Numerical Simulation of the Ca(OH)2/CaO Thermochemical Heat Storage Process in an Internal Heating Fixed-Bed Reactor
Abstract
:1. Introduction
2. Reactor Description and Numerical Model
2.1. Reactor Description
2.2. Numerical Model
2.2.1. Chemical Reaction
2.2.2. Governing Equations for Water Vapor
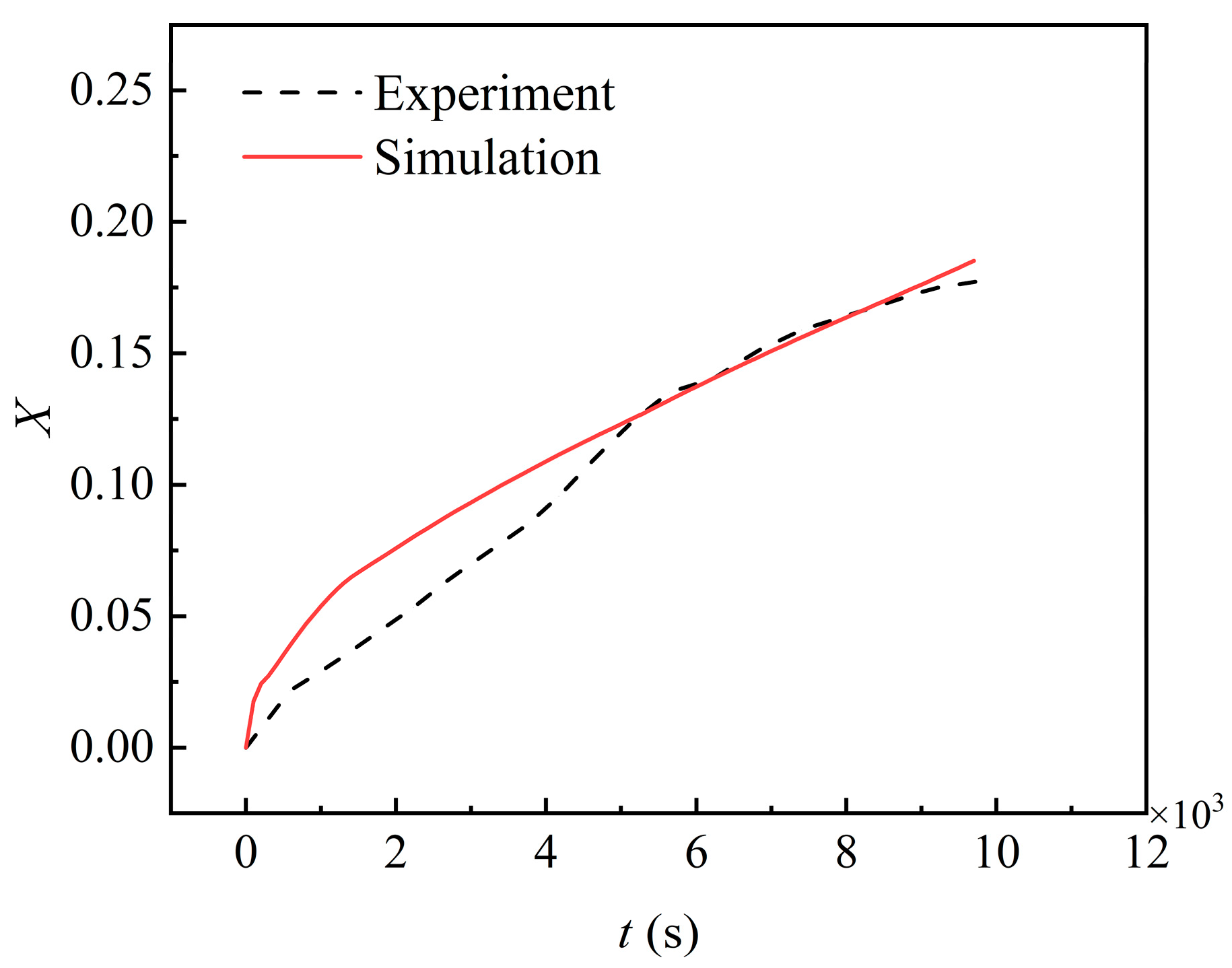
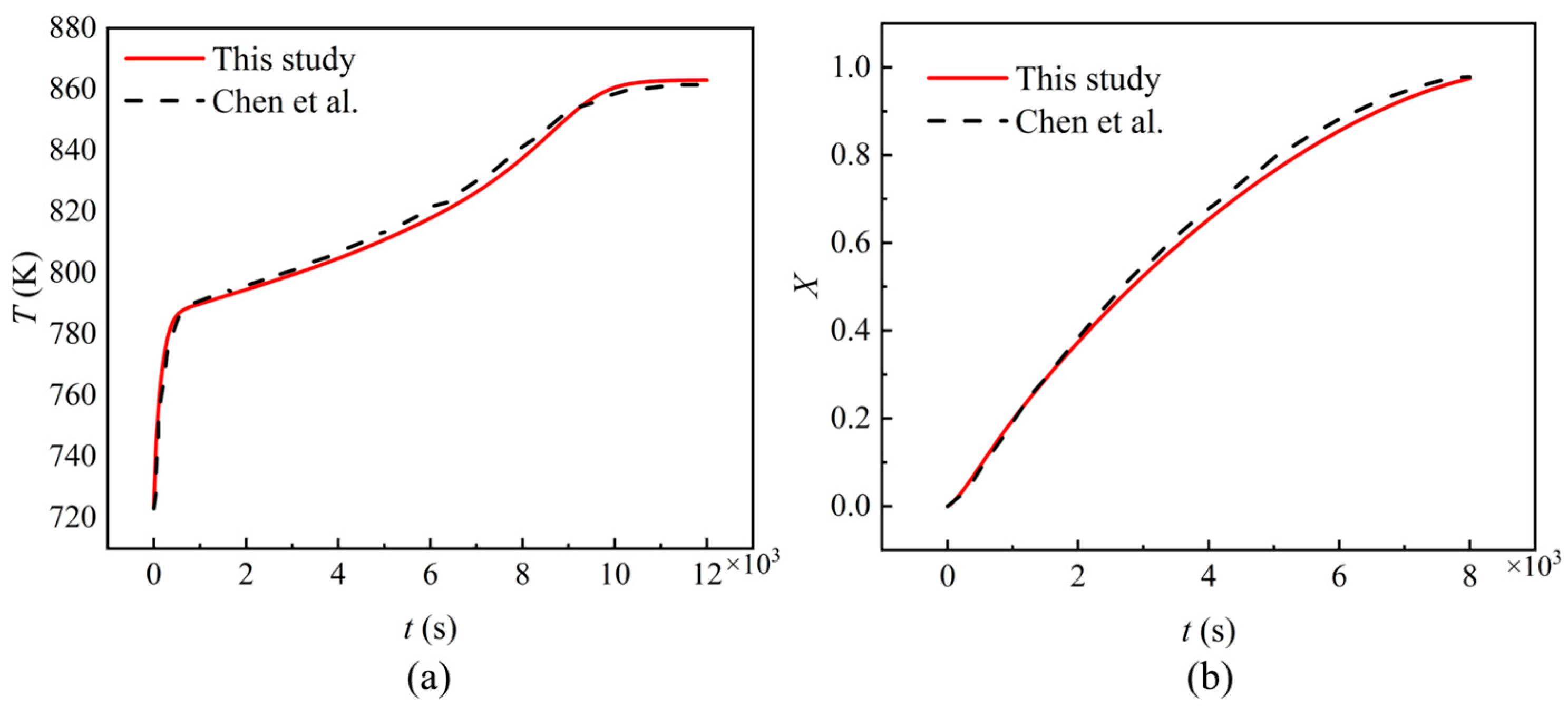
2.2.3. Validation
3. Results and Discussion
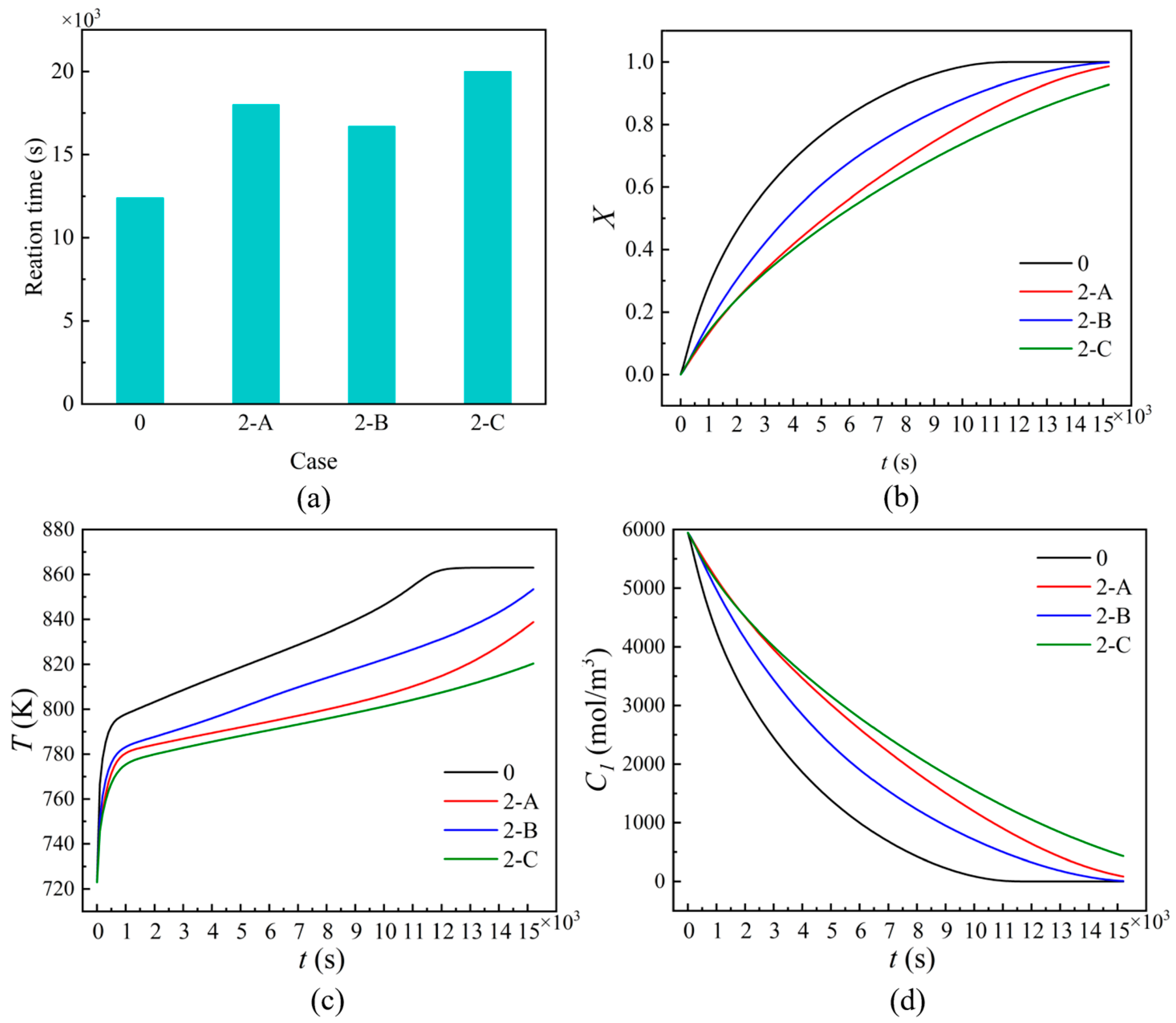
3.1. Effects of the Heating Mode
3.2. Temperature and Reaction Extent Distribution
3.3. Effects of Different Conditions
3.3.1. Solid Particle Porosity
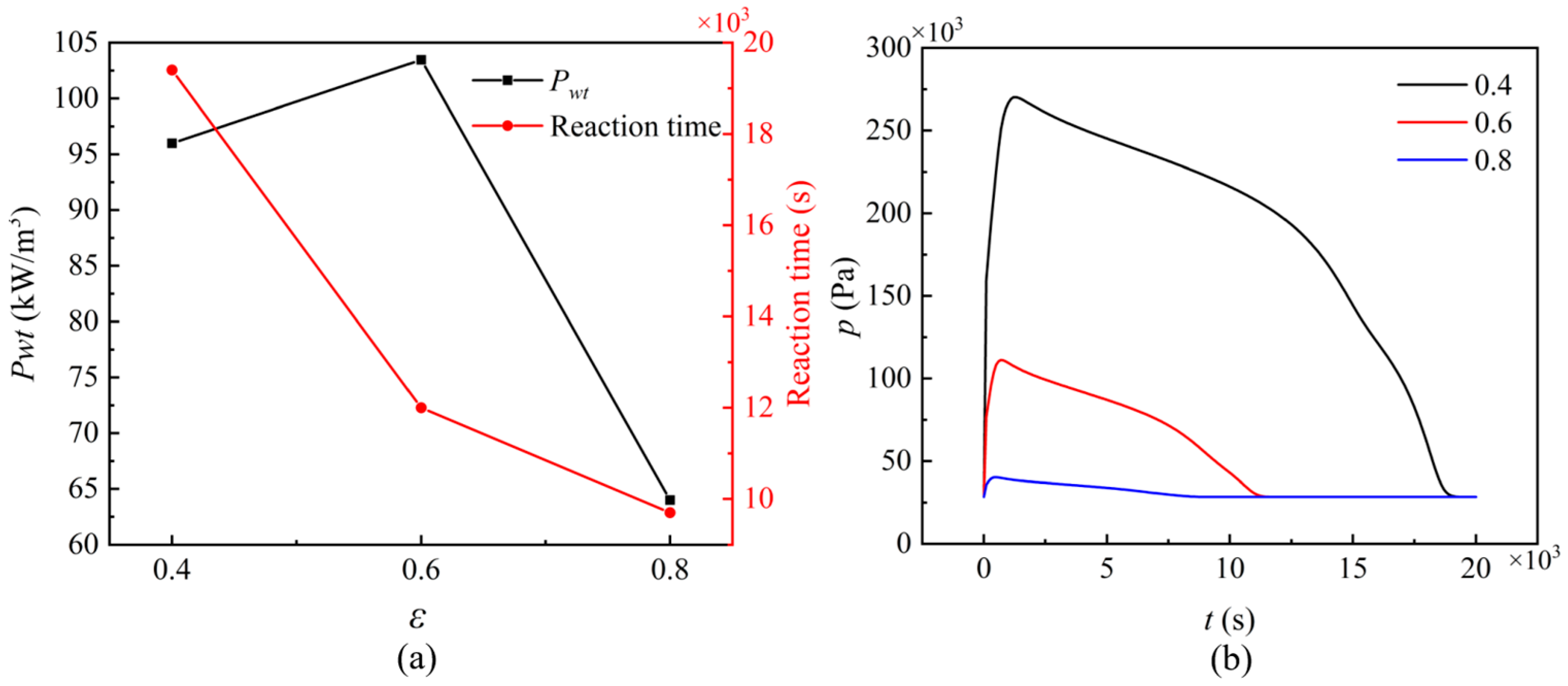
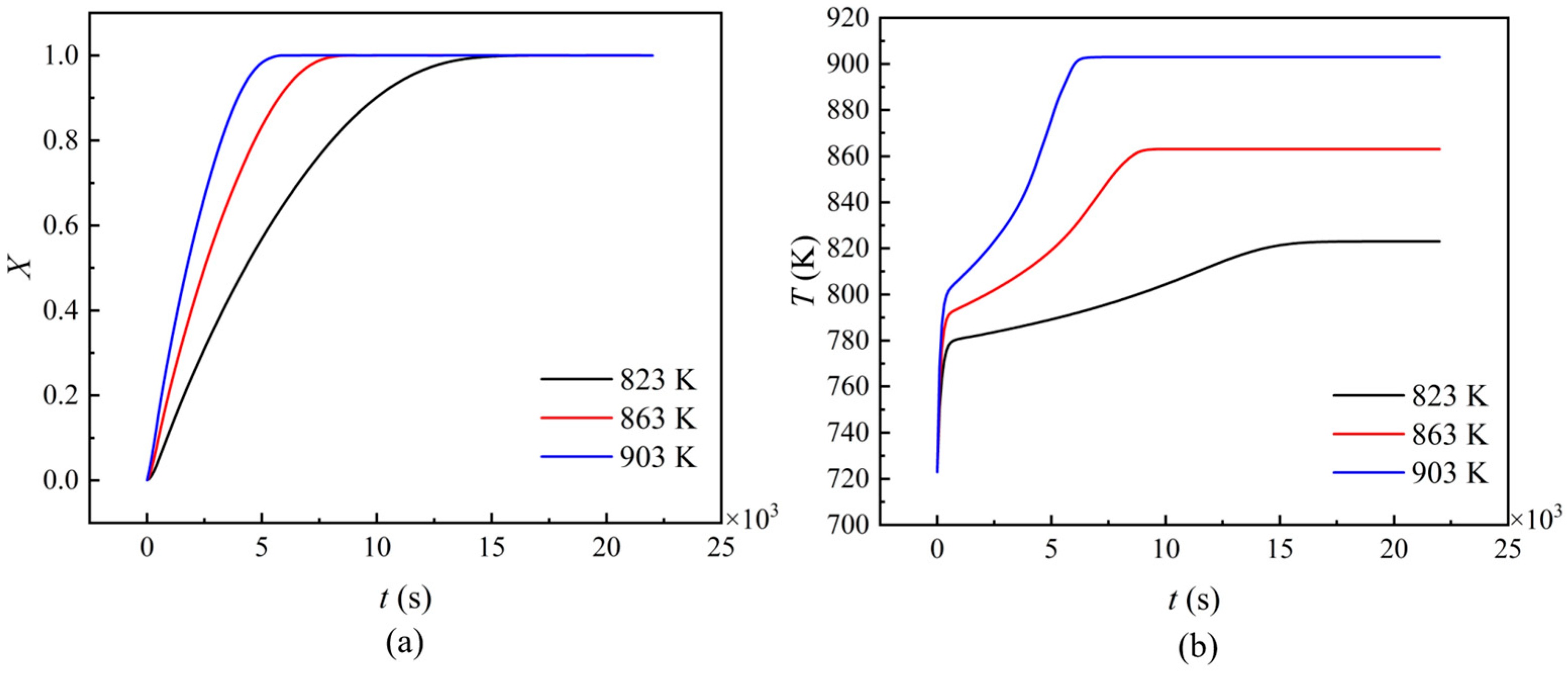
3.3.2. Wall Temperature
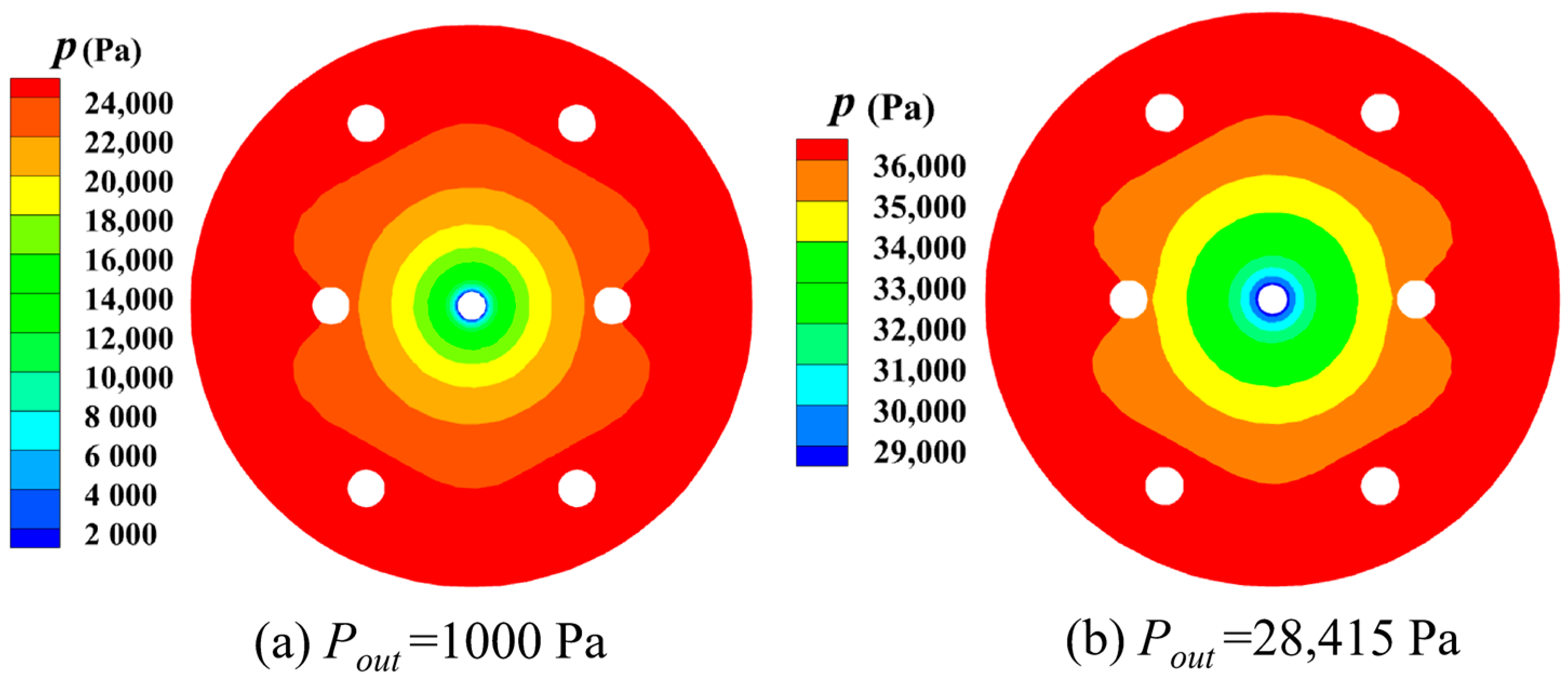
3.3.3. Outlet Pressure
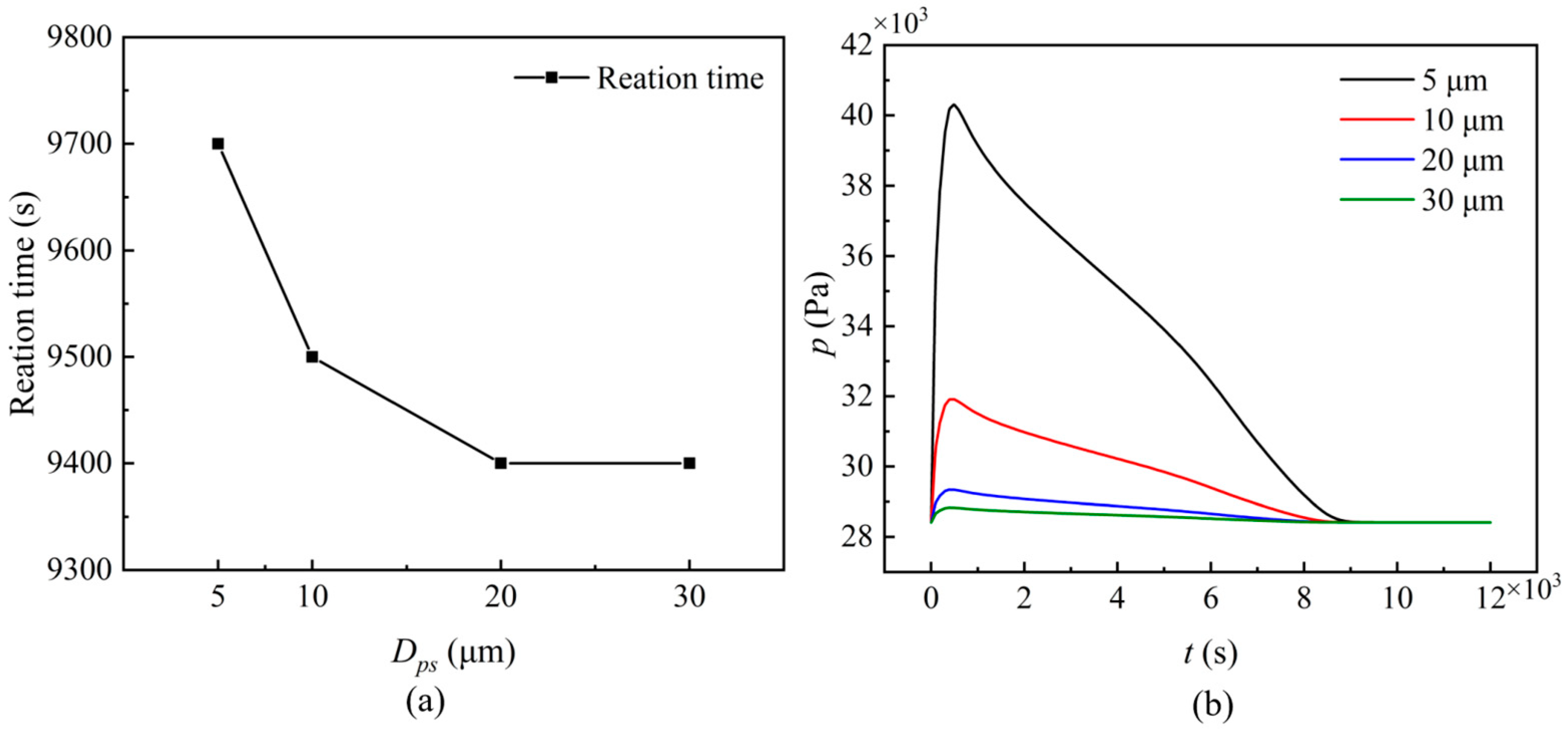
3.3.4. Solid Particle Size
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behabtu, H.A.; Messagie, M.; Coosemans, T.; Berecibar, M.; Anlay Fante, K.; Kebede, A.A.; Mierlo, J.V. A review of energy storage technologies’ application potentials in renewable energy sources grid integration. Sustainability 2020, 12, 10511. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, H.Y.; Qi, J.H.; Han, K.H.; He, S.Y.; Guo, C.; Cheng, S.; Gao, M. Thermodynamic and exergy analysis of a novel PEMFC-ORC-MH combined integrated energy system. Energy Conv. Manag. 2022, 264, 115709. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Pan, Z.H.; Zhao, C.Y. Gas-solid thermochemical heat storage reactors for high-temperature applications. Energy 2017, 130, 155–173. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, Y.L.; Xu, H.X.; Du, R.Q.; Wang, Y. Review on air and water thermal energy storage of buildings with phase change materials. Exp. Comput. Multiph. Flow 2021, 3, 77–99. [Google Scholar] [CrossRef]
- Li, H.T.; Wang, N.N.; Zhao, B.; Feng, H.M.; Han, K.H.; He, S.Y.; Gao, M. Simulation study on the effect of fins on the heat transfer performance of horizontal dual-inner-tube latent thermal energy storage heat exchangers. J. Energy Storag. 2022, 49, 104125. [Google Scholar] [CrossRef]
- Schaube, F.; Koch, L.; Woerner, A.; Mueller-Steinhagen, H. A thermodynamic and kinetic study of the de-and rehydration of Ca(OH)2 at high H2O partial pressures for thermo-chemical heat storage. Thermochim. Acta 2012, 538, 9–20. [Google Scholar] [CrossRef]
- Schmidt, M.; Gutierrez, A.; Linder, M. Thermochemical energy storage with CaO/Ca(OH)2-Experimental investigation of the thermal capability at low vapor pressures in a lab scale reactor. Appl. Energy 2017, 188, 672–681. [Google Scholar] [CrossRef]
- Wang, K.; Yan, T.; Li, R.K.; Pan, W.G. A review for Ca(OH)2/CaO thermochemical energy storage systems. J. Energy Storag. 2022, 50, 104612. [Google Scholar] [CrossRef]
- Schmidt, M.; Szczukowski, C.; Roßkopf, C.; Linder, M.; Wörner, A. Experimental results of a 10 kW high temperature thermochemical storage reactor based on calcium hydroxide. Appl. Therm. Eng. 2014, 62, 553–559. [Google Scholar] [CrossRef]
