Arsenic Immobilization for Paddy Field and Improvement of Rice (Oryza sativa L.) Growth through Cerium–Manganese Modified Wheat Straw Biochar Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Design
2.3. Chemical Analysis
2.3.1. Analysis of Basic Physical and Chemical Properties
2.3.2. Plant Sampling and Analysis
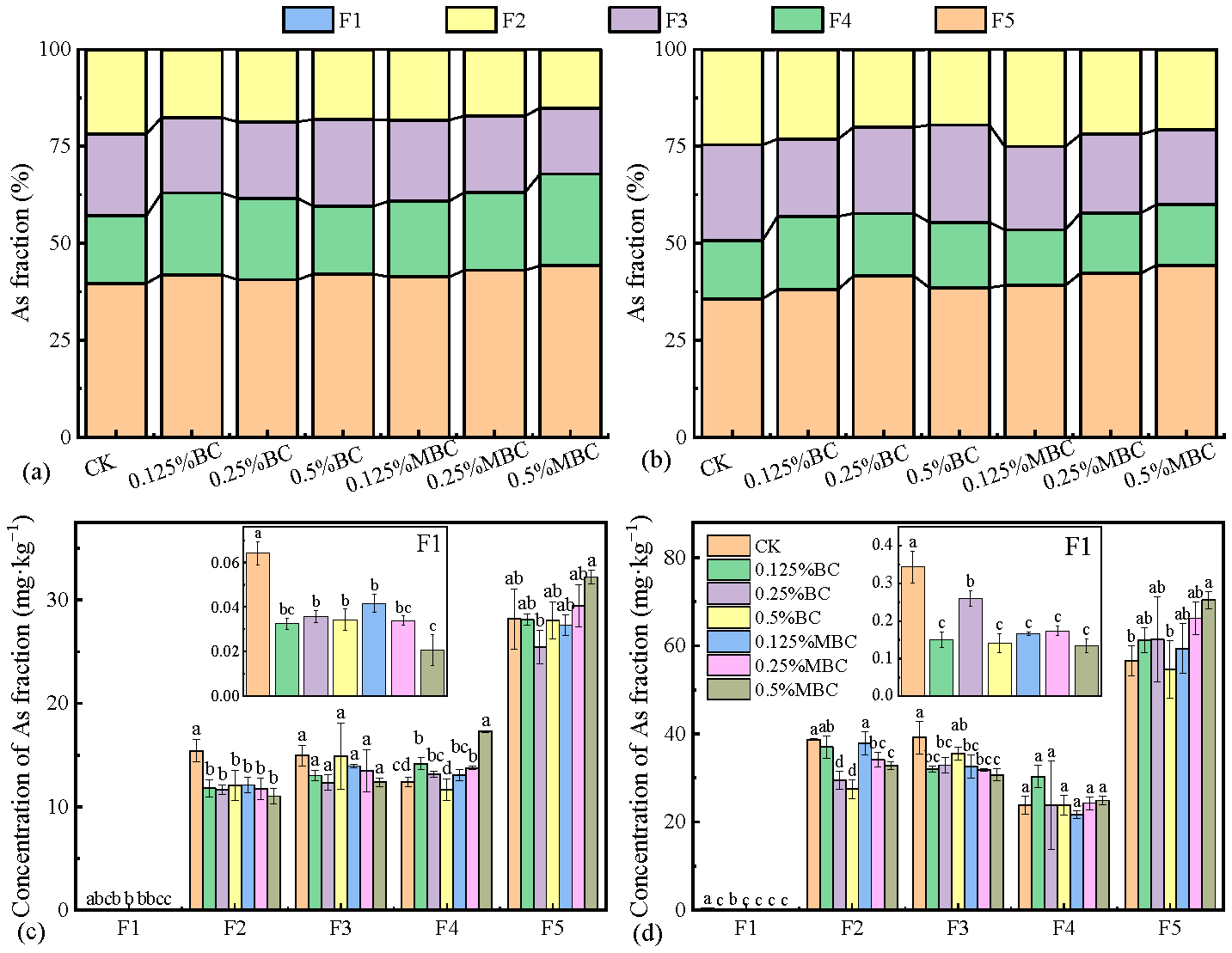
2.3.3. As Fraction
2.4. Data Analysis
3. Results
3.1. Soil Physiochemical Properties
3.2. Ws-As in Soils
3.3. As Fractions
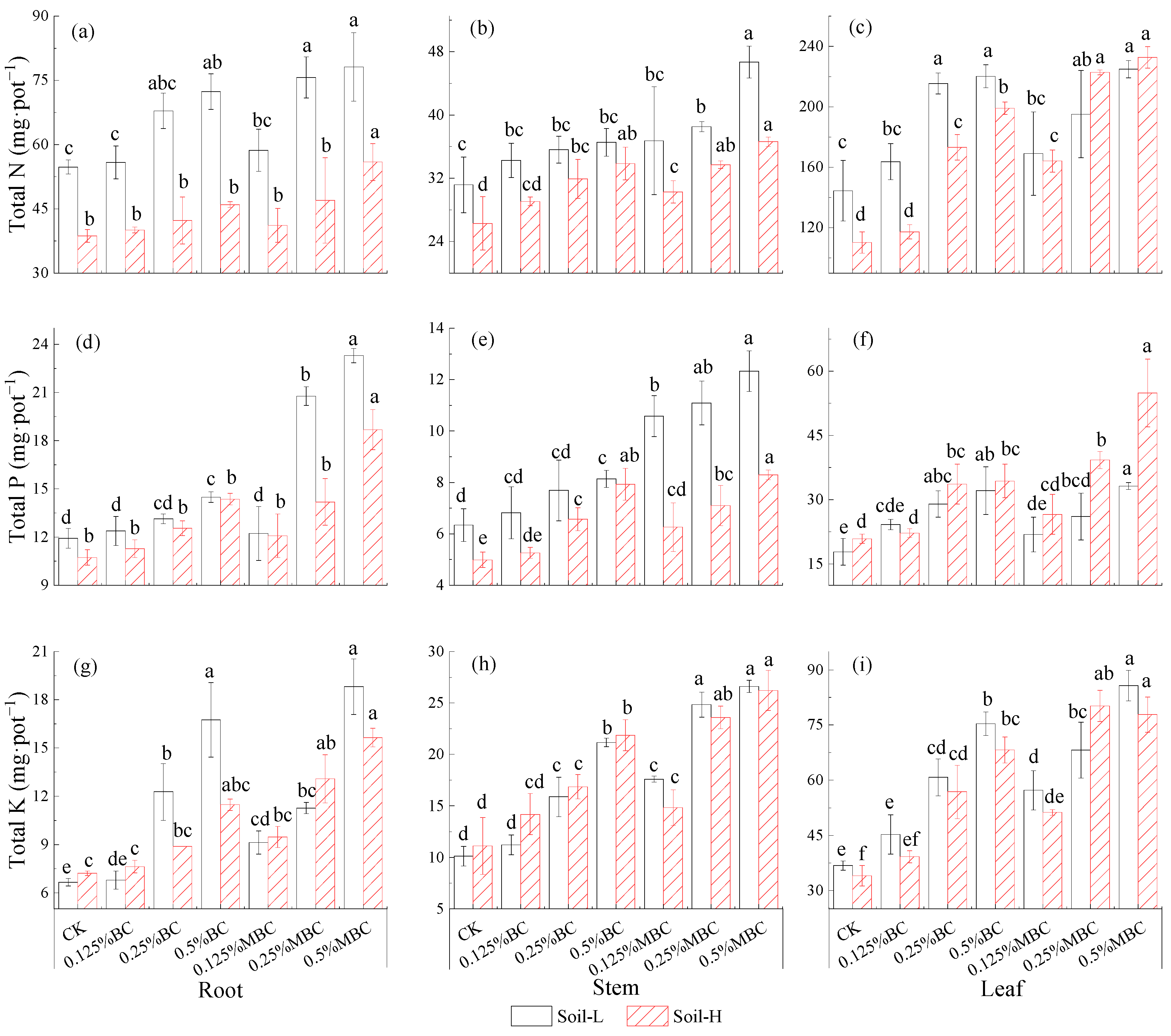
3.4. Rice Biomass and Nutrient Accumulation
3.5. As Uptake, Enrichment, and Transfer Coefficients in Rice Plants
3.6. Relationships between Soil Physicochemical Properties and As Bioavailability
4. Discussion
4.1. MBC-Mediated Effects on Rice Growth and Nutrient Uptake
4.2. As Immobilization Effect by MBC Application
4.3. The Possible Mechanism of As Passivation by MBC Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alarcon-Herrera, M.T.; Martin-Alarcon, D.A.; Gutierrez, M.; Reynoso-Cuevas, L.; Martin-Dominguez, A.; Olmos-Marquez, M.A.; Bundschuh, J. Co-occurrence, possible origin, and health-risk assessment of arsenic and fluoride in drinking water sources in Mexico: Geographical data visualization. Sci. Total Environ. 2020, 698, 134168. [Google Scholar] [CrossRef]
- Orak, N.H. A Hybrid Bayesian Network Framework for Risk Assessment of Arsenic Exposure and Adverse Reproductive Outcomes. Ecotoxicol. Environ. Saf. 2020, 192, 110270. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Zhai, W.; Dai, Y.; Zhao, W.; Yuan, H.; Qiu, D.; Chen, J.; Gustave, W.; Maguffin, S.C.; Chen, Z.; Liu, X.; et al. Simultaneous immobilization of the cadmium, lead and arsenic in paddy soils amended with titanium gypsum. Environ. Pollut. 2020, 258, 113790. [Google Scholar] [CrossRef]
- Lyu, P.; Li, L.; Huang, X.; Xie, J.; Ye, J.; Tian, Y.; Huang, J.; Zhu, C. Ternary Ca-Mg-Al layered double-hydroxides for synergistic remediation of As, Cd, and Pb from both contaminated soil and groundwater: Characteristics, effectiveness, and immobilization mechanisms. J. Hazard. Mater. 2022, 442, 130030. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Yin, D.; Wang, X.; Chen, C.; Peng, B.; Tan, C.; Li, H. Varying effect of biochar on Cd, Pb and As mobility in a multi-metal contaminated paddy soil. Chemosphere 2016, 152, 196–206. [Google Scholar] [CrossRef]
- Carey, A.M.; Norton, G.J.; Deacon, C.; Scheckel, K.G.; Lombi, E.; Punshon, T.; Guerinot, M.L.; Lanzirotti, A.; Newville, M.; Choi, Y.; et al. Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol. 2011, 192, 87–98. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Yang, J.; Zhou, S.; Wang, L.; Bolan, N. Evaluation of hydroxyapatite derived from flue gas desulphurization gypsum on simultaneous immobilization of lead and cadmium in contaminated soil. J. Hazard. Mater. 2020, 400, 123038. [Google Scholar] [CrossRef]
