Experimental Investigation and Mechanism Analysis of Direct Aqueous Mineral Carbonation Using Steel Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Section
2.3. Calculation of Carbonation Performance
2.4. Characterization of the Samples
3. Results and Discussion
3.1. Carbonation Performance of Different Steel Slags
3.2. Characterization of the Samples
3.2.1. TG Analysis
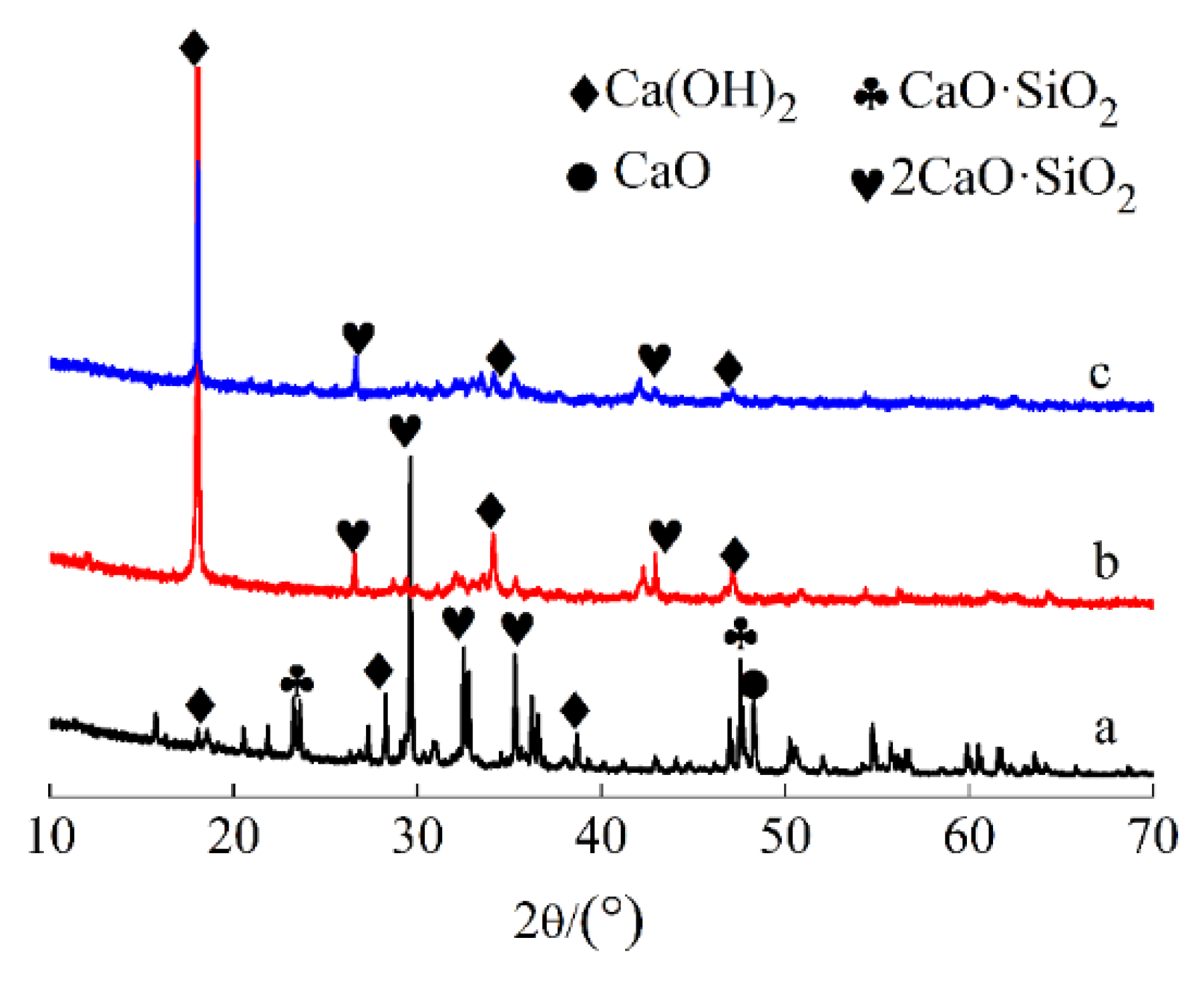
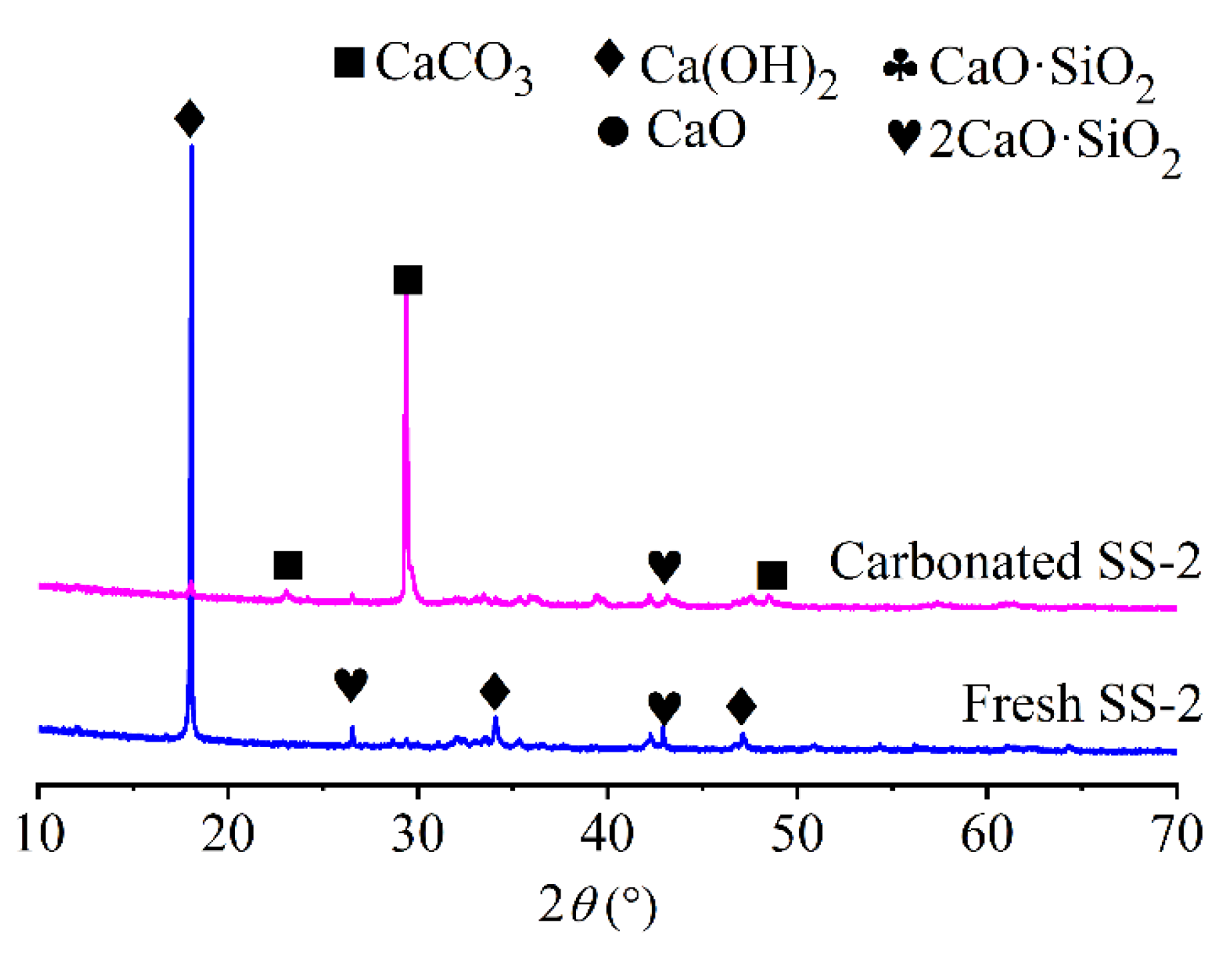
3.2.2. XRD Analysis
3.2.3. SEM-EDS Analysis
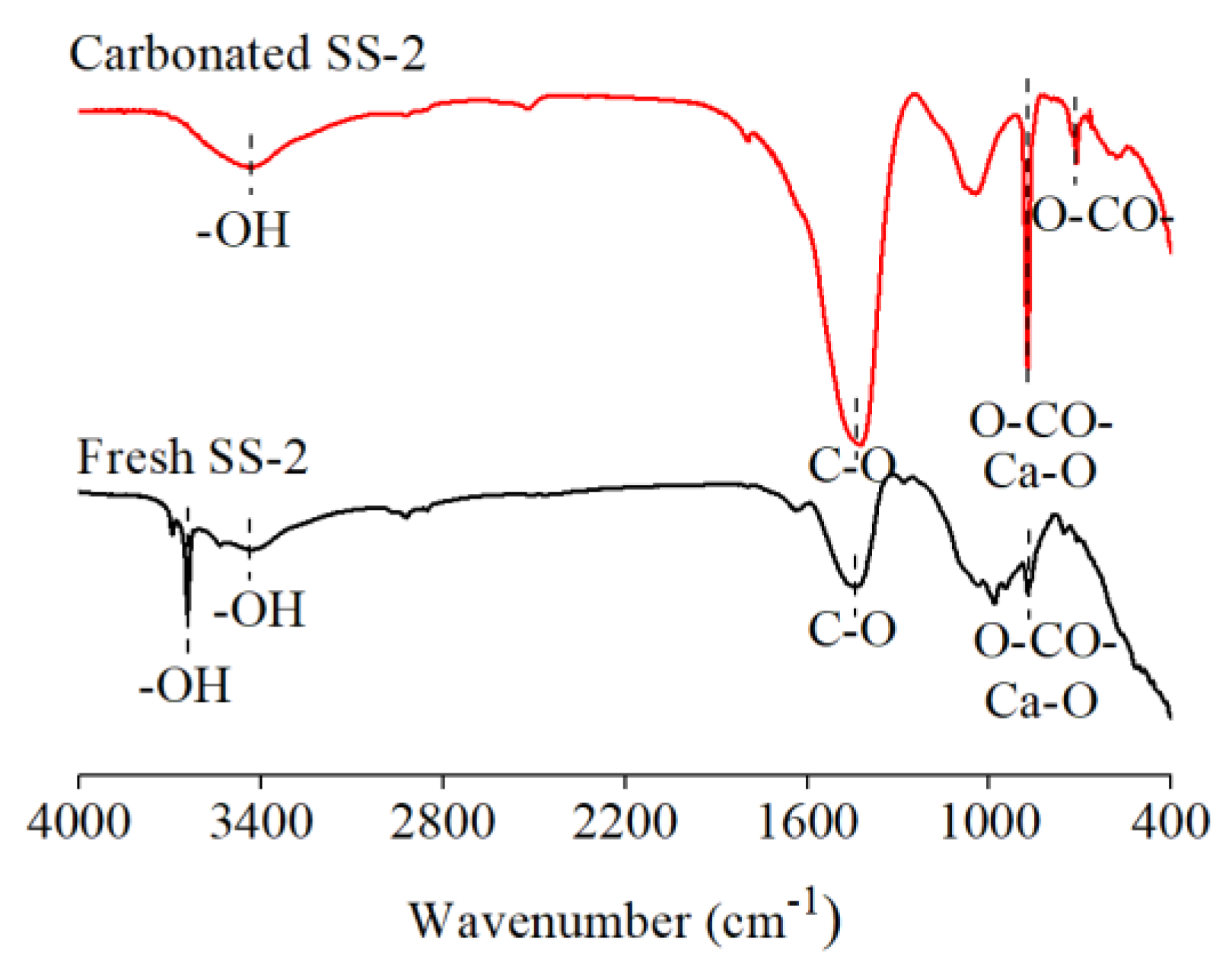
3.2.4. FTIR Spectra
