The Sustained Response of Dissolved Inorganic Carbon to Urban Constructed Wetland in the Fenhe River, China: A Case Study
Abstract
:1. Introduction
2. Materials and Methods
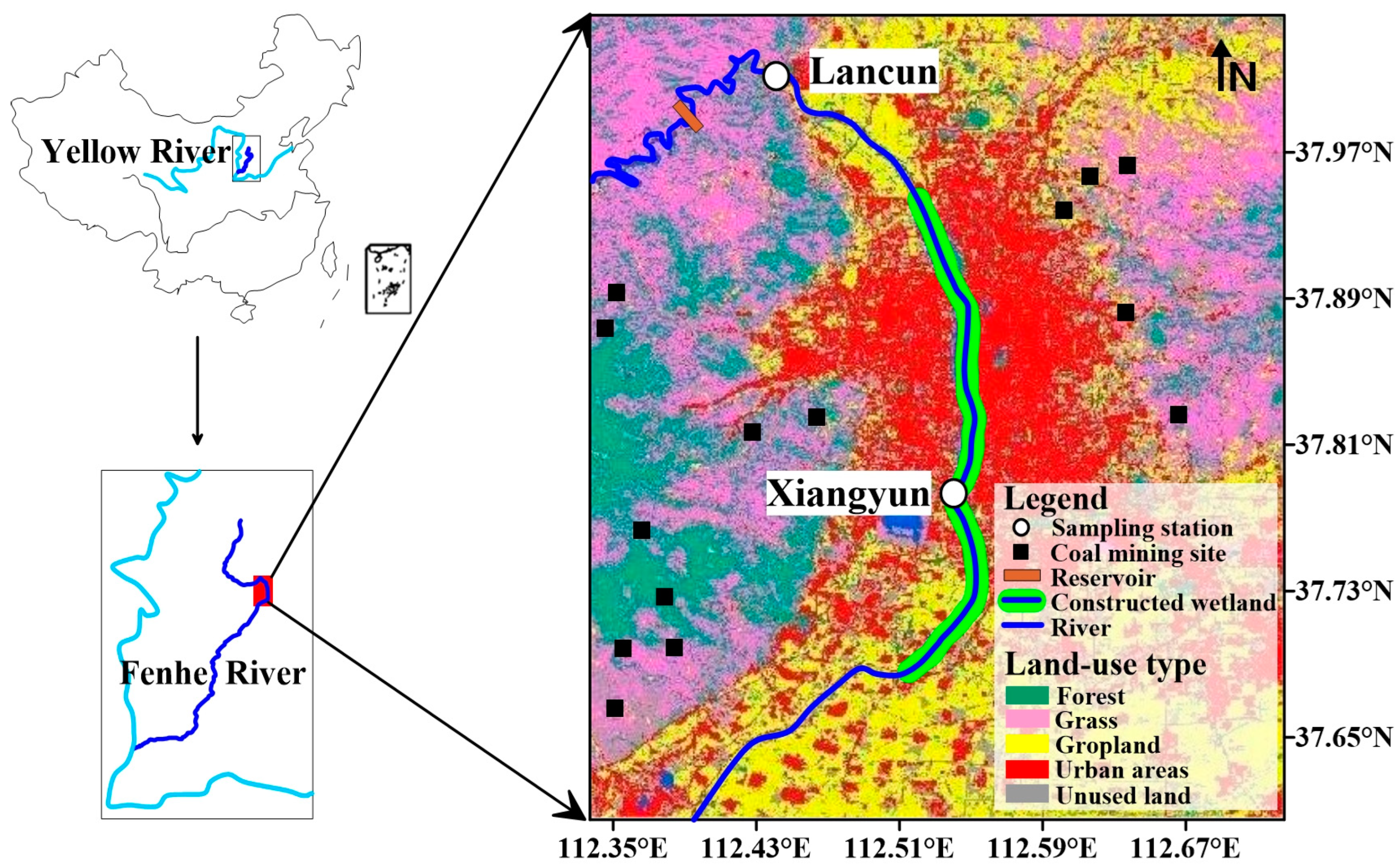
2.1. Study Area