- Ranjha, Q.; Oztekin, A. Numerical analyses of three-dimensional fixed reaction bed for thermochemical energy storage. Renew. Energy 2017, 111, 825–835. [Google Scholar] [CrossRef]
- Dai, L.; Long, X.-F.; Lou, B.; Wu, J. Thermal cycling stability of thermochemical energy storage system Ca(OH)2/CaO. Appl. Therm. Eng. 2018, 133, 261–268. [Google Scholar] [CrossRef]
- Azpiazu, M.N.; Morquillas, J.M.; Vazquez, A. Heat recovery from a thermal energy storage based on the Ca(OH)2/CaO cycle. Appl. Therm. Eng. 2003, 23, 733–741. [Google Scholar] [CrossRef]
- Fujii, I.; Tsuchiya, K.; Higano, M.; Yamada, J. Studies of an energy storage system by use of the reversible chemical reaction: CaO + H2O = Ca(OH)2. Sol. Energy 1985, 34, 367–377. [Google Scholar] [CrossRef]
- Ranjha, Q.; Vahedi, N.; Oztekin, A. High-temperature thermochemical energy storage-heat transfer enhancements within reaction bed. Appl. Therm. Eng. 2019, 163, 114407. [Google Scholar] [CrossRef]
- Chen, J.T.; Xia, B.Q.; Zhao, C.Y. Topology optimization for heat transfer enhancement in thermochemical heat storage. Int. J. Heat Mass Transf. 2020, 154, 119785. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; He, P.; Tao, W.-Q. Numerical study and enhancement of Ca(OH)2/CaO dehydration process with porous channels embedded in reactors. Energy 2019, 181, 417–428. [Google Scholar] [CrossRef]
- Ye, H.; Tao, Y.B.; Wu, Z.H. Performance improvement of packed bed thermochemical heat storage by enhancing heat transfer and vapor transmission. Appl. Energy 2022, 326, 119946. [Google Scholar] [CrossRef]
- Rosskopf, C.; Haas, M.; Faik, A.; Linder, M.; Woerner, A. Improving powder bed properties for thermochemical storage by adding nanoparticles. Energy Conv. Manag. 2014, 86, 93–98. [Google Scholar] [CrossRef]
- Schaube, F.; Kohzer, A.; Schuetz, J.; Woerner, A.; Mueller-Steinhagen, H. De-and rehydration of Ca(OH)2 in a reactor with direct heat transfer for thermo-chemical heat storage. Part A: Experimental results. Chem. Eng. Res. Des. 2013, 91, 856–864. [Google Scholar] [CrossRef]
