Sequential Application of Different Types of Coagulants as an Innovative Method of Phosphorus Inactivation, on the Example of Lake Mielenko, Poland
Abstract
:1. Introduction
2. Methodology
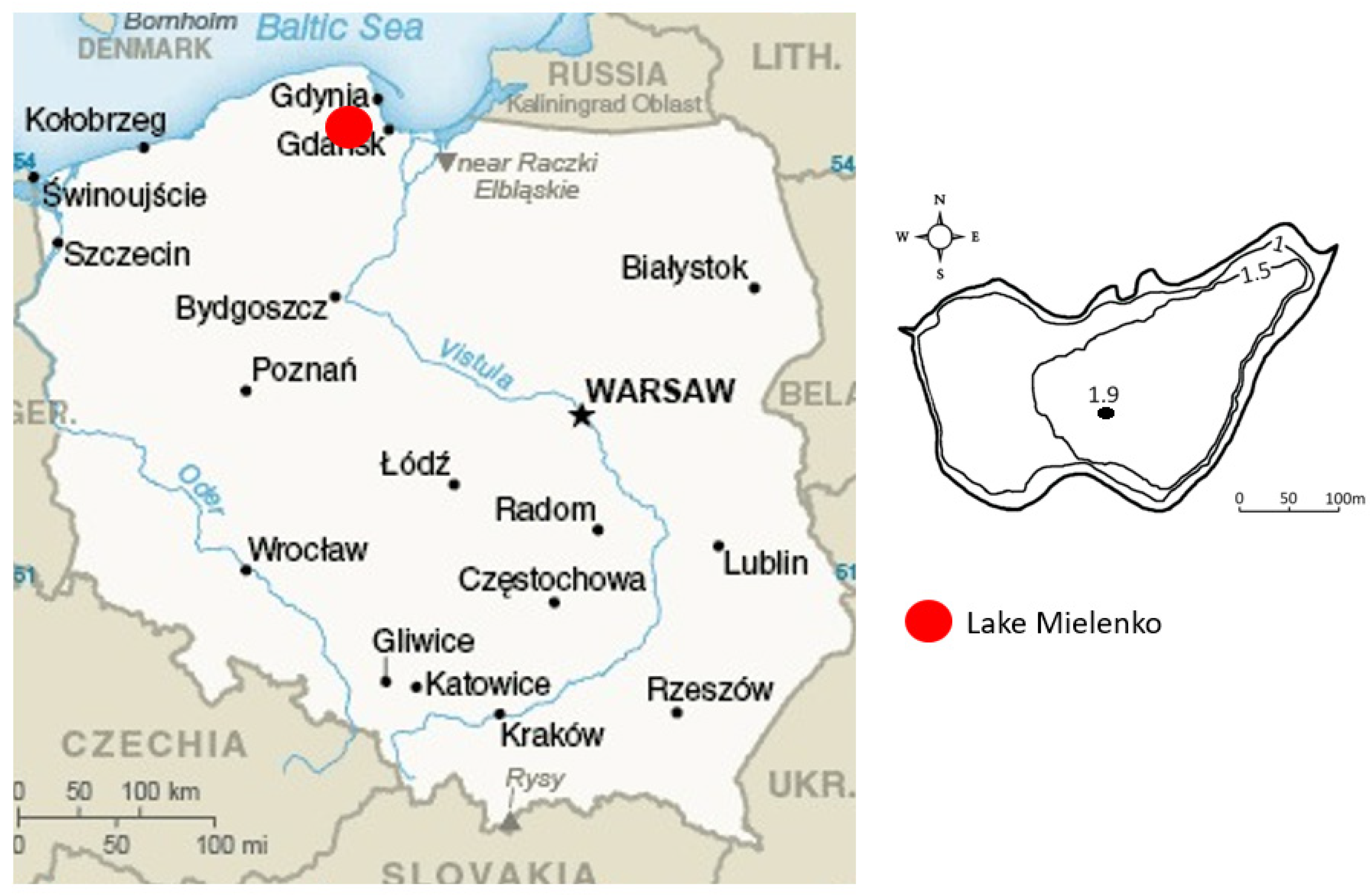
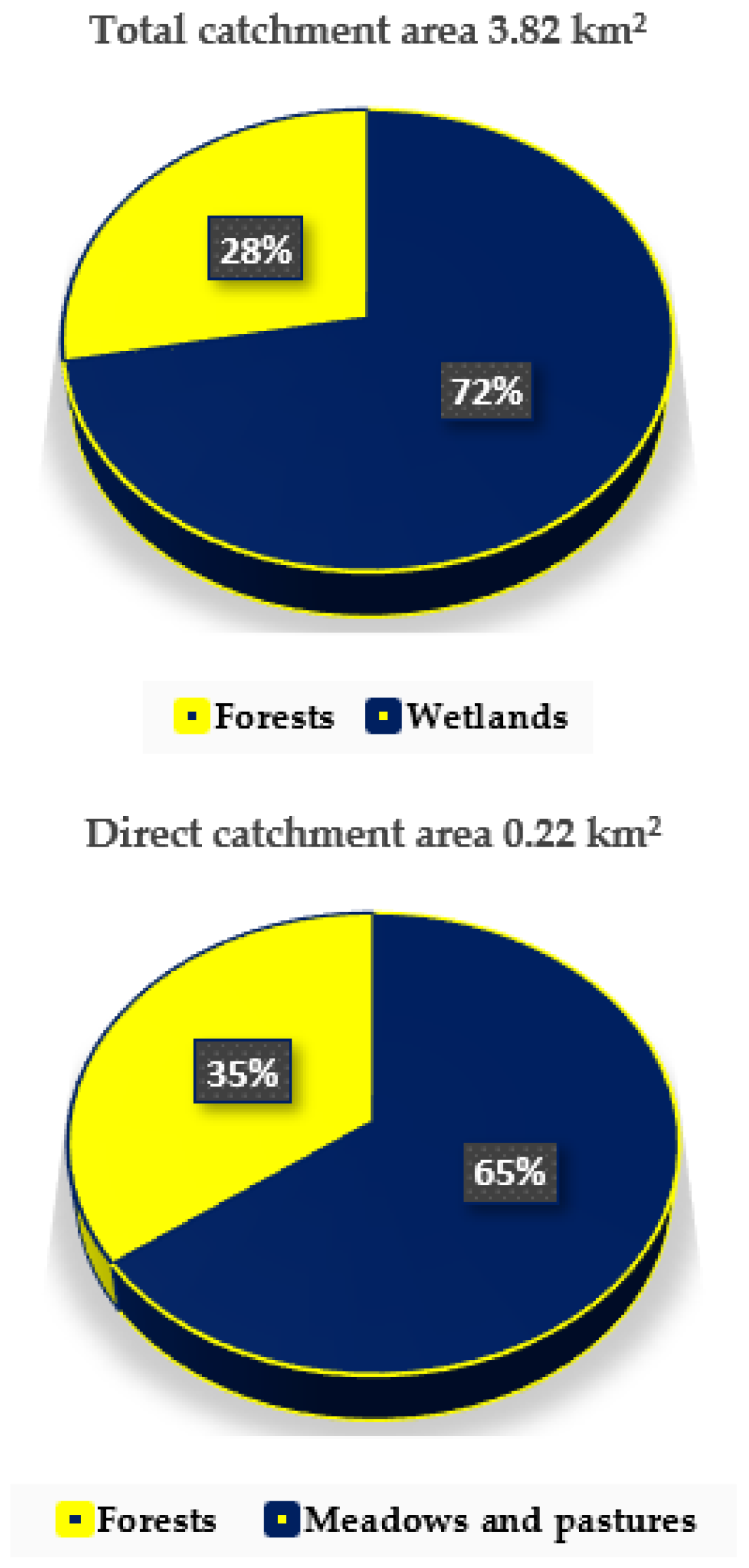
2.1. Description of This Study Area
2.2. The Collection of Water Samples and Laboratory Analysis
2.3. Restoration of Lake Mielenko
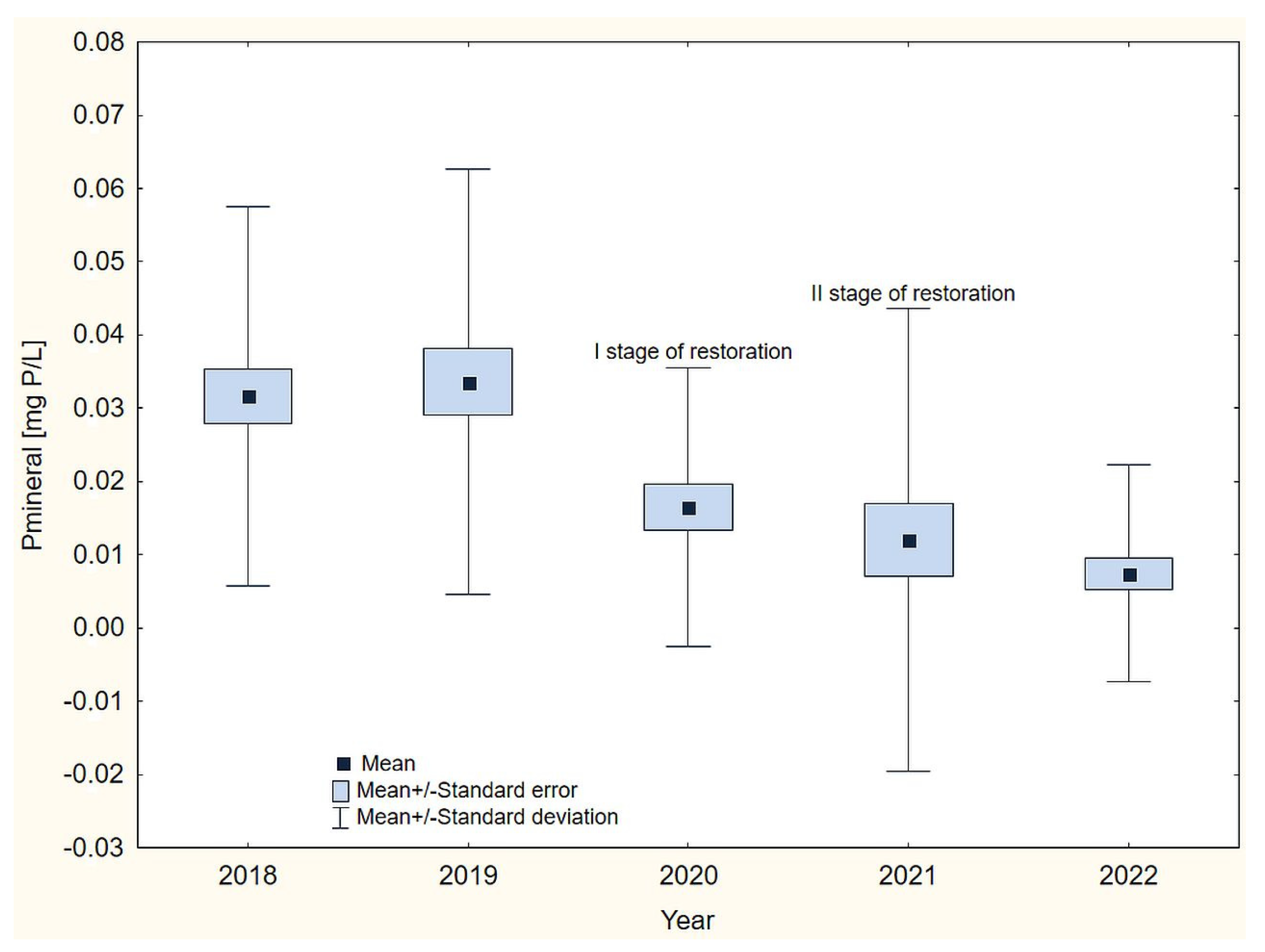
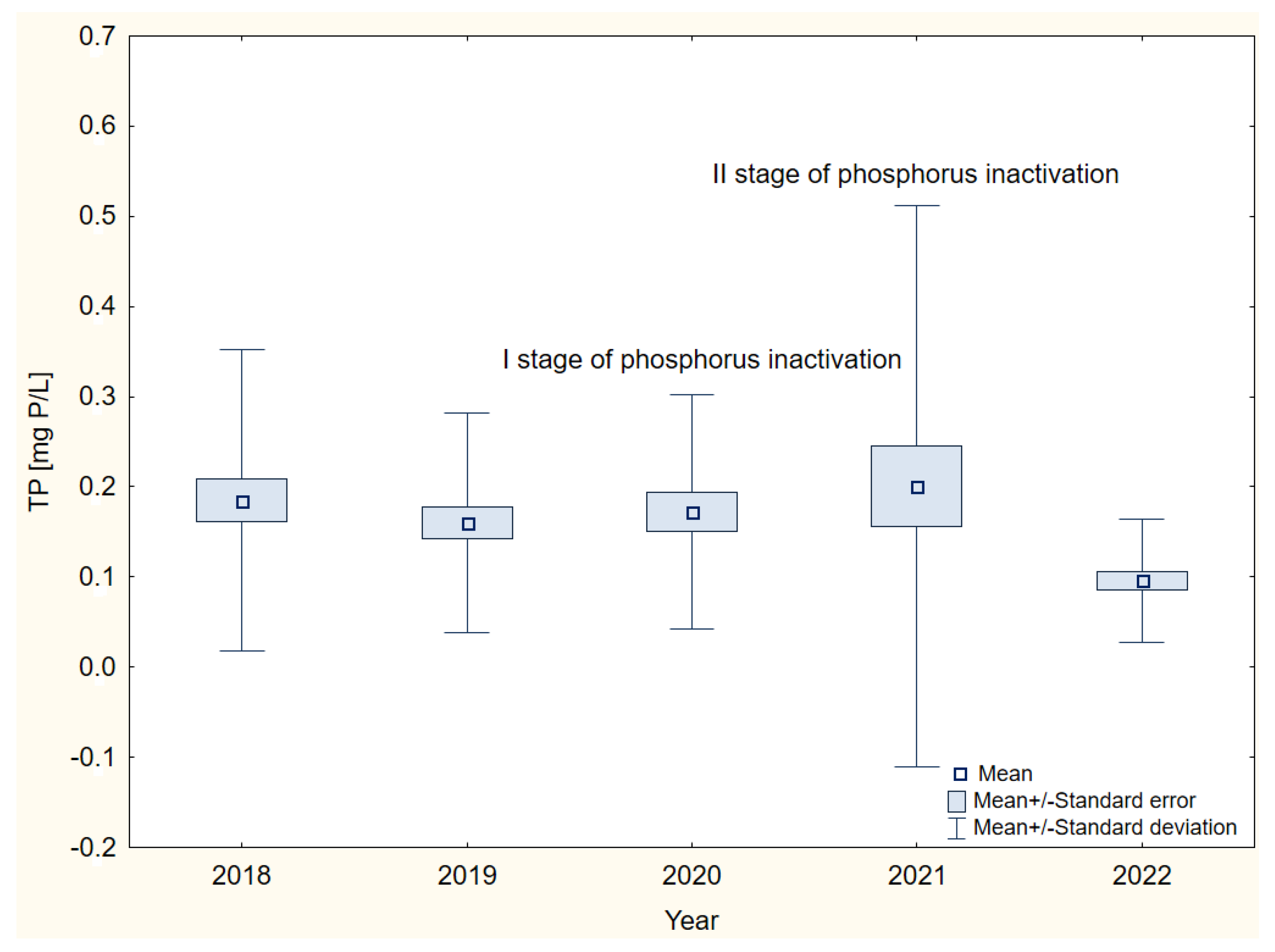
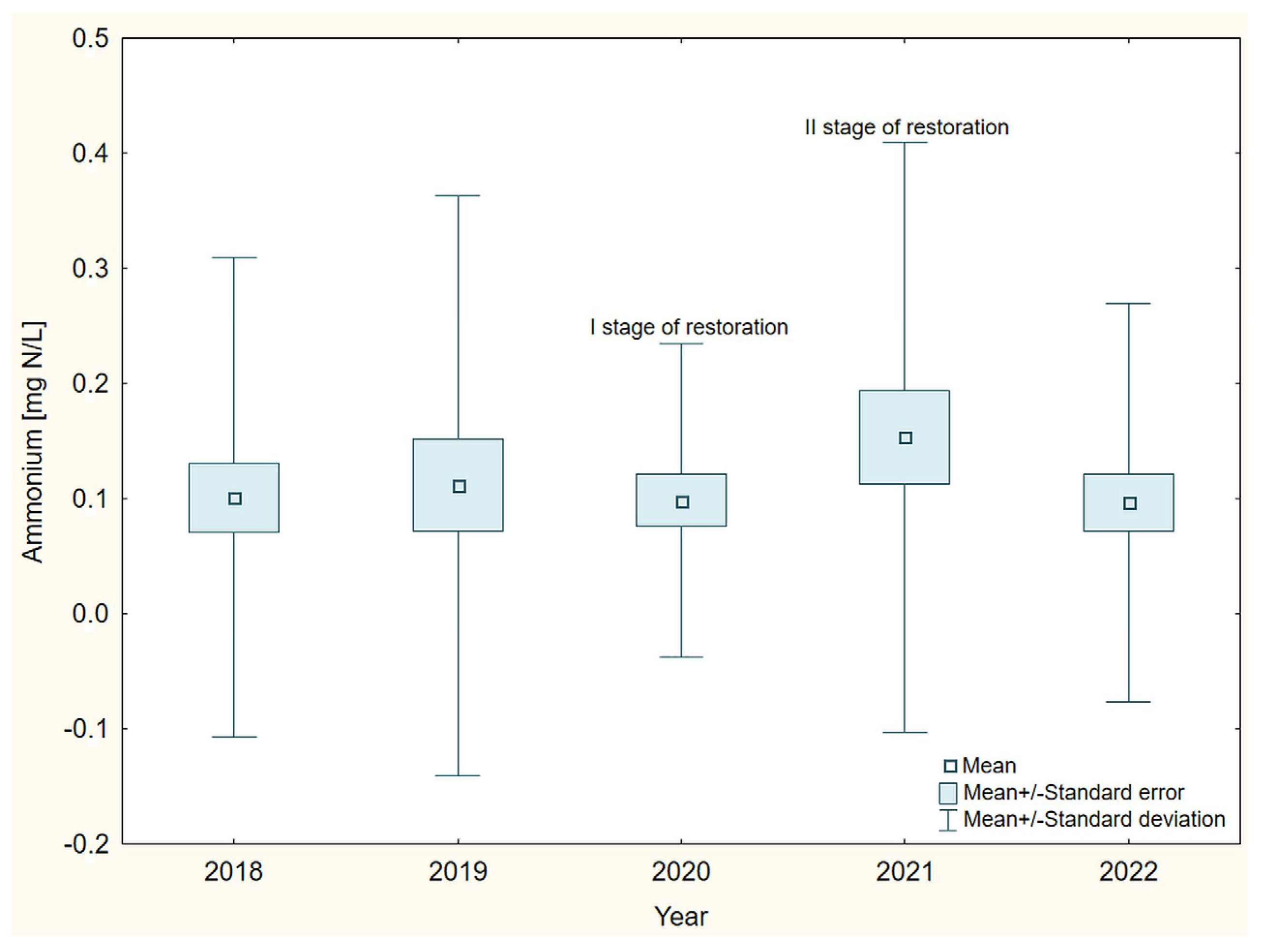
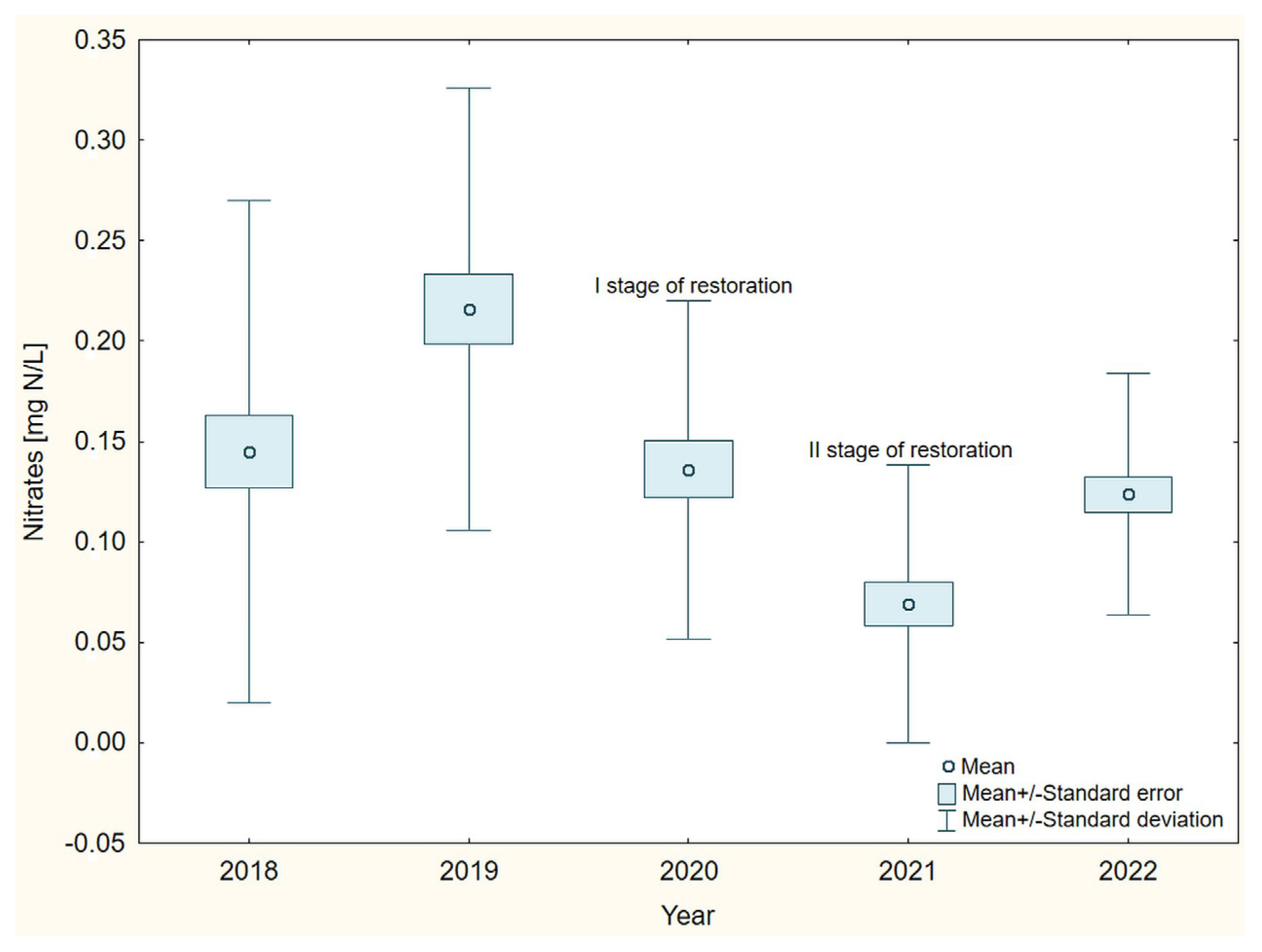
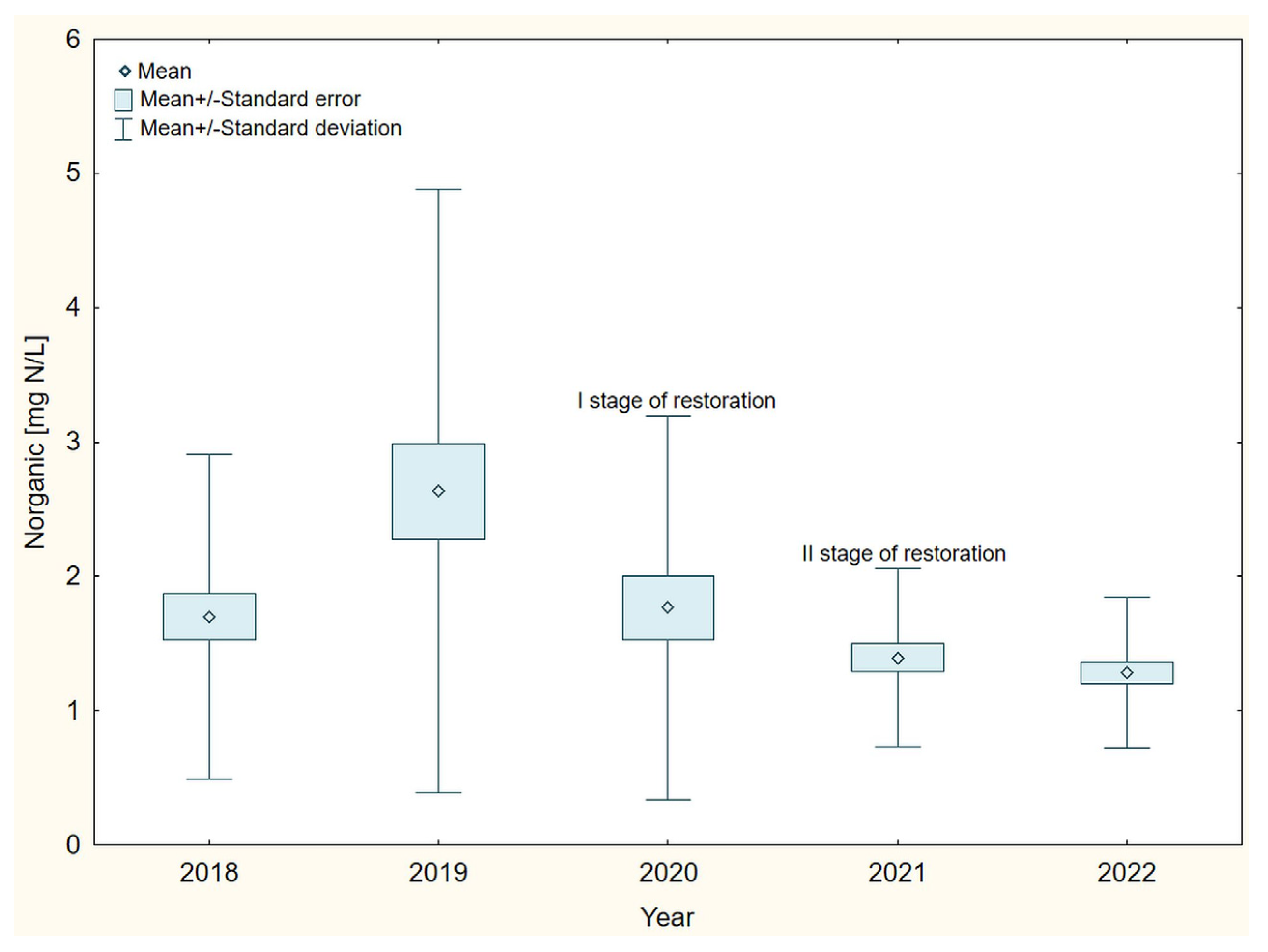
