Use of Zeolite (Chabazite) Supplemented with Effective Microorganisms for Wastewater Mitigation of a Marine Fish Farm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Zeolite and Effective Microorganisms
2.3. Experimental Design
2.4. Sampling and Analytical Determinations
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; FAO Fisheries and Aquaculture Department, Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Pearson, T.H.; Black, K.D. Environmental Impact of Aquaculture; Sheffield Academic Press: Sheffield, UK, 2001. [Google Scholar]
- Holby, O.; Hall, P.O.J. Chemical fluxes and mass balances in a marine fish cage farm. II Phosphorus. Mar. Ecol. Prog. Ser. 1991, 70, 263–272. [Google Scholar] [CrossRef]
- Handy, R.D.; Poxton, M.G. Nitrogen pollution in mariculture: Toxicity and excretion of nitrogenous compounds by marine fish. Rev. Fish Biol. 1993, 3, 205–241. [Google Scholar] [CrossRef]
- Porrello, S.; Lenzi, M.; Ferrari, G.; Persia, E.; Tomassetti, P. Loading of nutrients from a land-based fish farm (Orbetello, Italy) at different times. Aquac. Int. 2005, 13, 97–108. [Google Scholar] [CrossRef]
- Troell, M.; Halling, C.; Neori, A.; Chopin, T.; Buschmann, A.H.; Kautsky, N.; Yarish, C. Integrated mariculture: Asking the right questions. Aquaculture 2003, 226, 69–90. [Google Scholar] [CrossRef]
- Porrello, S.; Lenzi, M.; Tomassetti, P.; Persia, E.; Finoia, M.G.; Mercatali, I. Reduction of aquaculture wastewater eutrophication by phytotreatment ponds system. II: Nitrogen and phosphorus content in macroalgae and sediment. Aquaculture 2003, 219, 531–544. [Google Scholar] [CrossRef]
- Machias, A.; Karakassis, I.; Labropoulou, M.; Somarakis, S.; Papadopoulou, K.N.; Papaconstantinou, C. Changes in wild fish assemblages after the establishment of a fish 54 farming zone in an oligotrophic marine ecosystem. Estuar. Coast. Shelf Sci. 2004, 60, 771–779. [Google Scholar] [CrossRef]
- Fernandez-Jover, D.; Sanchez-Jerez, P.; Bayle-Sempere, J.T.; Arechavala-Lopez, P.; Martinez Rubio, L. Coastal fish farms are settlement sites for juvenile fish. Mar. Environ. Res. 2009, 68, 89–96. [Google Scholar] [CrossRef]
- Navarrete-Mier, F.; Sanz-Lazaro, C.; Marin, A. Does bivalve mollusc polyculture reduce marine fin fish farming environmental impact? Aquaculture 2010, 306, 101–107. [Google Scholar] [CrossRef]
- Sara, G.; Reid, G.K.; Rinaldi, A.; Palmeri, V.; Troell, M. Growth and reproductive simulation of candidate shellfish species at fish cages in the Southern Mediterranean: Dynamic Energy Budget (DEB) modelling for integrated multi-trophic aquaculture. Aquaculture 2012, 324–325, 259–266. [Google Scholar] [CrossRef]
- Atkins, R.P.; Deeley, D.M.; McAlpine, K.W. Managing the aquatic environment. Fertil. Res. 1993, 36, 171–175. [Google Scholar] [CrossRef]
- De Leo, G.A.; Batoli, M.; Naldi, M.; Viaroli, P. A First Generation Stochastic Bioeconomic Analysis of Algal Bloom Control in a Coastal Lagoon (Sacca di Goro, Po River Delta). Mar. Ecol. 2002, 23 (Suppl. S1), 92–100. [Google Scholar] [CrossRef]
- Sherman, J.D. Ion exchange separations with molecular sieve zeolites. In Zeolites: Science and Technology; Springer: Dordrecht, Netherlands, 1983; Chapters 583–623. [Google Scholar] [CrossRef]
- Passaglia, E. Zeoliti Naturali, Zeolititi e Loro Applicazioni; Arvan: Padova, Italy, 2008. (In Italian) [Google Scholar]
- Comba, S.; Martin, M.; Marchisio, D.; Sethi, R.; Barberis, E. Reduction of Nitrate and Ammonium Adsorption Using Microscale Iron Particles and Zeolitite. Water Air Soil Pollut. 2012, 223, 1079–1089. [Google Scholar] [CrossRef]
- Faccini, B.; Di Giuseppe, D.; Malferrari, D.; Coltorti, M.; Abbondanzi, F.; Campisi, T. Ammonium-exchanged zeolitite preparation for agricultural uses: From laboratory tests to large-scale application in ZeoLIFE project prototype. Period. Mineral. 2015, 84, 303–321. [Google Scholar]
- Colombani, N.; Mastrocicco, M.; Di Giuseppe, D.; Faccini, B.; Coltorti, M. Batch and column experiments on nutrient leaching in soils amended with Italian natural zeolitites. Catena 2015, 127, 64–71. [Google Scholar] [CrossRef]