3.3. Effect of Operational Parameters on the Carbonation of Steel Slag
3.3.1. Influence of Particle Size
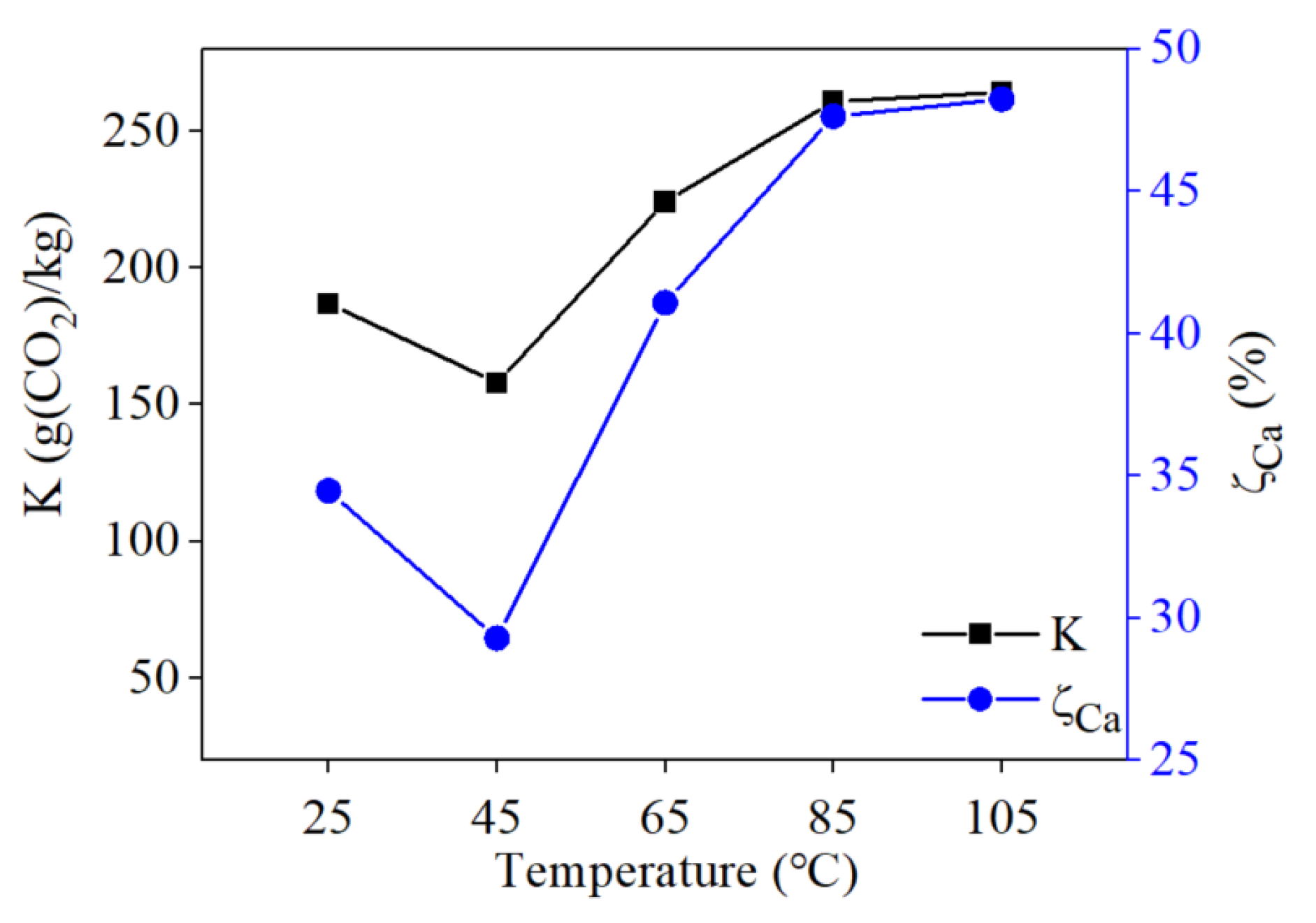
3.3.2. Influence of Reaction Temperature
3.3.3. Influence of Initial CO2 Pressure
3.3.4. Influence of the Liquid-to-Solid Ratio
