.M.P.D.; Silva, H.V.D.; Silva, A.C.F.D. Nutrition of tree species in tropical dry forest and rainforest environments. Rev. Ceres 2020, 67, 70–80. [Google Scholar] [CrossRef]
- Giweta, M. Role of litter production and its decomposition, and factors affecting the processes in a tropical forest ecosystem: A review. J. Ecol. Environ. 2020, 44, 11. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, W.; Zhou, G.; Bai, Y.; Li, J.; Tang, X.; Chen, D.; Liu, Q.; Ma, W.; Xiong, G.; et al. Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2018, 115, 4033–4038. [Google Scholar] [CrossRef]

| Species | DAP (cm) | HT (m) | VOL (m3) |
|---|---|---|---|
| CA | 14.93 b | 14.56 b | 0.1272 b |
| CI | 14.17 b | 17.12 b | 0.1419 b |
| SA | 18.67 a | 18.37 b | 0.2273 b |
| GR | 21.43 a | 22.14 a | 0.3467 a |
| UG | 20.50 a | 23.56 a | 0.3575 a |
| UR | 19.20 a | 19.49 b | 0.2488 b |
| Macronutrients | Micronutrients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | B | Cu | Fe | Mn | Zn | |
| Species | g kg−1 | mg kg−1 | |||||||||
| CA | 12.77 a | 0.67 a | 12.80 a | 19.03 a | 2.20 a | 1.07 a | 41.67 a | 29.67 a | 352.33 b | 150.33 a | 26.33 b |
| CI | 14.97 a | 0.83 a | 14.87 a | 9.23 b | 2.37 a | 0.97 a | 51.67 a | 57.33 a | 385.00 b | 196.33 a | 42.33 a |
| SA | 14.33 a | 1.00 a | 9.10 b | 10.70 b | 3.07 a | 0.97 a | 57.33 a | 49.00 a | 587.67 a | 151.00 a | 14.33 c |
| GR | 14.00 a | 0.93 a | 7.73 b | 11.17 b | 2.07 a | 1.03 a | 46.33 a | 43.67 a | 502.33 a | 153.67 a | 11.00 c |
| UG | 13.73 a | 0.90 a | 9.77 b | 9.40 b | 2.10 a | 1.13 a | 58.67 a | 37.33 a | 303.33 b | 109.67 a | 8.00 c |
| UR | 12.43 a | 1.03 a | 11.80 a | 12.60 b | 2.73 a | 1.03 a | 51.67 a | 47.00 a | 420.67 b | 107.00 a | 17.67 c |
| Macronutrients | Micronutrients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | B | Cu | Fe | Mn | Zn | |
| Species | g kg−1 | mg kg−1 | |||||||||
| CA | 1.03 a | 0.27 a | 1.93 a | 1.80 a | 0.37 a | 0.47 a | 1.67 a | 9.67 a | 36.33 b | 16.33 a | 4.67 b |
| CI | 1.40 a | 0.33 a | 1.10 b | 1.93 a | 0.27 a | 0.57 a | 4.67 a | 9.67 a | 111.00 b | 7.33 a | 25.67 a |
| SA | 0.83 a | 0.30 a | 0.93 b | 0.93 b | 0.33 a | 0.40 a | 1.33 a | 10.00 a | 24.33 a | 7.00 a | 4.33 c |
| GR | 0.73 a | 0.30 a | 0.70 b | 1.27 b | 0.17 a | 0.40 a | 1.67 a | 10.33 a | 78.67 a | 8.67 a | 2.67 c |
| UG | 0.83 a | 0.10 a | 0.53 b | 0.83 b | 0.23 a | 0.57 a | 1.67 a | 9.67 a | 66.67 b | 8.33 a | 7.33 c |
| UR | 1.00 a | 0.17 a | 1.17 b | 1.10 b | 0.23 a | 0.40 a | 1.33 a | 9.33 a | 56.00 b | 8.33 a | 7.33 c |
| Macronutrients | Micronutrients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | B | Cu | Fe | Mn | Zn | |
| Species | g kg−1 | mg kg−1 | |||||||||
| CA | 2.33 a | 0.20 d | 3.83 a | 9.37 b | 1.90 b | 0.43 a | 8.33 b | 10.33 a | 42.33 d | 77.33 a | 21.00 a |
| CI | 3.23 a | 0.23 d | 5.83 a | 12.90 b | 1.60 c | 0.53 a | 13.33 a | 10.33 a | 99.00 c | 90.67 a | 22.67 a |
| SA | 3.07 a | 1.37 a | 4.97 a | 19.53 a | 2.63 a | 0.50 a | 9.33 b | 8.33 b | 25.33 d | 72.67 a | 19.00 a |
| GR | 3.07 a | 0.87 b | 4.63 a | 25.53 a | 2.07 b | 0.60 a | 17.33 a | 8.00 b | 320.33 a | 43.00 b | 3.67 b |
| UG | 3.03 a | 0.47 c | 3.97 a | 13.90 b | 1.37 c | 0.53 a | 7.67 b | 8.33 b | 187.33 b | 23.67 b | 4.67 b |
| UR | 3.13 a | 0.80 b | 4.43 a | 8.70 b | 1.23 c | 0.57 a | 9.00 b | 8.33 b | 135.67 c | 21.67 b | 5.33 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, O.A.P.; Silva, D.B.d.; Teixeira-Filho, M.C.M.; Silva, T.B.; Campos, C.N.S.; Baio, F.H.R.; Azevedo, G.B.d.; Faria, G.A.; Teodoro, L.P.R.; Teodoro, P.E. Macro- and Micronutrient Contents and Their Relationship with Growth in Six Eucalyptus Species. Sustainability 2023, 15, 15771. https://doi.org/10.3390/su152215771
da Silva OAP, Silva DBd, Teixeira-Filho MCM, Silva TB, Campos CNS, Baio FHR, Azevedo GBd, Faria GA, Teodoro LPR, Teodoro PE. Macro- and Micronutrient Contents and Their Relationship with Growth in Six Eucalyptus Species. Sustainability. 2023; 15(22):15771. https://doi.org/10.3390/su152215771
Chicago/Turabian Styleda Silva, Otavio Ananias Pereira, Dayane Bortoloto da Silva, Marcelo Carvalho Minhoto Teixeira-Filho, Tays Batista Silva, Cid Naudi Silva Campos, Fabio Henrique Rojo Baio, Gileno Brito de Azevedo, Gláucia Amorim Faria, Larissa Pereira Ribeiro Teodoro, and Paulo Eduardo Teodoro. 2023. "Macro- and Micronutrient Contents and Their Relationship with Growth in Six Eucalyptus Species" Sustainability 15, no. 22: 15771. https://doi.org/10.3390/su152215771