- Briggs, M.R.P.; Funge-Smith, S.J. The effects of zeolites and other alumino-silicate clays on water quality at various salinities. Aquac. Res. 1996, 27, 301–311. [Google Scholar] [CrossRef]
- Markoska, V.; Reka, A. Preliminary Examinations from Wastewater Treatment by Zeolite from Waste Materials. In Proceedings of the 8. Simpozijum “Reciklazne Tehnologijie I Odrzivi Razvoj”, Brosko Jezero, Serbia, 3–5 July 2013; pp. 239–242. [Google Scholar]
- Kumar, L.; Kaur, R.; Sharma, J. The efficiency of zeolites in water treatment for combating ammonia—An experimental study on Yamuna River water & treated sewage effluents. Inorg. Chem. Commun. 2021, 134, 108978. [Google Scholar]
- Muscarella, S.M.; Badalucco, L.; Cano, B.; Laudicina, V.A.; Mannina, G. Ammonium adsorption, desorption and recovery by acid and alkaline treated zeolite. Bioresour. Technol. 2021, 341, 125812. [Google Scholar] [CrossRef] [PubMed]
- Novembre, D.; Gimeno, D.; Calista, M.; Mancinelli, V.; Miccadei, E. On the suitability of phillipsite-chabazite zeolite rock for ammonia uptake in water: A case study from Pescara River (Italy). Sci. Rep. 2022, 12, 9284. [Google Scholar] [CrossRef]
- Aly, H.A.; Abdel-Rahim, M.M.; Lofty, A.M.; Abdelaty, B.S.; Sallam, G.M. Tjhe Applicability of Activated Carbon, Natural Zeolites and Porbiotics and Its Effects on Ammonia Removal Efficiency and Fry Performance of European Seabass Dicentrarchus labrax. J. Aquacultere Res. Dev. 2016, 7, 459–466. [Google Scholar]
- López-Ruiz, J.L.; Gómez-Garrudo, M.E. Zeolites in marine nitrogen transformations. Aquac. Eng. 1994, 13, 147–152. [Google Scholar] [CrossRef]
- Colella, C. Ion exchange equilibria in zeolite minerals. Miner. Depos. 1996, 31, 554–562. [Google Scholar] [CrossRef]
- Amend, D.F.; Croy, T.R.; Goven, B.A.; Johnson, K.A.; McCarthy, D.H. Transportation of fish in closed systems: Methods to control ammonia, carbon dioxide, pH and bacterial growth. Trans. Am. Fish. Soc. 1982, 111, 603–611. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Sorensen, J.; Blackburn, T.H.; Lomholt, J.P. Distribution of oxygen in marine sediments measured with microelectrodes. Limnol. Oceanogr. 1980, 25, 403–411. [Google Scholar] [CrossRef]
- Herbert, R.A.; Nedwell, D.B. Role of environmental factors in regulating nitrate respiration in intertidal sediments. In Denitrification in Soil and Sediment; Revsbech, N.P., Sorensen, J., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 77–90. [Google Scholar]
- Lenzi, M.; Gennaro, P.; Franchi, E.; Marsili, L. Assessment of fish-farms wastewater synergistic impact on a Mediterranean non-tidal lagoon. J. Aquac. Fish. 2019, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.E.; Larsen, S.C. Zeolite and mesoporous silica nanomaterials: Greener syntheses, environmental applications and biological toxicity. Environ. Sci. Nano 2014, 1, 200–213. [Google Scholar] [CrossRef]
- Flanigen, E.M. Adsorption properties of molecular sieve zeolites. In Zeo-Agriculture: Use of Natural Zeolites in Agriculture and Aquaculture; Westview Press: Boulder, CO, USA, 1983; pp. 55–68. [Google Scholar]
- Weihrauch, D.; Wilkie, M.P.; Walsh, P.J. Ammonia and urea transporters in gills of fish and aquatic crustaceans. J. Exp. Biol. 2008, 212, 1716–1730. [Google Scholar] [CrossRef] [PubMed]
- Malferrari, D.; Laurora, A.; Brigatti, M.F.; Coltorti, M.; Di Giuseppe, D.; Faccini, B.; Passaglia, E.; Vezzalini, M.G. Open-field experimentation of the innovative and integrated zeolite cycle: Project definition and material characterization. Rend. Fis. Acc. Lincei 2013, 24, 141–150. [Google Scholar] [CrossRef]
- Higa, T.; Parr, J.F. Beneficial and Effective Microorganisms for a Sustainable Agriculture and Environment; International Nature Farming Research Center: Atami, Japan, 1994; Volume 1. [Google Scholar]
- Zakaria, Z.; Gairola, S.; Shariff, N.M. Effective Microorganisms (EM) Technology for Water Quality Restoration and Potential for Sustainable Water Resources and Management. Int. Congr. Environ. Model. Softw. 2010, 142. Available online: https://scholarsarchive.byu.edu/cgi/viewcontent.cgi?article=2187&context=iemssconference (accessed on 11 November 2023).