- Lyu, P.; Li, L.; Huang, X.; Wang, G.; Zhu, C. Pre-magnetic bamboo biochar cross-linked CaMgAl layered double-hydroxide composite: High-efficiency removal of As(III) and Cd(II) from aqueous solutions and insight into the mechanism of simultaneous purification. Sci. Total Environ. 2022, 823, 153743. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Luo, Y.; Dai, X.; Wang, Y.; Chen, Y.; Shi, L. Comparison of Phytoremediation Potential of Nerium indicum with Inorganic Modifier Calcium Carbonate and Organic Modifier Mushroom Residue to Lead–Zinc Tailings. Int. J. Environ. Res. Public. Health 2022, 19, 10353. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Dai, X.; Chen, Y.; Luo, Y.; Yao, H.; Ouyang, D.; Li, Z.; Wang, Z. Organic–inorganic composite modifiers enhance restoration potential of Nerium oleander L. to lead–zinc tailing: Application of phytoremediation. Environ. Sci. Pollut. Res. 2023, 30, 56569–56579. [Google Scholar] [CrossRef]
- Sohi, S.P. Agriculture. Carbon storage with benefits. Science 2012, 338, 1034–1035. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Advances in application of cotton-based adsorbents for heavy metals trapping, surface modifications and future perspectives. Ecotoxicol. Environ. Saf. 2020, 201, 110825. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Wang, J.; Zhang, C.; Chen, R. Stabilization of heavy metal-contaminated soils by biochar: Challenges and recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef]
- Wu, P.; Ata-Ul-Karim, S.T.; Singh, B.P.; Wang, H.; Wu, T.; Liu, C.; Fang, G.; Zhou, D.; Wang, Y.; Chen, W. A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 2019, 1, 23–43. [Google Scholar] [CrossRef]
- Kamali, M.; Jahaninafard, D.; Mostafaie, A.; Davarazar, M.; Gomes, A.P.D.; Tarelho, L.A.C.; Dewil, R.; Aminabhavi, T.M. Scientometric analysis and scientific trends on biochar application as soil amendment. Chem. Eng. J. 2020, 395, 125128. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2016, 17, 685–716. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.-H.; Tsang, D.C.W.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Beesley, L.; Marmiroli, M.; Pagano, L.; Pigoni, V.; Fellet, G.; Fresno, T.; Vamerali, T.; Bandiera, M.; Marmiroli, N. Biochar addition to an arsenic contaminated soil increases arsenic concentrations in the pore water but reduces uptake to tomato plants (Solanum lycopersicum L.). Sci. Total Environ. 2013, 454–455, 598–603. [Google Scholar] [CrossRef]