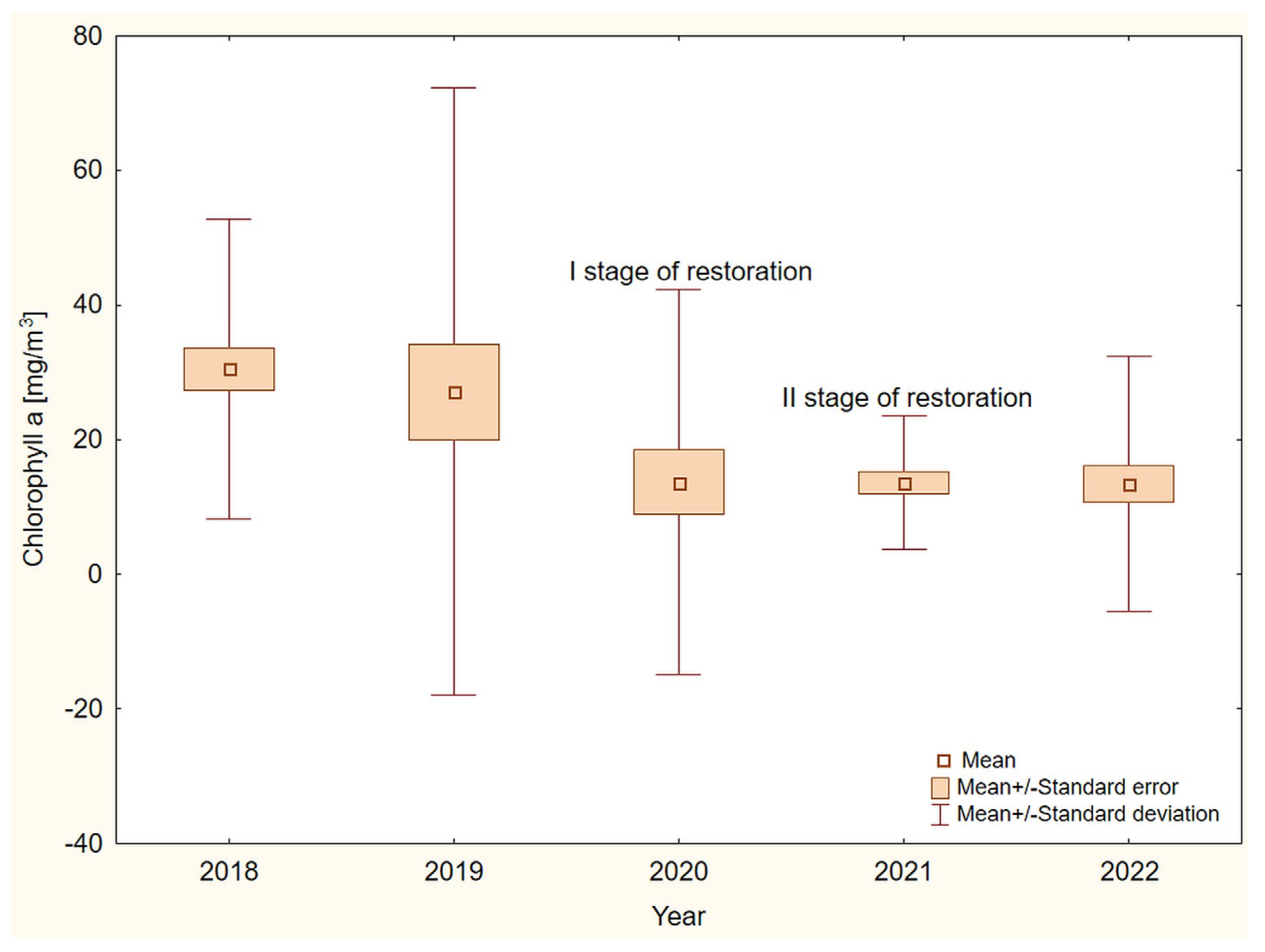
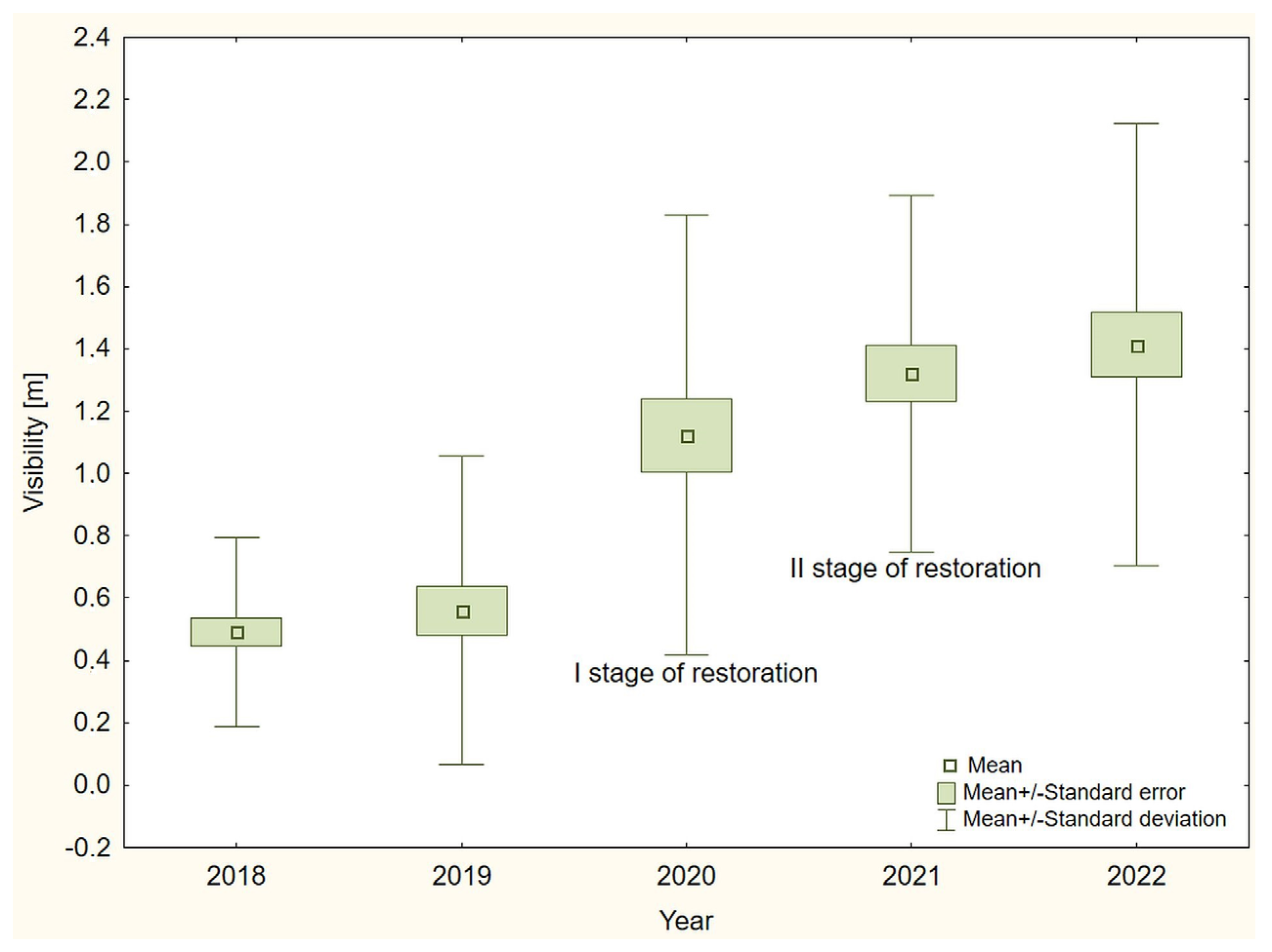
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Wu, X.; Hao, H.; He, Z. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ. Sci. B 2008, 9, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-S.; Shen, S.-L.; Zhou, A.; Lyu, H.-M. Assessment and management of lake eutrophication: A case study in Lake Erhai, China. Sci. Total. Environ. 2021, 751, 141618. [Google Scholar] [CrossRef] [PubMed]
- Chislock, M.F.; Doster, E.; Zitomer, R.A.; Wilson, A.E. Eutrophication: Causes, Consequences, and