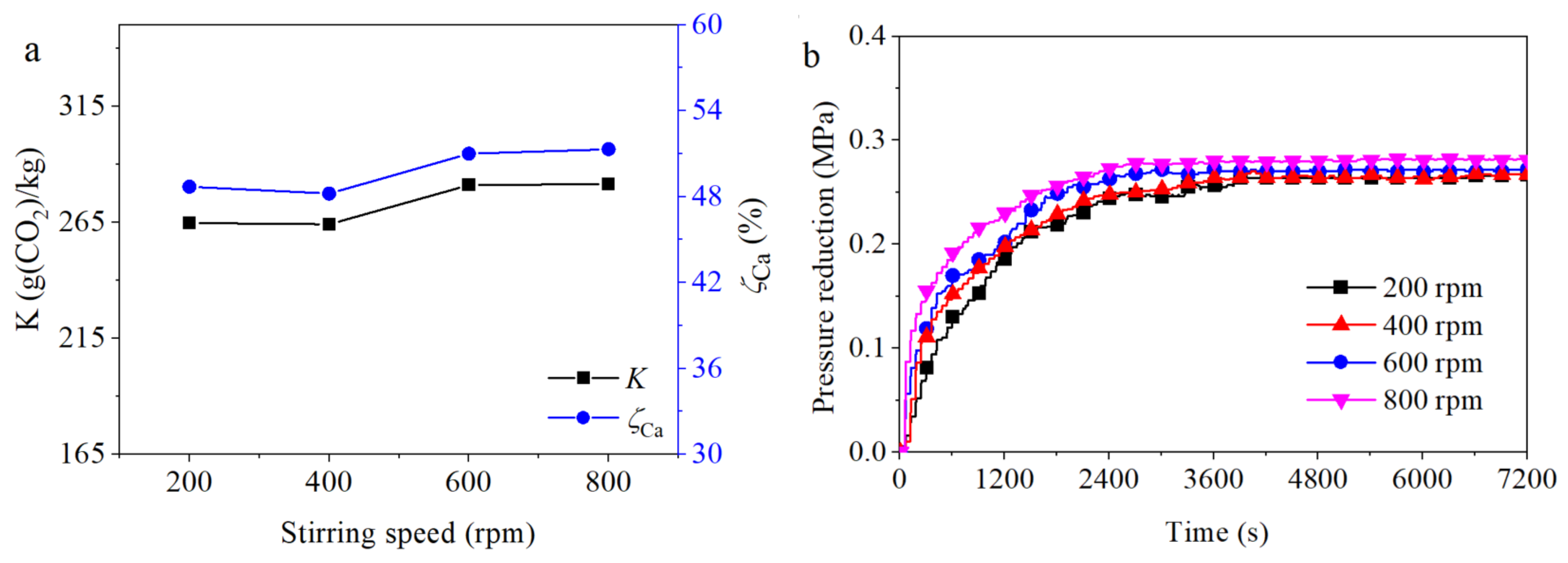
3.3.5. Influence of Rotational Speed
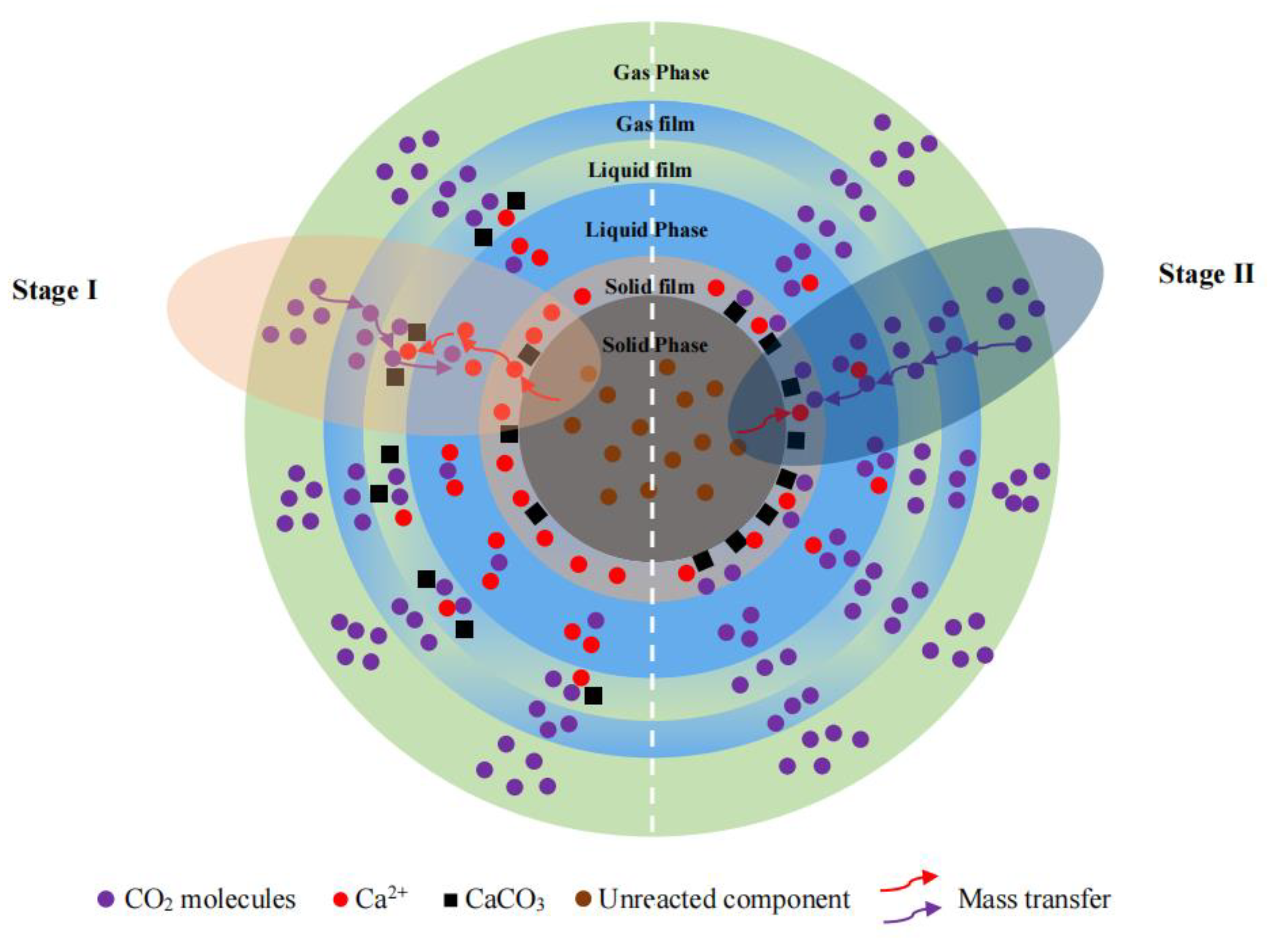
3.4. Mechanism Analysis of Steel Slag for CO2 Sequestration
- Dissolution of CO2 in the liquid phase to form H2CO3 (H2O + CO2 → H2CO3);
- Dissociation of H2CO3 to form HCO3− or CO32− ions (H2CO3 → HCO3− + H+; HCO3− → CO32− + H+);
- Irreversible hydration of CaO in steel slag (CaO + H2O → Ca(OH)2), dissolution and ionization of Ca(OH)2 (Ca(OH)2 → Ca2 + +2OH−);
- Reaction between CO32− and Ca2+ ions to form CaCO3 (Ca2 + + CO32− → CaCO3).