- Schaube, F.; Utz, I.; Woerner, A.; Mueller-Steinhagen, H. De- and rehydration of Ca(OH)2 in a reactor with direct heat transfer for thermo-chemical heat storage. Part B: Validation of model. Chem. Eng. Res. Des. 2013, 91, 865–873. [Google Scholar] [CrossRef]
- Shi, T.; Xu, H.; Qi, C.; Lei, B.; Wu, Y.; Zhao, C. Multi-physics modeling of thermochemical heat storage with enhance heat transfer. Appl. Therm. Eng. 2021, 198, 117508. [Google Scholar] [CrossRef]
- Afflerbach, S.; Kappes, M.; Gipperich, A.; Trettin, R.; Krumm, W. Semipermeable encapsulation of calcium hydroxide for thermochemical heat storage solutions. Sol. Energy 2017, 148, 1–11. [Google Scholar] [CrossRef]
- Afflerbach, S.; Afflerbach, K.; Trettin, R.; Krumm, W. Improvement of a semipermeable shell for encapsulation of calcium hydroxide for thermochemical heat storage solutions Material design and evaluation in laboratory and reactor scale. Sol. Energy 2021, 217, 208–222. [Google Scholar] [CrossRef]
- Xia, B.Q.; Zhao, C.Y.; Yan, J.; Khosa, A.A. Development of granular thermochemical heat storage composite based on calcium oxide. Renew. Energy 2020, 147, 969–978. [Google Scholar] [CrossRef]