- Qiao, J.T.; Liu, T.X.; Wang, X.Q.; Li, F.B.; Lv, Y.H.; Cui, J.H.; Zeng, X.D.; Yuan, Y.Z.; Liu, C.P. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef]
- Arabi, Z.; Rinklebe, J.; El-Naggar, A.; Hou, D.; Sarmah, A.K.; Moreno-Jimenez, E. (Im)mobilization of arsenic, chromium, and nickel in soils via biochar: A meta-analysis. Environ. Pollut. 2021, 286, 117199. [Google Scholar] [CrossRef]
- Yang, X.; Shaheen, S.M.; Wang, J.; Hou, D.; Ok, Y.S.; Wang, S.L.; Wang, H.; Rinklebe, J. Elucidating the redox-driven dynamic interactions between arsenic and iron-impregnated biochar in a paddy soil using geochemical and spectroscopic techniques. J. Hazard. Mater. 2022, 422, 126808. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wang, F.; Wang, L.; Liu, J.; Hashimoto, Y.; Hosomi, M. Arsenic immobilization and removal in contaminated soil using zero-valent iron or magnetic biochar amendment followed by dry magnetic separation. Sci. Total Environ. 2021, 768, 144521. [Google Scholar] [CrossRef]
- Thomas, E.; Borchard, N.; Sarmiento, C.; Atkinson, R.; Ladd, B. Key factors determining biochar sorption capacity for metal contaminants: A literature synthesis. Biochar 2020, 2, 151–163. [Google Scholar] [CrossRef]
- Wan, X.; Li, C.; Parikh, S.J. Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ. Pollut. 2020, 261, 114157. [Google Scholar] [CrossRef]
- Yan, S.; Zhou, K.; Li, Y.; He, Q.; Xia, L.; Liu, S.; Li, H.; Liang, D.; Song, S. Efficient removal of As(V) from diluted aqueous solutions by Fe/La oxide magnetic microspheres. J. Clean. Prod. 2020, 273, 123134. [Google Scholar] [CrossRef]
- Yi, S.; Sun, Y.; Hu, X.; Xu, H.; Gao, B.; Wu, J. Porous nano-cerium oxide wood chip biochar composites for aqueous levofloxacin removal and sorption mechanism insights. Environ. Sci. Pollut. Res. Int. 2018, 25, 25629–25637. [Google Scholar] [CrossRef]
- Liu, K.; Li, F.; Cui, J.; Yang, S.; Fang, L. Simultaneous removal of Cd(II) and As(III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: Synergistic effects and mechanisms. J. Hazard. Mater. 2020, 395, 122623. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, J.; Zhao, N.; Yang, X.; Chen, C.; Shang, J. Goethite modified biochar as a multifunctional amendment for cationic Cd(II), anionic As(III), roxarsone, and phosphorus in soil and water. J. Clean. Prod. 2020, 247, 119579. [Google Scholar] [CrossRef]
- Liu, G.; Meng, J.; Huang, Y.; Dai, Z.; Tang, C.; Xu, J. Effects of carbide slag, lodestone and biochar on the immobilization, plant uptake and translocation of As and Cd in a contaminated paddy soil. Environ. Pollut. 2020, 266 Pt 1, 115194. [Google Scholar] [CrossRef]