4. Conclusions
- (1)
- Particle size, temperature, pressure, and liquid-to-solid ratio significantly influence both the sequestration rate K and carbonation rate of steel slag, while the influence of rotational speed is minor. The optimal carbonation performance of steel slag is observed under the following conditions: D < 75 μm, T = 105 °C, p = 0.5 MPa, and L/S = 5 mL/g. Under these conditions, the sequestration and carbonation rates reached 283 g(CO2)/kg and 51.61%, respectively.
- (2)
- Various characterization techniques, including XRD, SEM-EDS, TG, and FTIR, were employed to analyze the steel slag samples before and after carbonation, confirming the formation of calcium carbonate. From a thermodynamic perspective, the sequence of reactivity among the four calcium-based active components in steel slag with CO2 is as follows: CaO > Ca(OH)2 > 2CaO·SiO2 > CaO·SiO2.
- (3)
- The direct aqueous carbonation process of steel slag can be divided into two stages: in the initial stage, the rate-limiting step is the mass transfer of CO2; as time progresses, the mass transfer of Ca2+ becomes the controlling factor for the carbonation rate.
- (4)
- CO2 sequestration through the direct aqueous carbonation of steel slag as depicted in this study will consume a large amount of fresh water. In the follow-up research process, seawater can be used as a substitute for fresh water, as the reaction medium and its impacts on CO2 mineralization using steel slag needs further research.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moro, C.; Francioso, V.; Lopez-Arias, M.; Velay-Lizancos, M. The impact of CO2 uptake rate on the environmental performance of cementitious composites: A new dynamic Global Warming Potential analysis. J. Clean. Prod. 2022, 375, 134155. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, J.; Li, Y.; Liu, M.; Han, J. An aging giant at the center of global warming: Population dynamics and its effect on CO2 emissions in China. J. Environ. Manag. 2023, 327, 116906. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhou, S.; Peng, T.; Ou, X. A review of CO2 emissions reduction technologies and low-carbon development in the iron and steel industry focusing on China. Renew. Sustain. Energy Rev. 2021, 143, 110846. [Google Scholar] [CrossRef]
- Hu, J.; Tang, Q.; Wu, Z.; Zhang, B.; He, C.; Chen, Q. Optimization and assessment method for total energy system retrofit in the petrochemical industry considering clean energy substitution for fossil fuel. Energy Convers. Manag. 2023, 284, 116967. [Google Scholar] [CrossRef]
- Liu, D.; Wang, P.; Sun, Y.; Zhang, H.; Xu, S. Co-abatement of carbon and air pollutants emissions in China’s iron and steel industry under carbon neutrality scenarios. Renew. Sustain. Energy Rev. 2024, 191, 114140. [Google Scholar] [CrossRef]
- Xiao, B.; Wen, Z.; Miao, S.; Gao, Q. Utilization of steel slag for cemented tailings backfill: Hydration, strength, pore structure, and cost analysis. Case Stud. Constr. Mater. 2021, 15, e00621. [Google Scholar] [CrossRef]
- Ferrara, G.; Belli, A.; Keulen, A.; Tulliani, J.-M.; Palmero, P. Testing procedures for CO2 uptake assessment of accelerated carbonation products: Experimental application on basic oxygen furnace steel slag samples. Constr. Build. Mater. 2023, 406, 133384. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, C.; Li, Z.; Liu, G.; Zhang, W.; Xie, G.; Xing, F. Carbonation of steel slag at low CO2 concentrations: Novel biochar cold-bonded steel slag artificial aggregates. Sci. Total Environ. 2023, 902, 166065. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Cui, K.; Wang, H.; Fu, T. Carbon capture and storage technology by steel-making slags: Recent progress and future challenges. Chem. Eng. J. 2023, 455, 140552. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Loria, P.; Bright, M.B.H. Lessons captured from 50 years of CCS projects. Electr. J. 2021, 34, 106998. [Google Scholar] [CrossRef]