- Lenzi, M.; Leporatti Persiano, M.; Ciarapica, M. Quality Improvement of Eutrophic Environment Degraded by Organic Matter, in Experiences Conducted in Sea-Water Microcosms. Eur. J. Biol. Biotechnol. 2021, 2, 29–32. [Google Scholar] [CrossRef]
- APAT; IRSA-CNR. Metodi Analitici per le Acque; Manuali e Linee Guida: Rome, Italy, 2003; Volume 1, p. 1153. ISBN 88-448-0083-7. [Google Scholar]
- Aspila, K.I.; Agemiam, H.; Chau, A.S.Y. A semiautomatic method for the determination of inorganic, organic and total phosphate in sediments. Analyst 1976, 101, 187–197. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- StataCorp Stata 14 Base Reference Manual; Stata Press: College Station, TX, USA, 2015.
- Wheeler, P.A.; Bjornsater, B.R. Seasonal fluctuations in tissue nitrogen, phosphorus and N:P for five macroalgal species common to the Pacific Northwest coast. J. Phycol. 1992, 28, 1–6. [Google Scholar] [CrossRef]
- Novicki, B.L.; Requintina, E.; van Kueren, D.; Kelly, J.R. Nitrogen losses through sediment denitrification in Boston Harbour and Massachusetts Bay. Estuaries 1997, 20, 626–639. [Google Scholar]
- Porrello, S.; Lenzi, M.; Persia, E.; Tomassetti, P.; Finoia, M.G. Reduction of aquaculture wastewater eutrophication by phytotreatment ponds system. I: Dissolved and particulate nitrogen and phosphorus. Aquaculture 2003, 219, 515–529. [Google Scholar] [CrossRef]