Controls in Aquatic Ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Carpenter, S.R. Eutrophication of aquatic ecosystems: Biostability and soil phosphorus. Proc. Natl. Acad. Sci. USA 2009, 12, 10002–10005. [Google Scholar] [CrossRef] [PubMed]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of U.S. Freshwaters: Analysis of potential Economic Damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sonarghare, P.C.; Masram, S.C.; Sonparote, U.R.; Khaparue, K.P.; Kharkate, S.K. Causes and effects of eutrophication on aquatic life. Essence Int. J. Environ. Rehabil. Conserv. 2020, 11, 213–218. [Google Scholar] [CrossRef]
- Garg, J.; Garg, H.K. Nutrient loading and its consequences in a lake ecosystem. Trop. Ecol. 2002, 43, 355–358. [Google Scholar]
- Ashour, M.F.Y.; Guan, D.; Zheng, G. Influencing factors analysis for algal blooms in cold reservoir. J. Phys. Conf. Ser. 2021, 1732, 012147. [Google Scholar] [CrossRef]
- Gawrońska, H.; Brzozowska, R.; Grochowska, J.; Lossow, K. Possibilities to reduce internal loading to lake water by artificial aeration. Pol. J. Environ. Stud. 2003, 12, 171–179. [Google Scholar]
- Wilson, A.E.; Sarnelle, O.; Tillmanns, A.R. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: Meta-analyses of laboratory experiments. Limnol. Oceanogr. 2006, 51, 1915–1924. [Google Scholar] [CrossRef]
- Arend, K.K.; Beletsky, D.; DePinto, J.U.; Ludsin, S.A.; Roberts, J.J.; Rucinski, D.K.; Scavia, D.; Schwab, D.J.; Höök, T.O. Seasonal and interannual effects of hypoxia of fish habitat quality in central Lake Erie. Freshw. Biol. 2011, 56, 366–383. [Google Scholar] [CrossRef]
- Radosavljevic, J.; Slowinski, S.; Shafii, M.; Akbarzadeh, Z.; Rezanezhad, F.; Parsons, C.T.; Withers, W.; Van Cappellen, P. Salinization as a driver of eutrophication symptoms in an urban lake (Lake Wilcox, Ontario, Canada). Sci. Total Environ. 2022, 846, 157336. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Bu, W.; Hu, X.; Liu, B.; Liang, K.; Chen, F. Species divergence in seedling leaf traits and tree growth response to nitrogen and phosphorus additions in an evergreen broadleaved forest of subtropical China. J. For. Res. 2023, 34, 137–150. [Google Scholar] [CrossRef]
- Water Framework Directive. Directive 2000/60/EC of European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32000L0060 (accessed on 20 August 2023).