- Cheng, C.; Huang, W.; Xu, H.; Liu, Z.; Li, X.; Shi, H.; Yu, Y.; Qu, Z.; Yan, N. CO2 sequestration and CaCO3 recovery with steel slag by a novel two-step leaching and carbonation method. Sci. Total Environ. 2023, 891, 164203. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research progress on CO2 capture and utilization technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Santos, R.M.; Ling, D.; Sarvaramini, A.; Guo, M.; Elsen, J.; Larachi, F.; Beaudoin, G.; Blanpain, B.; Van Gerven, T. Stabilization of basic oxygen furnace slag by hot-stage carbonation treatment. Chem. Eng. J. 2012, 203, 239–250. [Google Scholar] [CrossRef]
- Ghouleh, Z.; Guthrie, R.I.L.; Shao, Y. High-strength KOBM steel slag binder activated by carbonation. Constr. Build. Mater. 2015, 99, 175–183. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Lv, Z.; Tong, Y.; Ran, J.; Qin, C. Evaluation on direct aqueous carbonation of industrial/mining solid wastes for CO2 mineralization. J. Ind. Eng. Chem. 2023, 122, 359–365. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; El-Naas, M.H.; Zevenhoven, R.; Al-Sobhi, S.A. Enhanced CO2 capture through reaction with steel-making dust in high salinity water. Int. J. Greenh. Gas Control. 2019, 91, 102819. [Google Scholar] [CrossRef]
- Chang, E.E.; Pan, S.-Y.; Chen, Y.-H.; Tan, C.-S.; Chiang, P.-C. Accelerated carbonation of steelmaking slags in a high-gravity rotating packed bed. J. Hazard. Mater. 2012, 227–228, 97–106. [Google Scholar] [CrossRef]
- Barin, I.B. Ca-CeTe. In Thermochemical Data of Pure Substances, 3rd ed.; Wiley-VCH: New York, NY, USA, 1995; pp. 416–523. [Google Scholar]
- Wang, Z.; Cui, L.; Liu, Y.; Hou, J.; Li, H.; Zou, L.; Zhu, F. High-efficiency CO2 sequestration through direct aqueous carbonation of carbide slag: Determination of carbonation reaction and optimization of operation parameters. Front. Environ. Sci. Eng. 2023, 18, 12. [Google Scholar] [CrossRef]
- Yadav, S.; Mehra, A. Experimental study of dissolution of minerals and CO2 sequestration in steel slag. Waste Manag. 2017, 64, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-Y.; Hung, C.-H.; Chan, Y.-W. Integrated CO2 Fixation, Waste Stabilization, and Product Utilization via High-Gravity Carbonation Process Exemplified by Circular Fluidized Bed Fly Ash. ACS Sustain. Chem. Eng. 2016, 4, 3045–3052. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Comans, R.N.J. Carbonation of steel slag for CO2 sequestration: Leaching of products and reaction mechanisms. Environ. Sci. Technol. 2006, 40, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Gao, X. Properties of coal gangue-Portland cement mixture with carbonation. Fuel 2019, 245, 1–12. [Google Scholar] [CrossRef]
- Yang, C.; Yang, X.; Zhao, T.; Liu, F. An indirect CO2 utilization for the crystallization control of CaCO3 using alkylcarbonate. J. CO2 Util. 2021, 45, 101448. [Google Scholar] [CrossRef]
- Putra, M.D.; Ristianingsih, Y.; Jelita, R.; Irawan, C.; Nata, I.F. Potential waste from palm empty fruit bunches and eggshells as a heterogeneous catalyst for biodiesel production. RSC Adv. 2017, 7, 55547–55554. [Google Scholar] [CrossRef]
- Li, X.; Mehdizadeh, H.; Ling, T.C. Environmental, economic and engineering performances of aqueous carbonated steel slag powders as alternative material in cement pastes: Influence of particle size. Sci. Total Environ. 2023, 903, 166210. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Yellishetty, M.; Bui, H.H.; Xu, T. A laboratory-scale study of the aqueous mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2015, 103, 665–674. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Wang, X.; Grigore, M.; French, D.; Gözükara, Y.M.; Yu, J.; Zeng, M. CO2 sequestration by direct mineralisation using fly ash from Chinese Shenfu coal. Fuel Process. Technol. 2017, 156, 429–437. [Google Scholar] [CrossRef]
- Omale, S.O.; Choong, T.S.Y.; Abdullah, L.C.; Siajam, S.I.; Yip, M.W. Utilization of Malaysia EAF slags for effective application in direct aqueous sequestration of carbon dioxide under ambient temperature. Heliyon 2019, 5, e02602. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvi Dananjayan, R.R.; Kandasamy, P.; Andimuthu, R. Direct mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2016, 112, 4173–4182. [Google Scholar] [CrossRef]