- Carvalho, S.; Falcao, M.; Curdia, J.; Moura, A.; Serpa, D.; Gaspar, A.B.; Dinis, M.T.; Pousao-Ferreira, P.; Cancela da Fonseca, L. Benthic dynamics within a land-based semi-intensive aquaculture fish-farm: Thenimportance of settlement ponds. Aquacult. Int. 2009, 17, 517–587. [Google Scholar] [CrossRef]
- Bosma, R.H.; Verdegem, M.C.J. Sustainable aquaculture in ponds: Principles, practices and limits. Livest. Sci. 2011, 139, 58–68. [Google Scholar] [CrossRef]
- Ehler, D.; Songsangjinda, P.; Keawtawee, T.; Chaiyakam, K. Nitrogen dynamics in the settlement ponds of a small-scale recirculating shrimp farm (Penaeus monodon) in rural Thailand. Aquac. Int. 2007, 15, 55–66. [Google Scholar] [CrossRef]
- Golterman, H.L. Phosphate release from anoxic sediments or “what did Mortimer really write?”. Hydrobiologia 2001, 450, 99–106. [Google Scholar] [CrossRef]
- Tal, Y.; Schreier, H.J.; Sowers, K.R.; Stubblefield, J.D.; Place, A.R.; Zohar, Y. Environmentally sustainable land-based marine aquaculture. Aquaculture 2009, 286, 28–35. [Google Scholar] [CrossRef]
- Stahlberg, C.; Bastviken, D.; Svensson, B.H.; Rahm, L. Mineralisation of organic matter in coastal sediment at different frequency and duration of resuspension. Estuar. Coastal Shelf Sci. 2006, 70, 317–325. [Google Scholar] [CrossRef]
- Fenchel, T. Aspects of decomposer food chains in marine benthos. Ver. Deutsh. Zool. Ges. 1992, 65, 14–22. [Google Scholar]

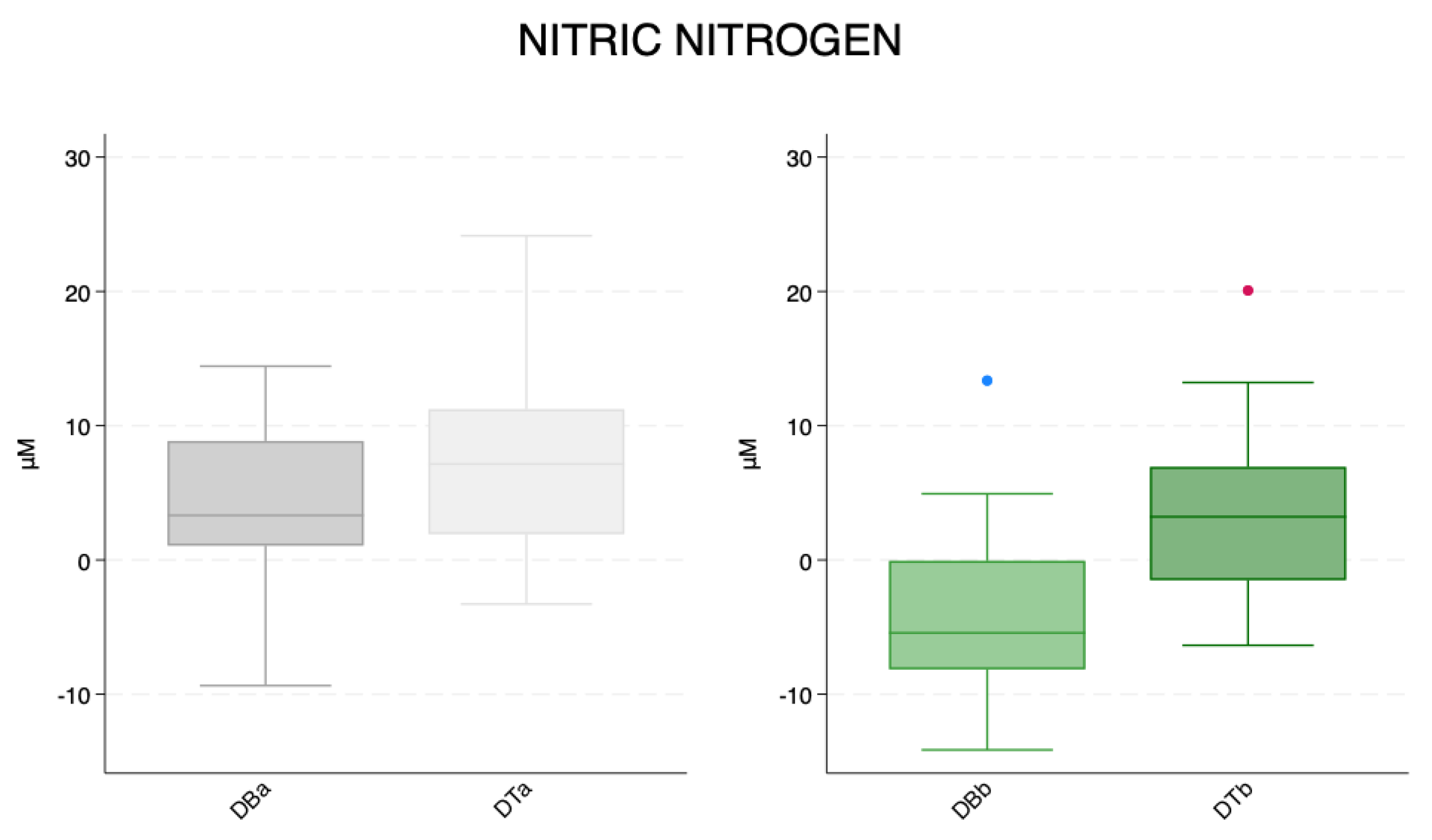
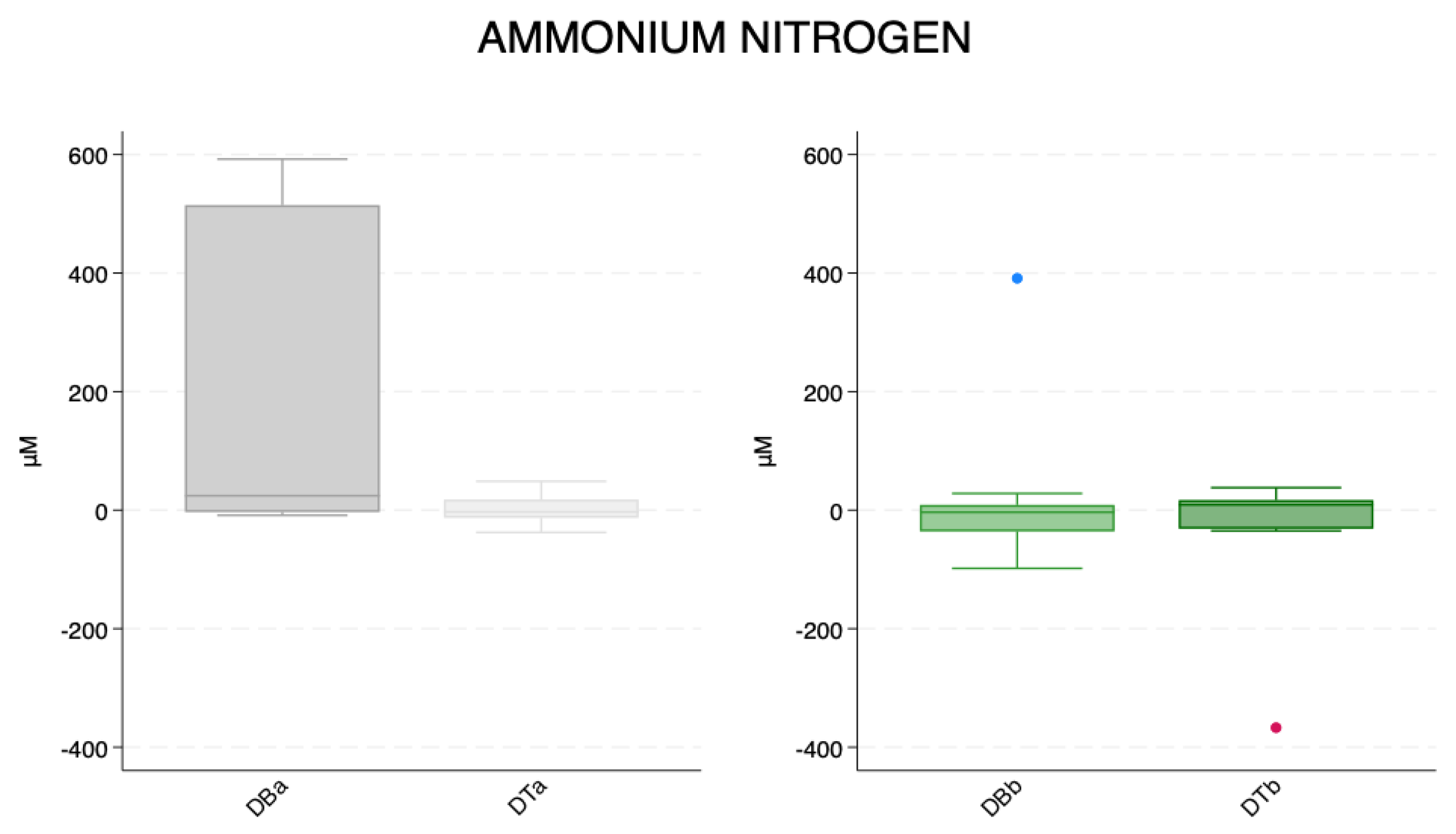


| Mean ± SD | N | Min | Max | Median | ||
|---|---|---|---|---|---|---|
| μM | ||||||