- Liang, T.; Li, L.; Zhu, C.; Liu, X.; Li, H.; Su, Q.; Ye, J.; Geng, B.; Tian, Y.; Sardar, M.F.; et al. Adsorption of As(V) by the Novel and Efficient Adsorbent Cerium-Manganese Modified Biochar. Water 2020, 12, 2720. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemical Analysis Method; China Agricultural Science and Technology Press: Beijing, China, 2000; 495p. [Google Scholar]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–332. [Google Scholar] [CrossRef]
- Li, L.; Zhu, C.; Liu, X.; Li, F.; Li, H.; Ye, J. Biochar amendment immobilizes arsenic in farmland and reduces its bioavailability. Environ. Sci. Pollut. Res. Int. 2018, 25, 34091–34102. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.Y.; Deng, X.; Herbert, S.; Xing, B.S. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Tian, X.; Wang, D.; Chai, G.; Zhang, J.; Zhao, X. Does biochar inhibit the bioavailability and bioaccumulation of As and Cd in co-contaminated soils? A meta-analysis. Sci. Total Environ. 2021, 762, 143117. [Google Scholar] [CrossRef]
- Chen, Z.; Pei, J.; Wei, Z.; Ruan, X.; Hua, Y.; Xu, W.; Zhang, C.; Liu, T.; Guo, Y. A novel maize biochar-based compound fertilizer for immobilizing cadmium and improving soil quality and maize growth. Environ. Pollut. 2021, 277, 116455. [Google Scholar] [CrossRef]
- Islam, M.S.; Magid, A.; Chen, Y.; Weng, L.; Ma, J.; Arafat, M.Y.; Khan, Z.H.; Li, Y. Effect of calcium and iron-enriched biochar on arsenic and cadmium accumulation from soil to rice paddy tissues. Sci. Total Environ. 2021, 785, 147163. [Google Scholar] [CrossRef]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.-G.; Liu, S.-B.; Liu, H.-Y.; Zeng, G.-M.; Tan, X.-F.; Yang, C.-P.; Ding, Y.; Yan, Z.-L.; Cai, X.-X. Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem. Eng. J. 2017, 314, 223–231. [Google Scholar] [CrossRef]
- Du, Y.; Fan, H.; Wang, L.; Wang, J.; Wu, J.; Dai, H. α-Fe2O3 nanowires deposited diatomite: Highly efficient absorbents for the removal of arsenic. J. Mater. Chem. A 2013, 1, 7729. [Google Scholar] [CrossRef]
- Olivera, S.; Chaitra, K.; Venkatesh, K.; Muralidhara, H.B.; Inamuddin; Asiri, A.M.; Ahamed, M.I. Cerium dioxide and composites for the removal of toxic metal ions. Environ. Chem. Lett. 2018, 16, 1233–1246. [Google Scholar] [CrossRef]
- Pillewan, P.; Mukherjee, S.; Roychowdhury, T.; Das, S.; Bansiwal, A.; Rayalu, S. Removal of As(III) and As(V) from water by copper oxide incorporated mesoporous alumina. J. Hazard. Mater. 2011, 186, 367–375. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Gao, M.; Song, Z. Effect of Fe-Mn-Ce modified biochar composite on microbial diversity and properties of arsenic-contaminated paddy soils. Chemosphere 2020, 250, 126249. [Google Scholar] [CrossRef]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Luo, M.; Lin, H.; He, Y.; Zhang, Y. The influence of corncob-based biochar on remediation of arsenic and cadmium in yellow soil and cinnamon soil. Sci. Total Environ. 2020, 717, 137014. [Google Scholar] [CrossRef]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.R.; Tang, J.F.; Cotner, J.B.; Xu, Y.Y. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Xia, D.; Jiang, X.; Fu, D.; Shen, L.; Wang, H.; Li, Q.B. Enhanced bioreduction of iron and arsenic in sediment by biochar amendment influencing microbial community composition and dissolved organic matter content and composition. J. Hazard. Mater. 2016, 311, 20–29. [Google Scholar] [CrossRef]
- Mladenov, N.; Zheng, Y.; Miller, M.P.; Nemergut, D.R.; Legg, T.; Simone, B.; Hageman, C.; Moshiur Rahman, M.; Matin Ahmed, K.; Mcknight, D.M. Dissolved organic matter sources and consequences for iron and arsenic mobilization in Bangladesh aquifers. Environ. Sci. Technol. 2010, 44, 123–128. [Google Scholar] [CrossRef]
- Hossain, M.; Mestrot, A.; Norton, G.J.; Deacon, C.; Islam, M.R.; Meharg, A.A. Arsenic dynamics in paddy soil under traditional manuring practices in Bangladesh. Environ. Pollut. 2021, 268 Pt A, 115821. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252 Pt A, 846–855. [Google Scholar] [CrossRef]
- Xu, W.; Shafi, M.; Penttinen, P.; Hou, S.; Wang, X.; Ma, J.; Zhong, B.; Guo, J.; Xu, M.; Ye, Z.; et al. Bioavailability of heavy metals in contaminated soil as affected by different mass ratios of biochars. Environ. Technol. 2020, 41, 3329–3337. [Google Scholar] [CrossRef]
- Zhang, W.; Cho, Y.; Vithanage, M.; Shaheen, S.M.; Rinklebe, J.; Alessi, D.S.; Hou, C.-H.; Hashimoto, Y.; Withana, P.A.; Ok, Y.S. Arsenic removal from water and soils using pristine and modified biochars. Biochar 2022, 4, 55. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Mosa, A.; Zimmerman, A.R.; Ma, L.Q.; Harris, W.G.; Migliaccio, K.W. Manganese oxide-modified biochars: Preparation, characterization, and sorption of arsenate and lead. Bioresour. Technol. 2015, 181, 13–17. [Google Scholar] [CrossRef]
- Lian, F.; Liu, X.; Gao, M.; Li, H.; Qiu, W.; Song, Z. Effects of Fe-Mn-Ce oxide-modified biochar on As accumulation, morphology, and quality of rice (Oryza sativa L.). Environ. Sci. Pollut. Res. Int. 2020, 27, 18196–18207. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Natasha; Mosa, A.; El-Naggar, A.; Faysal Hossain, M.; Abdelrahman, H.; Khan Niazi, N.; Shahid, M.; Zhang, T.; Fai Tsang, Y.; et al. Manganese oxide-modified biochar: Production, characterization and applications for the removal of pollutants from aqueous environments—A review. Bioresour. Technol. 2022, 346, 126581. [Google Scholar] [CrossRef]