- Guo, R.; Takasu, H.; Funayama, S.; Shinoda, Y.; Tajika, M.; Harada, T.; Kato, Y. Mechanical stability and heat transfer improvement of CaO-based composite pellets for thermochemical energy storage. Chem. Eng. Sci. 2022, 255, 117674. [Google Scholar] [CrossRef]
- Seitz, G.; Helmig, R.; Class, H. A numerical modeling study on the influence of porosity changes during thermochemical heat storage. Appl. Energy 2020, 259, 114152. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage. Appl. Energy 2016, 175, 277–284. [Google Scholar] [CrossRef]

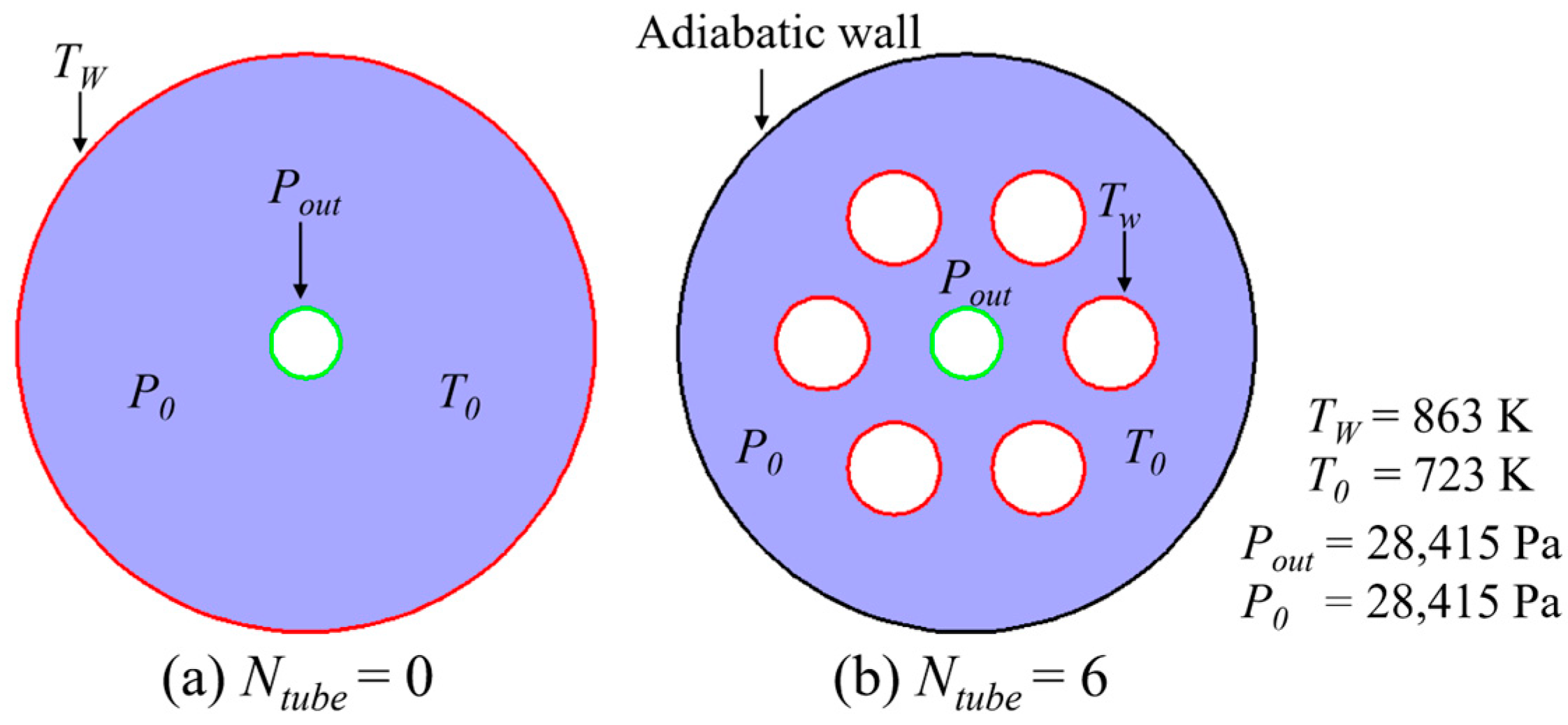
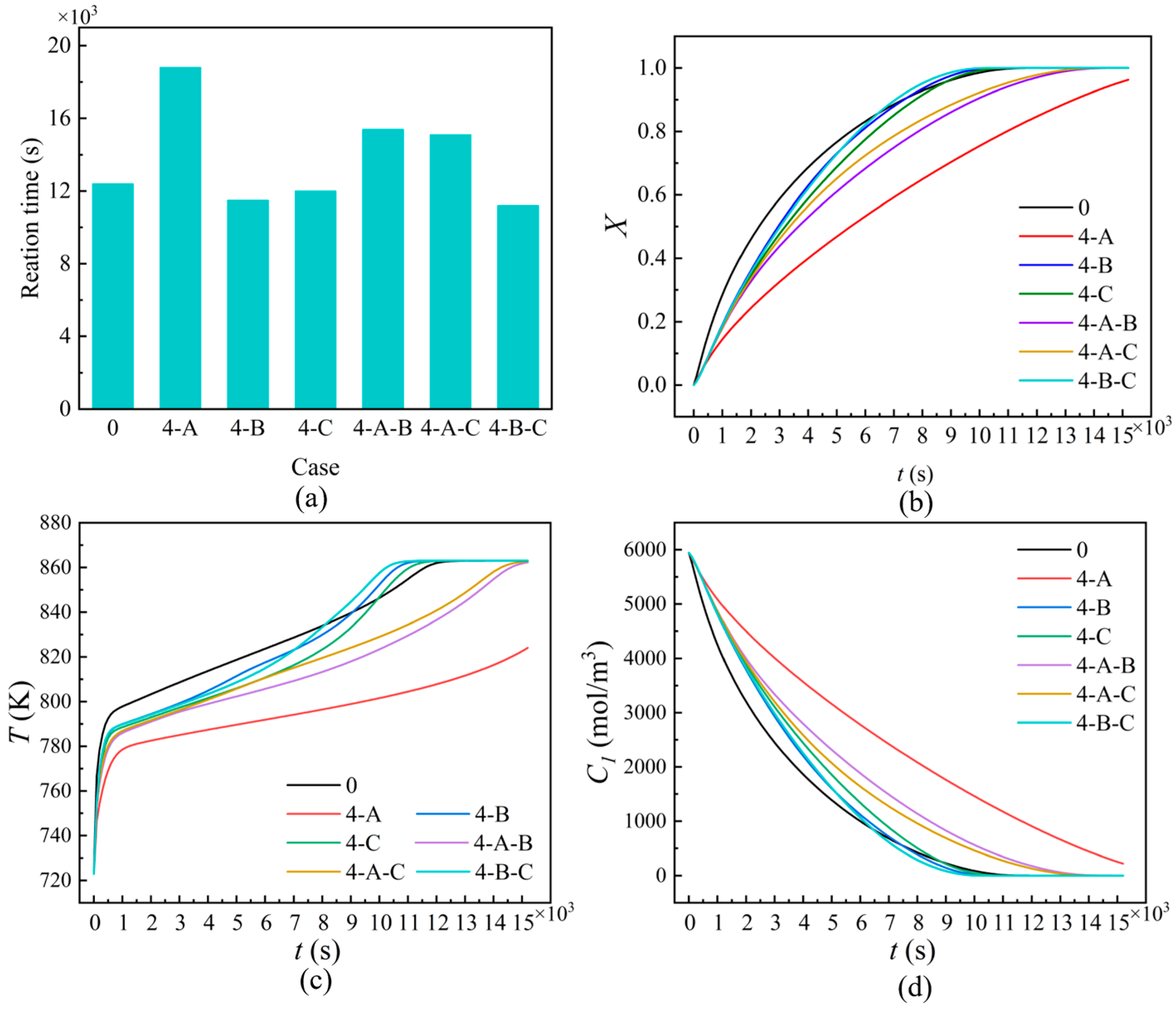

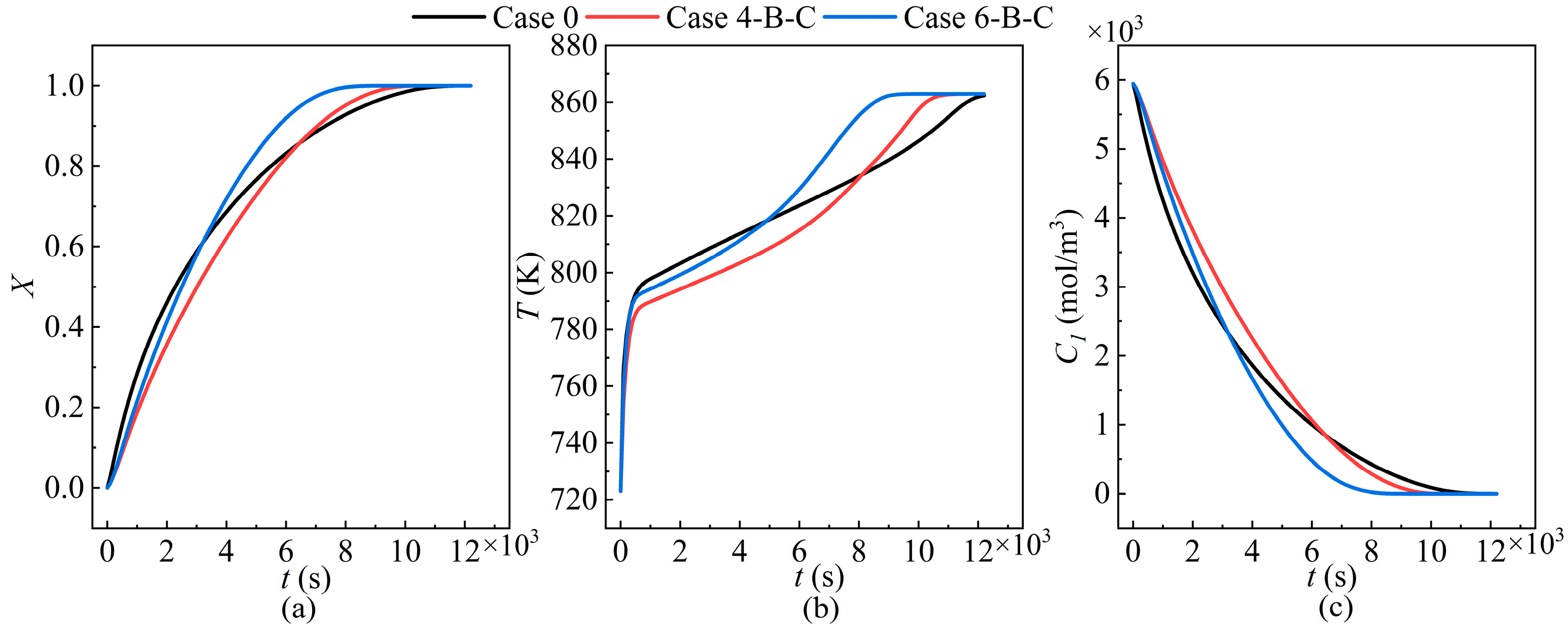
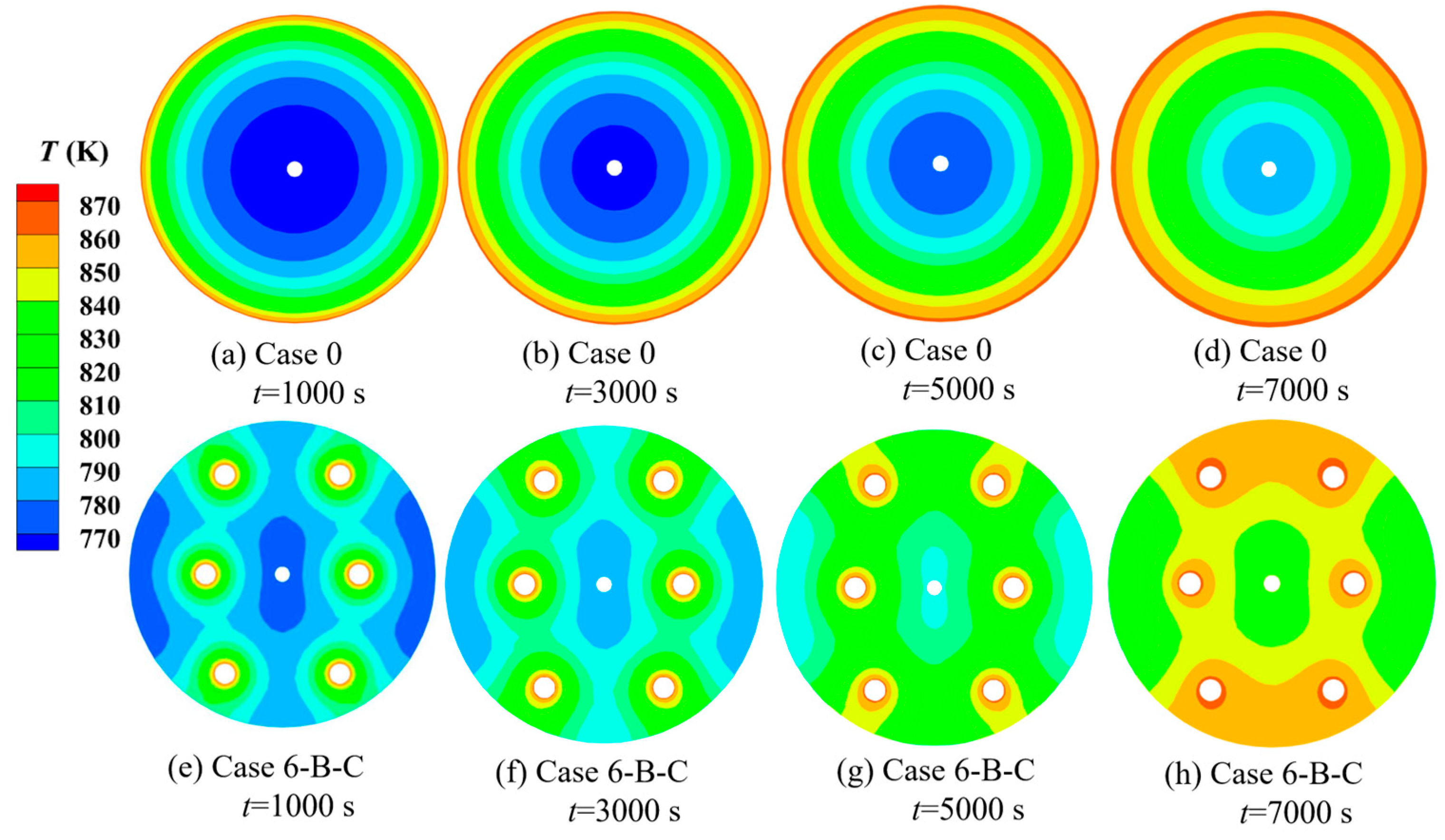
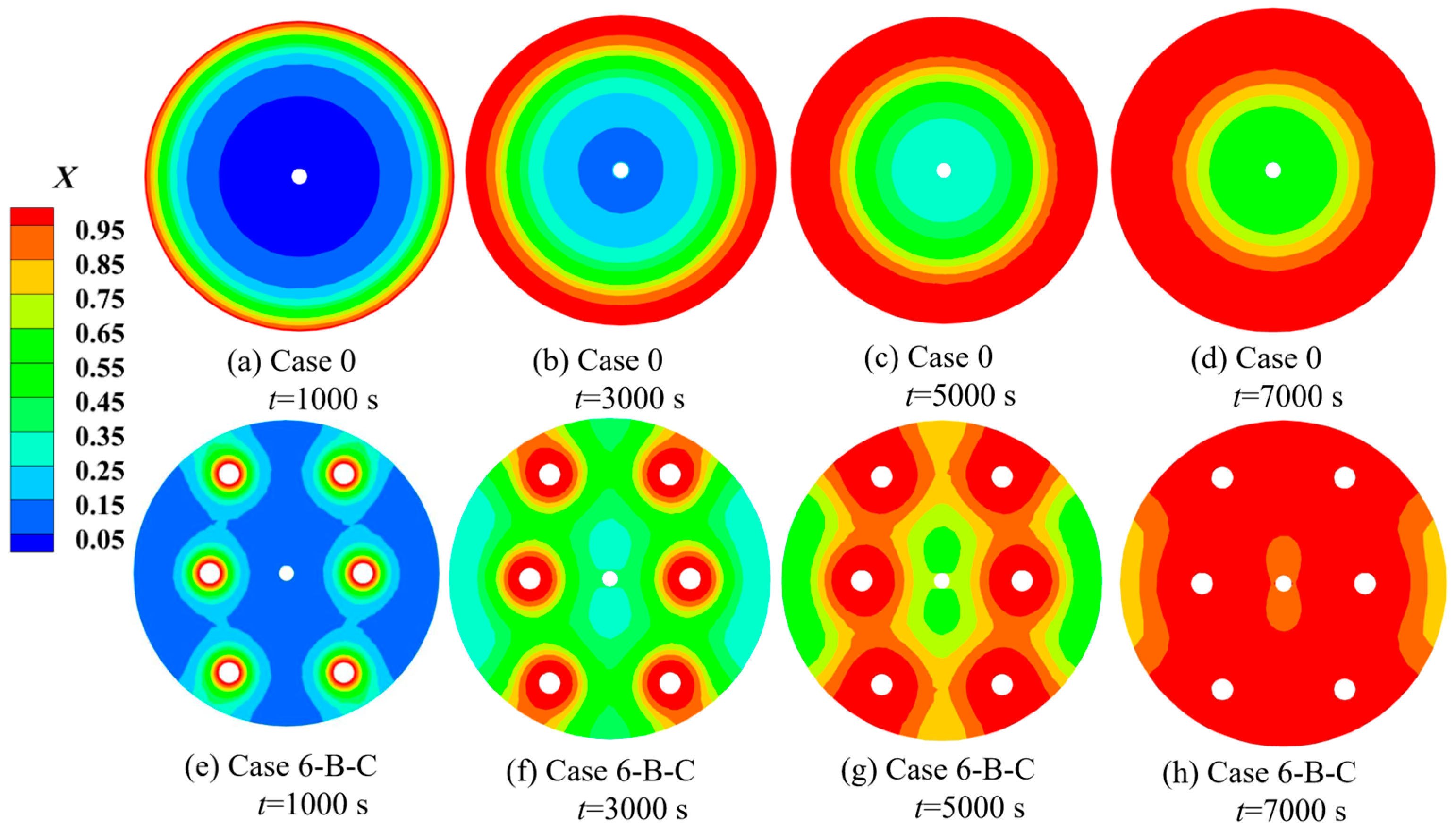

| Cases | Ntube | d1 (mm) | d2 (mm) | Cases | Ntube | d1 (mm) | d2 (mm) |
|---|---|---|---|---|---|---|---|
| 0 | 0 | – | – | 6-A | 6 | 12.5 | 12.5 |
| 2-A | 2 | 12.5 | – | 6-B | 25 | 25 | |
| 2-B | 25 | – | 6-C | 37.5 | 37.5 | ||