| T1a | N-NO3 | 85.9 ± 39.2 | 10 | 29.43 | 125.29 | 108 |
| N-NH4 | 182.2 ± 53.1 | 10 | 122.86 | 265.86 | 183.25 | |
| DIN | 268.1 ± 87.3 | 10 | 152.29 | 371 | 299.96 | |
| SRP | 2.65 ± 0.46 | 10 | 2 | 3.23 | 2.71 | |
| T1b | N-NO3 | 76.6 ± 34.1 | 10 | 31.07 | 109.64 | 93.71 |
| N-NH4 | 250.4 ± 150.9 | 10 | 118.21 | 603.64 | 211.21 | |
| DIN | 327.0 ± 177.3 | 10 | 158.14 | 711.71 | 307.21 | |
| SRP | 2.25 ± 0.37 | 10 | 1.52 | 2.77 | 2.31 | |
| T2a | N-NO3 | 93.1 ± 35.9 | 10 | 48.57 | 128.86 | 112.57 |
| N-NH4 | 184.3 ± 42.6 | 10 | 125.43 | 248.36 | 193.61 | |
| DIN | 277.4 ± 75.6 | 10 | 174.86 | 368.93 | 313.75 | |
| SRP | 2.39 ± 0.24 | 10 | 1.97 | 2.68 | 2.45 | |
| T2b | N-NO3 | 80.5 ± 35.1 | 10 | 31.64 | 116.57 | 99.18 |
| N-NH4 | 216.9 ± 99.0 | 10 | 103.43 | 408.57 | 199.39 | |
| DIN | 297.5 ± 126.9 | 10 | 135.07 | 512.79 | 303.79 | |
| SRP | 2.05 ± 0.21 | 10 | 1.65 | 2.32 | 2.1 | |
| B1a | N-NO3 | 88.9 ± 38.0 | 10 | 38.29 | 134.57 | 111 |
| N-NH4 | 182.9 ± 59.6 | 10 | 110.07 | 285.71 | 186.43 | |
| DIN | 271.8 ± 91.3 | 10 | 159.5 | 395 | 303.71 | |
| SRP | 2.43 ± 0.24 | 10 | 1.97 | 2.71 | 2.5 | |
| B1b | N-NO3 | 82.8 ± 30.1 | 10 | 47.21 | 115.43 | 99.39 |
| N-NH4 | 218.2 ± 31.8 | 10 | 127.5 | 441.86 | 180.25 | |
| DIN | 300.9 ± 136.3 | 10 | 174.71 | 557.29 | 287.43 | |
| SRP | 2.34 ± 0.40 | 10 | 1.84 | 2.81 | 2.29 | |
| B2a | N-NO3 | 92.4 ± 37.7 | 10 | 39.36 | 139.14 | 112.5 |
| N-NH4 | 396.3 ± 282.7 | 10 | 124.93 | 780.21 | 270.57 | |
| DIN | 488.7 ± 313.1 | 10 | 170.5 | 895.29 | 399.39 | |