- Georgakopoulos, E.; Santos, R.M.; Chiang, Y.W.; Manovic, V. Influence of process parameters on carbonation rate and conversion of steelmaking slags—Introduction of the ‘carbonation weathering rate’. Greenh. Gases Sci. Technol. 2016, 6, 470–491. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, S.J.; Kawatra, S.K.; Eisele, T.C.; Sutter, L.L. Carbon dioxide sequestration in cement kiln dust through mineral carbonation. Environ. Sci. Technol. 2009, 43, 1986–1992. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Stramazzo, A. CO2 sequestration through aqueous accelerated carbonation of BOF slag: A factorial study of parameters effects. J. Environ. Manag. 2016, 167, 185–195. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Lai, C.; Sun, X. Beyond the standard two-film theory: Computational fluid dynamics simulations for carbon dioxide capture in a wetted wall column. Chem. Eng. Sci. 2018, 184, 103–110. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S.; Ma, L.; Zhao, S.; Liu, H.; Dai, Q.; Yang, Y.; Xu, C.; Xin, X.; Zhang, X.; et al. Mechanism analysis of carbide slag capture of CO2 via a gas-liquid-solid three-phase fluidization system. J. Clean. Prod. 2021, 279, 123712. [Google Scholar] [CrossRef]






| Sample | wt (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | Na2O | K2O | P2O5 | SO3 | |
| SS-1 | 22.21 ± 0.89 | 1.35 ± 0.04 | 0.39 ± 0.01 | 64.73 ± 2.14 | 6.25 ± 0.18 | 1.07 ± 0.04 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.23 ± 0.01 |
| SS-2 | 9.47 ± 0.21 | 1.98 ± 0.07 | 24.44 ± 0.76 | 51.79 ± 2.31 | 5.95 ± 0.25 | 0.71 ± 0.02 | 0.08 ± 0.00 | 0.05 ± 0.00 | 1.45 ± 0.03 | 0.41 ± 0.01 |
| SS-3 | 16.80 ± 0.45 | 4.42 ± 0.10 | 24.90 ± 0.63 | 39.10 ± 1.78 | 4.38 ± 0.32 | 1.00 ± 0.05 | 0.34 ± 001 | 0.40 ± 0.01 | 1.47 ± 0.05 | 1.32 ± 0.03 |
| Variables | Feedstock | D (μm) | T (°C) | p (MPa) | L/S (mL/g) | r (rpm) |
|---|---|---|---|---|---|---|
| Steel slags | SS-1, SS-2, SS-3 | 120~75 | 65 | 2 | 15 | 200 |
| D | SS-2 | >180, 180~150, 150~120, 120~75, <75 | 65 | 2 | 15 | 200 |
| T | SS-2 | <200 | 25, 45, 65, 85, 105 | 2 | 15 | 200 |
| p | SS-2 | <200 | 105 | 0.1, 0.5, 1.0,1.5, 2 | 15 | 200 |
| L/S | SS-2 | <200 | 105 | 0.5 | 1, 5, 10, 15, 20 | 200 |
| r | SS-2 | <200 | 105 | 0.5 | 15 | 200, 400, 600, 800 |
| Phase | Reaction Equation | ΔrGmθ (kJ/mol) |
|---|---|---|
| CaO | CaO + CO2 → CaCO3 | −178.32 + 0.16(T + 273.15) |
| Ca(OH)2 | Ca(OH)2 + CO2 → CaCO3 + H2O | −113.15 + 0.13(T + 273.15) |
| CaO·SiO2 | CaO·SiO2 + H2O + CO2 → CaCO3 + SiO2·H2O | −92.77 + 0.16(T + 273.15) |
| 2CaO·SiO2 | 1/2(2CaO·SiO2) + 1/2H2O + CO2 → CaCO3 + 1/2SiO2·H2O | −111.27 + 0.16(T + 273.15) |
| Sample Parameter | Unit | >180 | 180~150 | 150~120 | 120~75 | <75 |
|---|---|---|---|---|---|---|
| BET surface area | m2/g | 2.005 ± 0.081 | 10.227 ± 0.113 | 10.966 ± 0.152 | 13.909 ± 0.094 | 14.162 ± 0.172 |
| Total pore volume | cm3/g | 0.005 ± 0.000 | 0.022 ± 0.001 | 0.024 ± 0.001 | 0.029 ± 0.001 | 0.038 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.; Cui, L.; Liu, Y.; Zou, L.; Hou, J.; Li, C.; Wu, G.; Xu, R.; Jiang, B.; Wang, Z. Experimental Investigation and Mechanism Analysis of Direct Aqueous Mineral Carbonation Using Steel Slag. Sustainability 2024, 16, 81. https://doi.org/10.3390/su16010081
Zhu F, Cui L, Liu Y, Zou L, Hou J, Li C, Wu G, Xu R, Jiang B, Wang Z. Experimental Investigation and Mechanism Analysis of Direct Aqueous Mineral Carbonation Using Steel Slag. Sustainability. 2024; 16(1):81. https://doi.org/10.3390/su16010081
Chicago/Turabian StyleZhu, Fuxia, Longpeng Cui, Yanfang Liu, Liang Zou, Jili Hou, Chenghao Li, Ge Wu, Run Xu, Bo Jiang, and Zhiqiang Wang. 2024. "Experimental Investigation and Mechanism Analysis of Direct Aqueous Mineral Carbonation Using Steel Slag" Sustainability 16, no. 1: 81. https://doi.org/10.3390/su16010081