- Luo, X.B.; Wang, C.C.; Luo, S.L.; Dong, R.Z.; Tu, X.M.; Zeng, G.S. Adsorption of As (III) and As (V) from water using magnetite Fe3O4-reduced graphite oxide-MnO2 nanocomposites. Chem. Eng. J. 2012, 187, 45–52. [Google Scholar] [CrossRef]
- Liu, X.; Gao, M.; Qiu, W.; Khan, Z.H.; Liu, N.; Lin, L.; Song, Z. Fe-Mn-Ce oxide-modified biochar composites as efficient adsorbents for removing As(III) from water: Adsorption performance and mechanisms. Environ. Sci. Pollut. Res. Int. 2019, 26, 17373–17382. [Google Scholar] [CrossRef]
- Xu, M.; Gao, P.; Wu, J.; Ma, J.; Zhang, X.; Yang, G.; Long, L.; Chen, C.; Song, C.; Xiao, Y. Biochar promotes arsenic sequestration on iron plaques and cell walls in rice roots. Chemosphere 2022, 288 Pt 1, 132422. [Google Scholar] [CrossRef]
- Liang, T.; Zhou, G.; Chang, D.; Wang, Y.; Gao, S.; Nie, J.; Liao, Y.; Lu, Y.; Zou, C.; Cao, W. Co-incorporation of Chinese milk vetch (Astragalus sinicus L.), rice straw, and biochar strengthens the mitigation of Cd uptake by rice (Oryza sativa L.). Sci. Total Environ. 2022, 850, 158060. [Google Scholar] [CrossRef]
- Ali, W.; Mao, K.; Zhang, H.; Junaid, M.; Xu, N.; Rasool, A.; Feng, X.; Yang, Z. Comprehensive review of the basic chemical behaviours, sources, processes, and endpoints of trace element contamination in paddy soil-rice systems in rice-growing countries. J. Hazard. Mater. 2020, 397, 122720. [Google Scholar] [CrossRef]
- Beesley, L.; Marmiroli, M. The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef]
- Khan, S.; Chao, C.; Waqas, M.; Arp, H.P.H.; Zhu, Y.G. Sewage Sludge Biochar Influence upon Rice (Oryza sativa L.) Yield, Metal Bioaccumulation and Greenhouse Gas Emissions from Acidic Paddy Soil. Environ. Sci. Technol. 2013, 47, 8624–8632. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, Q.; Zhu, H.; Li, H.; Yin, W.; Feng, K.; Wang, S.; Wang, X. Does biochar application in heavy metal-contaminated soils affect soil micronutrient dynamics? Chemosphere 2022, 290, 133349. [Google Scholar] [CrossRef]
- Rafique, M.; Ortas, I.; Rizwan, M.; Chaudhary, H.J.; Gurmani, A.R.; Hussain Munis, M.F. Residual effects of biochar and phosphorus on growth and nutrient accumulation by maize (Zea mays L.) amended with microbes in texturally different soils. Chemosphere 2020, 238, 124710. [Google Scholar] [CrossRef]
- Huang, X.; Lyu, P.; Li, L.; Xie, J.; Zhu, C. Effect of three aging processes on physicochemical and As(V) adsorption properties of Ce/Mn-modified biochar. Environ. Res. 2022, 214 Pt 1, 113839. [Google Scholar] [CrossRef]




| Properties | Soil-L | Soil-H | BC | MBC |