| SRP | 2.38 ± 0.19 | 10 | 2.06 | 2.65 | 2.4 | |
| B2b | N-NO3 | 79.2 ± 33.0 | 10 | 38.64 | 120.64 | 90.14 |
| N-NH4 | 238.7 ± 150.1 | 10 | 101.14 | 585.21 | 195.61 | |
| DIN | 317.9 ± 176.8 | 10 | 144.79 | 705.86 | 296.21 | |
| SRP | 2.36 ± 0.29 | 10 | 2.1 | 3.06 | 2.27 |
| N-NO3 | N-NH4 | DIN | SRP | |
|---|---|---|---|---|
| T2a-T1a | 8.39 | 5.29 | 3.4 | −9.964 |
| T2b-T1b | 5.03 | −13.35 | −9.04 | −8.621 |
| B2a-B1a | 4.02 | 116.62 | 79.82 | −1.86 |
| B2b-B1b | −4.26 | 9.44 | 5.6 | 0.97 |
| N-NO3 | N-NH4 | DIN | SRP | |
|---|---|---|---|---|
| ΔBa vs. ΔTa | 0.38 | 0.07 | 0.38 | 0.06 |
| ΔBb vs. ΔTb | 0.05 | 0.55 | 0.29 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenzi, M.; Leporatti Persiano, M.; Ciarapica, M.; D’Agostino, A. Use of Zeolite (Chabazite) Supplemented with Effective Microorganisms for Wastewater Mitigation of a Marine Fish Farm. Sustainability 2024, 16, 1353. https://doi.org/10.3390/su16041353
Lenzi M, Leporatti Persiano M, Ciarapica M, D’Agostino A. Use of Zeolite (Chabazite) Supplemented with Effective Microorganisms for Wastewater Mitigation of a Marine Fish Farm. Sustainability. 2024; 16(4):1353. https://doi.org/10.3390/su16041353
Chicago/Turabian StyleLenzi, Mauro, Marco Leporatti Persiano, Maurizio Ciarapica, and Antonella D’Agostino. 2024. "Use of Zeolite (Chabazite) Supplemented with Effective Microorganisms for Wastewater Mitigation of a Marine Fish Farm" Sustainability 16, no. 4: 1353. https://doi.org/10.3390/su16041353