- Shepherd, B.; Harper, D.; Millington, A. Modelling catchment-scale nutrient transport to water-courses in the UK. Hydrobiologia 1999, 395, 227–238. [Google Scholar] [CrossRef]
- Braskerud, B.C. Factors affecting phosphorus retention in small constructed wetlands treating agricultural non-point source pollution. Ecol. Eng. 2002, 19, 41–62. [Google Scholar] [CrossRef]
- Grochowska, J.; Tandyrak, R.; Wiśniewski, G. Long-term hydrochemical changes in a lake after the application of several protection measures in the catchment. Pol. J. Nat. Sci. 2014, 29, 251–263. [Google Scholar]
- Dondajewska, R.; Kozak, A.; Budzyńska, A.; Kowalczewska-Madura, K.; Gołdyn, R. Nature-based solutions for protection and restoration of degraded Bielsko Lake. Ecohydrol. Hydrobiol. 2018, 18, 401–411. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, W.; Jin, X.; Shan, B. Using biochar capping to reduce nitrogen release from sediments in eutrophic lakes. Sci. Total Environ. 2019, 646, 93–104. [Google Scholar] [CrossRef]
- Kowalczewska-Madura, K.; Dondajewska-Pielka, R.; Gołdyn, R. The assessment of external and internal nutrient loading as a basis for lake management. Water 2022, 14, 2844. [Google Scholar] [CrossRef]
- Kowalczewska-Madura, K.; Dondajewska, R.; Gołdyn, R. Influence of iron treatment on phosphorus internal loading from bottom sediments of the restored lake. Limnol. Rev. 2008, 8, 177–182. [Google Scholar]
- Zamparas, M.; Zacharias, I. Restoration of eutrophic freshwater by managing internal nutrient loads. A review. Sci. Total Environ. 2014, 496, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Klapper, H. Technologies for lake restoration. J. Limnol. 2003, 62, 73–90. [Google Scholar] [CrossRef]
- Dunalska, J.A.; Wiśniewski, G.; Mientki, C. Assessment of multi-year (1956–2003) hypolimnetic withdrawal from Lake Kortowskie, Poland. Lake Reserv. Manag. 2007, 23, 377–387. [Google Scholar] [CrossRef]
- Welch, E.B. The dilution/flushing technique in lake restoration. J. Am. Water Resour. Assoc. 1981, 17, 558–564. [Google Scholar] [CrossRef]
- Schönach, P.; Tapio, P.; Holmroos, H.; Horpilla, J.; Niemiströ, J.; Nygrĕn, N.A.; Tammeorg, O.; Massa, I. Persistency of artificial aeration at hypertrophic Lake Tuusulaujärvi: A sociohistorical analysis. Ambio 2017, 46, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Little, J.C. Designing hypolimnetic aeration and oxygenation systems—A review. Environ. Sci. Technol. 2006, 40, 7512–7520. [Google Scholar] [CrossRef]
- Patermo, M.; Schroeder, P.; Estes, T.; Francingues, N. Technical Guidelines for Environmental Dredging of Contaminated Sediments; DC 20314-1000; U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response: Washington, DC, USA, 2008; pp. 1–302.
- Abell, J.M.; Özkundakci, D.; Hamilton, D.P.; Reeves, P. Restoring shallow lakes impaired by eutrophication: Approaches, outcomes, and challenges. Crit. Rev. Environ. Sci. Technol. 2020, 52, 1199–1246. [Google Scholar] [CrossRef]
- Ripl, W. Restoration of eutrophic lakes by sediment treatment. In Restoration of Lakes, Streams, Floodplains, and Bogs in Europe; Springer: Dordrecht, The Netherlands, 2010; pp. 77–84. [Google Scholar] [CrossRef]
- Findlay, D.L.; Vanni, M.J.; Paterson, M.; Mills, K.H.; Kasian, S.E.M.; Findlay, W.J.; Salki, A.G. Dynamics of boreal lake ecosystem during a long-term manipulation of top predators. Ecosystems 2005, 8, 603–618. [Google Scholar] [CrossRef]
- Dhate, S. Role of macrophytes in improving water quality of an aquatic ecosystem. J. Appl. Sci. Environ. Manag. 2007, 11, 133–135. [Google Scholar]
- Deppe, T.; Benndorf, J. Phosphorus reduction in a shallow hyperthropic reservoir by in-lake dosage of ferrus iron. Water Res. 2002, 36, 4525–4534. [Google Scholar] [CrossRef]
- Lewandowski, J.; Schauser, I.; Hupfer, M. Long term effects of phosphorus precipitations with alum in hypereutrophic Lake Süsser See (Germany). Water Res. 2003, 37, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- Kondracki, J.A. Regional Geography of Poland, 3rd ed.; PWN Warsaw: Warsaw, Poland, 2011. [Google Scholar]
- Vollenweider, R.A. Advances in defining critical loading level for phosphorus in lake eutrophication. Mem. Inst. Ital. Hydrobiol. 1976, 33, 53–83. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Water Works Association: Denver, CO, USA, 2017; ISBN 978-0875532875. [Google Scholar]
- Kaca, E. Measurements of water flow volume and mass of substance contained in it, and its uncertainty on the example of fish ponds. Water-Environ. Rural. Areas 2003, 13, 31–57. (In Polish) [Google Scholar]
- Tibco Software Inc. STATISTICA, Version 13.3; Tibco Software Inc.: Palo Alto, CA, USA, 2021. [Google Scholar]
- Meerhoff, M.; Jeppesen, E. Shallow lakes and ponds. In Encyclopedia of Inland Waters; Academic Press: Cambridge, MA, USA, 2009; pp. 645–655. [Google Scholar] [CrossRef]
- Meerhoff, M.; González-Sagrario, M.d.L.A. Habitat complexity in shallow lakes and ponds: Importance, threats, and potential for restoration. Hydrobiologia 2022, 849, 3737–3760. [Google Scholar] [CrossRef]
- Scheffer, M. Ecology of Shallow Lakes; Population and Community Biology Series; Springer Science + Business Media: Dordrecht, The Netherlands, 2004; pp. 1–378. [Google Scholar]
- Jeppesen, E.; Canfield, D.E.; Bachmann, R.W.; Søndergaard, M.; Havens, K.E.; Johansson, L.S.; Lauridsen, T.L.; Sh, T.; Rutter, R.P.; Warren, G.; et al. Toward predicting climate change effects on lakes: A comparison of 1656 shallow lakes from Florida and Denmark reveals substantial differences in nutrient dynamics, metabolism, trophic structure, and top-down control. Inland Waters 2020, 10, 197–211. [Google Scholar] [CrossRef]
- Alhamarna, M.Z.; Tandyrak, R. Lake restoration approaches. Limnol. Rev. 2021, 21, 105–118. [Google Scholar] [CrossRef]