|---|---|---|---|---|
| pH | 6.59 | 6.42 | 9.26 | 6.06 |
| TN (Nitrogen) (g·kg−1) | 0.53 | 0.54 | 7.41 | 3.96 |
| TP (Phosphorus) (g·kg−1) | 0.51 | 0.60 | 2.64 | 1.00 |
| TK (Potassium) (g·kg−1) | 3.02 | 2.92 | 19.34 | 22.02 |
| TN (Soil organic matter) (g·kg−1) | 4.65 | 4.38 | 358 | 233 |
| As (mg·kg−1) | 68.99 | 158.52 | 0.11 | 0.10 |
| SBET (m2·g−1) | / | / | 5.53 | 6.88 |
| Treatments | SOC g kg−1 | TN g kg−1 | AP mg kg−1 | AK mg kg−1 | DOC mg kg−1 | pH / | |
|---|---|---|---|---|---|---|---|
| CK | 4.77 ± 0.09 d | 0.83 ± 0.02 bc | 7.37 ± 0.52 abc | 154.67 ± 2.40 e | 37.81 ± 1.10 e | 6.75 ± 0.04 b | |
| 0.125%BC | 5.00 ± 0.03 cd | 0.83 ± 0.03 c | 6.53 ± 0.15 c | 156.00 ± 3.51 e | 36.86 ± 1.31 b | 6.93 ± 0.09 a | |
| 0.25%BC | 5.44 ± 0.13 c | 0.85 ± 0.02 abc | 7.67 ± 0.58 ab | 154.67 ± 2.40 e | 38.08 ± 0.61 b | 6.94 ± 0.05 a | |
| L | 0.5%BC | 6.67 ± 0.06 a | 0.92 ± 0.01 ab | 8.30 ± 0.32 a | 168.00 ± 1.00 d | 43.56 ± 3.31 a | 6.97 ± 0.03 a |
| 0.125%MBC | 4.59 ± 0.14 d | 0.85 ± 0.00 abc | 7.73 ± 0.23 ab | 178.33 ± 0.33 c | 36.90 ± 0.99 b | 6.72 ± 0.01 bc | |
| 0.25%MBC | 5.05 ± 0.25 cd | 0.92 ± 0.01 a | 6.90 ± 0.10 bc | 191.67 ± 3.53 b | 34.66 ± 1.41 b | 6.61 ± 0.03 c | |
| 0.5%MBC | 6.05 ± 0.30 b | 0.93 ± 0.06 a | 7.80 ± 0.21 ab | 233.33 ± 5.36 a | 23.61 ± 0.82 c | 6.47 ± 0.01 d | |
| CK | 4.66 ± 0.25 c | 0.71 ± 0.00 c | 7.40 ± 0.15 c | 95.67 ± 4.98 d | 42.07 ± 1.64 bc | 6.82 ± 0.06 a | |
| 0.125%BC | 4.76 ± 0.13 c | 0.77 ± 0.02 c | 14.47 ± 1.97 a | 174.00 ± 14.64 b | 41.51 ± 1.02 bc | 6.84 ± 0.03 a | |
| 0.25%BC | 6.23 ± 0.06 b | 0.73 ± 0.03 c | 7.90 ± 0.76 c | 93.33 ± 1.45 d | 46.39 ± 2.24 b | 6.87 ± 0.02 a | |
| H | 0.5%BC | 7.10 ± 0.21 a | 0.95 ± 0.03 a | 10.17 ± 2.47 bc | 168.00 ± 2.65 b | 54.79 ± 2.18 b | 6.90 ± 0.04 a |
| 0.125%MBC | 5.10 ± 0.16 c | 0.77 ± 0.00 c | 12.33 ± 0.38 ab | 172.33 ± 0.67 b | 40.29 ± 1.30 cd | 6.76 ± 0.03 a | |
| 0.25%MBC | 5.11 ± 0.27 c | 0.74 ± 0.03 c | 9.20 ± 1.47 bc | 135.00 ± 6.11 c | 38.23 ± 1.80 cd | 6.65 ± 0.22 a | |
| 0.5%MBC | 6.24 ± 0.09 b | 0.88 ± 0.02 b | 11.43 ± 0.93 abc | 210.00 ± 5.57 a | 36.19 ± 1.40 d | 6.62 ± 0.01 a | |
| Soil | CK | 0.125%BC | 0.25%BC | 0.5%BC | 0.125%MBC | 0.25%MBC | 0.5%MBC |
|---|---|---|---|---|---|---|---|
| Soil-L | 21.80% | 17.63% | 18.67% | 18.12% | 18.21% | 17.20% | 15.14% |
| Soil-H | 24.61% | 23.13% | 20.11% | 19.51% | 25.05% | 21.92% | 20.75% |
| Treatments | Total Plant (g) | Root (g) | ||
|---|---|---|---|---|
| Soil-L | Soil-H | Soil-L | Soil-H | |
| CK | 7.39 ± 0.74 c | 6.47 ± 0.87 d | 1.42 ± 0.24 c | 1.47 ± 0.18 b |
| 0.125%BC | 9.45 ± 0.35 b | 7.27 ± 0.89 d | 1.67 ± 0.04 bc | 1.62 ± 0.06 b |
| 0.25%BC | 11.32 ± 0.61 a | 11.65 ± 0.61 b | 1.74 ± 0.19 bc | 1.72 ± 0.25 ab |