| 2-C | 37.5 | – | 6-A-B | 12.5 | 25 | ||
| 4-A | 4 | 12.5 | 12.5 | 6-A-C | 12.5 | 37.5 | |
| 4-B | 25 | 25 | 6-B-A | 25 | 12.5 | ||
| 4-C | 37.5 | 37.5 | 6-B-C | 25 | 37.5 | ||
| 4-A-B | 12.5 | 25 | 6-C-A | 37.5 | 12.5 | ||
| 4-A-C | 12.5 | 37.5 | 6-C-B | 37.5 | 25 | ||
| 4-B-C | 25 | 37.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Jiang, L.; Zhao, C. Numerical Simulation of the Ca(OH)2/CaO Thermochemical Heat Storage Process in an Internal Heating Fixed-Bed Reactor. Sustainability 2023, 15, 7141. https://doi.org/10.3390/su15097141
Yan J, Jiang L, Zhao C. Numerical Simulation of the Ca(OH)2/CaO Thermochemical Heat Storage Process in an Internal Heating Fixed-Bed Reactor. Sustainability. 2023; 15(9):7141. https://doi.org/10.3390/su15097141
Chicago/Turabian StyleYan, Jun, Lei Jiang, and Changying Zhao. 2023. "Numerical Simulation of the Ca(OH)2/CaO Thermochemical Heat Storage Process in an Internal Heating Fixed-Bed Reactor" Sustainability 15, no. 9: 7141. https://doi.org/10.3390/su15097141