- Grochowska, J.K.; Augustyniak-Tunowska, R. The influence of various restoration techniques on the content of selected ions in water of an urban lake. Sustainability 2023, 15, 12617. [Google Scholar] [CrossRef]
- Jilbërt, T.; Couture, R.-M.; Huser, B.J.; Salonen, K. Preface: Restoration of eutrophic lakes: Current practices and future challenges. Hydrobiologia 2020, 847, 4343–4357. [Google Scholar] [CrossRef]
- Augustyniak-Tunowska, R.; Grochowska, J.; Łopata, M.; Parszuto, K.; Tandyrak, R.; Tunowski, J. Sorption properties of the bottom sediment of a lake restored by phosphorus inactivation method 15 years after the termination of lake restoration procedures. Water 2019, 11, 2175. [Google Scholar] [CrossRef]
- Cyr, H.; McCabe, S.K.; Nürnberg, G.K. Phosphorus sorption experiments and the potential for internal phosphorus loading in littoral areas of a stratified lake. Water Res. 2009, 43, 1654–1666. [Google Scholar] [CrossRef]
- Golterman, H.L. The calcium and iron bound phosphate phase diagram. Hydrobiologia 1988, 159, 149–151. [Google Scholar] [CrossRef]
- Kowalczewska-Madura, K.; Dondajewska, R.; Gołdyn, R.; Kozak, A.; Messyasz, B. Internal phosphorus loading from the bottom sediments of a dimictic lake during its sustainable restoration. Water Air Soil Pollut. 2018, 229, 280. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, R.; Wang, J.; He, K.; Chen, J. Fluxes and mechanisms of phosphorus release from sediments in seasonal hypoxic reservoirs: A simulation-based experimental study. J. Soils Sediments 2021, 21, 3246–3258. [Google Scholar] [CrossRef]
- Tammeorg, O.; Nürnberg, G.; Niemistö, J.; Haldna, M.; Horppila, J. Internal phosphorus loading due to sediment anoxia in shallow areas: Implication for lake aeration treatments. Aquat. Sci. 2020, 82, 54. [Google Scholar] [CrossRef]
- Sakai, S.; Nakaya, M.; Sampei, Y.; Dettman, D.L.; Takayasu, K. Hydrogen sulfide and organic carbon at the sediment-water interface in coastal brackish Lake Nakaumi, SW Japan. Environ. Earth Sci. 2013, 68, 1999–2006. [Google Scholar] [CrossRef]
- Bojakowska, I. Phosphorus in lake sediments of Poland—Results of monitoring research. Limnol. Rev. 2016, 16, 15–25. [Google Scholar] [CrossRef]
- Gunnars, A.; Blomqvist, S. Phosphate exchange across the sediment-water interface when shifting from anoxic to oxic conditions: An experimental comparison of freshwater and brackish-marine systems. Biogeochemistry 1997, 37, 203–226. [Google Scholar] [CrossRef]
- Meyers, P.A.; Ishiwatri, R. Organic matter accumulation records in lake sediments. In Physics and Chemistry of Lakes; Lerman, A., Imboden, D.M., Gat, J.R., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1995. [Google Scholar]
- Meyers, P.A.; Lallier-Verges, E. Lacustrine sedimentary organic matter records of Lake Quaternary paleoclimates. J. Paleolimnol. 1999, 21, 345–372. [Google Scholar] [CrossRef]
- Krupińska, I. Impact of polyelectrolytes on the effectiveness of treatment of groundwater with increased natural organic matter content. Civ. Environ. Eng. Rep. 2018, 28, 17–29. [Google Scholar] [CrossRef]
- Krupińska, I.; Konkol, A. Impact of selected technological parameters on the course and effectiveness of the process coagulation in groundwater purification. Environ. Eng. 2015, 37, 1–38. (In Polish) [Google Scholar]
- Wágner, D.S.; Cazzaniga, C.; Steidl, M.; Dechesne, A.; Valverde-Pérez, B.; Plósz, B.G. Optimal influent N-to-P ratio for stable microalgal cultivation in water treatment and nutrient recovery. Chemosphere 2021, 262, 127939. [Google Scholar] [CrossRef] [PubMed]
- Tango, M.D.; Filho, J.A.Z.; Daniel, L.A.; de Souza Leite, L.; Hoffmann, M.T.; Mountinho, F.H.M. Effects of bicarbonate addition and N:P ratio on microalgae growth and resource recovery from domestic wastewater. AgriEngineering 2023, 5, 1178–1195. [Google Scholar] [CrossRef]


| TOC [mg/L] | BOD5 [mg/L] | Reaction [pH] | EC [µS/cm] | Seston [mg/L] | ||
|---|---|---|---|---|---|---|
| 2018 | Average | 26.97 | 8.9 | 8.15 | 419 | 10.2 |
| Standard Deviation | 4.19 | 1.9 | 0.54 | 29 | 5.4 | |
| Minimum | 21.55 | 7.0 | 7.55 | 382 | 4.3 | |
| Maximum | 33.14 | 12.5 | 9.14 | 475 | 20.8 | |
| 2019 | Average | 18.51 | 6.0 | 8.48 | 660 | 13.7 |
| Standard Deviation | 4.86 | 1.9 | 0.52 | 92 | 8.8 | |
| Minimum | 12.95 | 3.4 | 7.50 | 566 | 2.0 | |
| Maximum | 28.5 | 10.1 | 9.81 | 793 | 33.0 | |
| 2020 | Average | 17.91 | 5.4 | 7.83 | 689 | 8.0 |
| Standard Deviation | 7.88 | 1.1 | 0.51 | 47 | 3.9 | |
| Minimum | 11.01 | 3.9 | 6.71 | 610 | 1.4 | |
| Maximum | 35.43 | 7.3 | 8.50 | 737 | 14.5 | |
| 2021 | Average | 13.33 | 5.2 | 7.97 | 754 | 5.4 |
| Standard Deviation | 1.30 | 2.0 | 0.24 | 27 | 2.6 | |
| Minimum | 11.30 | 1.6 | 7.67 | 700 | 0.8 | |
| Maximum | 16.16 | 8.9 | 8.43 | 794 | 11.0 | |
| 2022 | Average | 14.91 | 4.6 | 8.13 | 647 | 5.0 |
| Standard Deviation | 1.89 | 1.6 | 0.40 | 77 | 2.5 | |
| Minimum | 11.51 | 2.4 | 7.51 | 517 | 1.6 | |
| Maximum | 18.24 | 7.5 | 8.78 | 736 | 9.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochowska, J.K.; Łopata, M.; Augustyniak-Tunowska, R.; Tandyrak, R. Sequential Application of Different Types of Coagulants as an Innovative Method of Phosphorus Inactivation, on the Example of Lake Mielenko, Poland. Sustainability 2023, 15, 16346. https://doi.org/10.3390/su152316346
Grochowska JK, Łopata M, Augustyniak-Tunowska R, Tandyrak R. Sequential Application of Different Types of Coagulants as an Innovative Method of Phosphorus Inactivation, on the Example of Lake Mielenko, Poland. Sustainability. 2023; 15(23):16346. https://doi.org/10.3390/su152316346
Chicago/Turabian StyleGrochowska, Jolanta Katarzyna, Michał Łopata, Renata Augustyniak-Tunowska, and Renata Tandyrak. 2023. "Sequential Application of Different Types of Coagulants as an Innovative Method of Phosphorus Inactivation, on the Example of Lake Mielenko, Poland" Sustainability 15, no. 23: 16346. https://doi.org/10.3390/su152316346