| 0.5%BC | 12.18 ± 0.54 a | 12.46 ± 1.08 b | 1.98 ± 0.21 ab | 1.96 ± 0.15 ab |
| 0.125%MBC | 8.58 ± 0.98 bc | 10.21 ± 0.79 c | 1.69 ± 0.21 bc | 1.63 ± 0.19 b |
| 0.25%MBC | 11.37 ± 0.96 a | 12.40 ± 0.38 b | 1.89 ± 0.18 ab | 1.76 ± 0.28 ab |
| 0.5%MBC | 12.57 ± 0.62 a | 18.58 ± 0.54 a | 2.13 ± 0.19 a | 2.21 ± 0.53 a |
| Type | Treatments | BCFsoil-root | BCFsoil-stem | BCFsoil-leaf | TFroot-stem | TFroot-leaf |
|---|---|---|---|---|---|---|
| CK | 0.85 ± 0.012 a | 0.08 ± 0.009 a | 0.05 ± 0.003 a | 0.09 ± 0.010 bcd | 0.07 ± 0.007 ab | |
| 0.125%BC | 0.71 ± 0.015 b | 0.07 ± 0.006 a | 0.04 ± 0.003 b | 0.1 ± 0.006 bc | 0.06 ± 0.009 ab | |
| 0.25%BC | 0.44 ± 0.027 d | 0.07 ± 0.003 a | 0.03 ± 0.003 bc | 0.14 ± 0.007 a | 0.07 ± 0.003 a | |
| Soil-L | 0.5%BC | 0.37 ± 0.015 e | 0.04 ± 0.000 b | 0.03 ± 0.000 bc | 0.11 ± 0.000 b | 0.08 ± 0.003 a |
| 0.125%MBC | 0.56 ± 0.027 c | 0.04 ± 0.006 b | 0.03 ± 0.003 cd | 0.08 ± 0.010 cd | 0.06 ± 0.009 b | |
| 0.25%MBC | 0.44 ± 0.042 d | 0.03 ± 0.006 b | 0.03 ± 0.003 cd | 0.07 ± 0.010 d | 0.06 ± 0.012 ab | |
| 0.5%MBC | 0.34 ± 0.009 e | 0.03 ± 0.003 b | 0.02 ± 0.000 d | 0.07 ± 0.009 d | 0.06 ± 0.006 ab | |
| CK | 1.23 ± 0.006 a | 0.13 ± 0.003 a | 0.07 ± 0.003 a | 0.11 ± 0.003 a | 0.06 ± 0.000 a | |
| 0.125%BC | 1.11 ± 0.039 b | 0.11 ± 0.006 b | 0.05 ± 0.003 b | 0.10 ± 0.009 a | 0.04 ± 0.000 bc | |
| 0.25%BC | 0.90 ± 0.024 c | 0.10 ± 0.003 c | 0.04 ± 0.006 b | 0.11 ± 0.009 a | 0.04 ± 0.003 b | |
| Soil-H | 0.5%BC | 0.79 ± 0.032 d | 0.05 ± 0.006 d | 0.02 ± 0.003 c | 0.07 ± 0.012 b | 0.03 ± 0.003 bc |
| 0.125%MBC | 0.63 ± 0.007 e | 0.02 ± 0.000 e | 0.02 ± 0.000 cd | 0.03 ± 0.000 c | 0.03 ± 0.000 c | |
| 0.25%MBC | 0.39 ± 0.022 f | 0.01 ± 0.003 e | 0.02 ± 0.003 cd | 0.03 ± 0.003 c | 0.04 ± 0.003 b | |
| 0.5%MBC | 0.26 ± 0.038 g | 0.01 ± 0.000 e | 0.01 ± 0.000 d | 0.03 ± 0.006 c | 0.04 ± 0.008 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.; Li, L. Arsenic Immobilization for Paddy Field and Improvement of Rice (Oryza sativa L.) Growth through Cerium–Manganese Modified Wheat Straw Biochar Application. Sustainability 2023, 15, 16161. https://doi.org/10.3390/su152316161
Liang T, Li L. Arsenic Immobilization for Paddy Field and Improvement of Rice (Oryza sativa L.) Growth through Cerium–Manganese Modified Wheat Straw Biochar Application. Sustainability. 2023; 15(23):16161. https://doi.org/10.3390/su152316161
Chicago/Turabian StyleLiang, Ting, and Lianfang Li. 2023. "Arsenic Immobilization for Paddy Field and Improvement of Rice (Oryza sativa L.) Growth through Cerium–Manganese Modified Wheat Straw Biochar Application" Sustainability 15, no. 23: 16161. https://doi.org/10.3390/su152316161