2.2. Station Setting and Sample Processing
2.3. Simulation of pCO2 Change Due to Temperature Effect and Biological Production
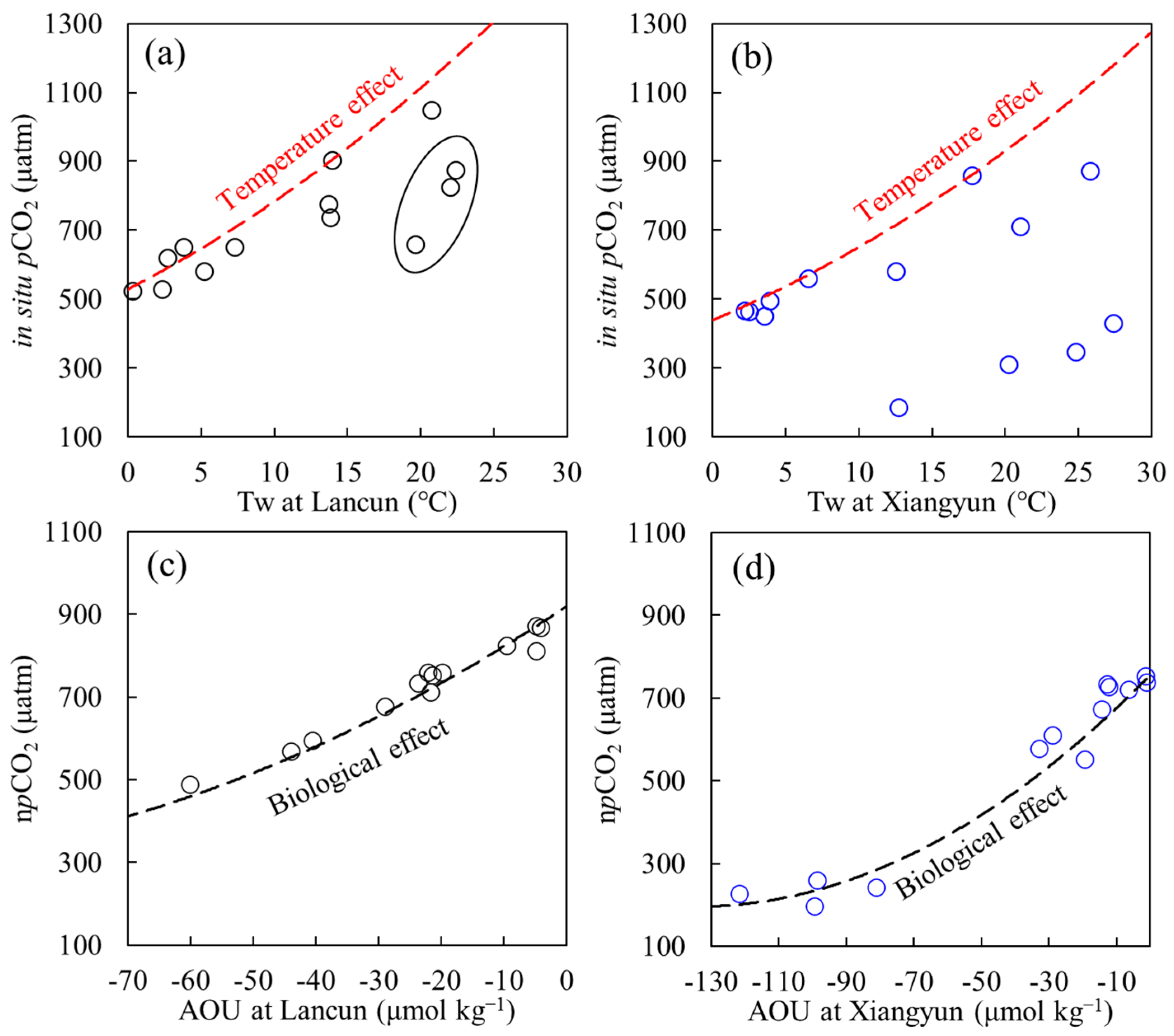
2.3.1. Temperature Effect
2.3.2. Biological Production
2.4. Statistical Analysis
3. Results
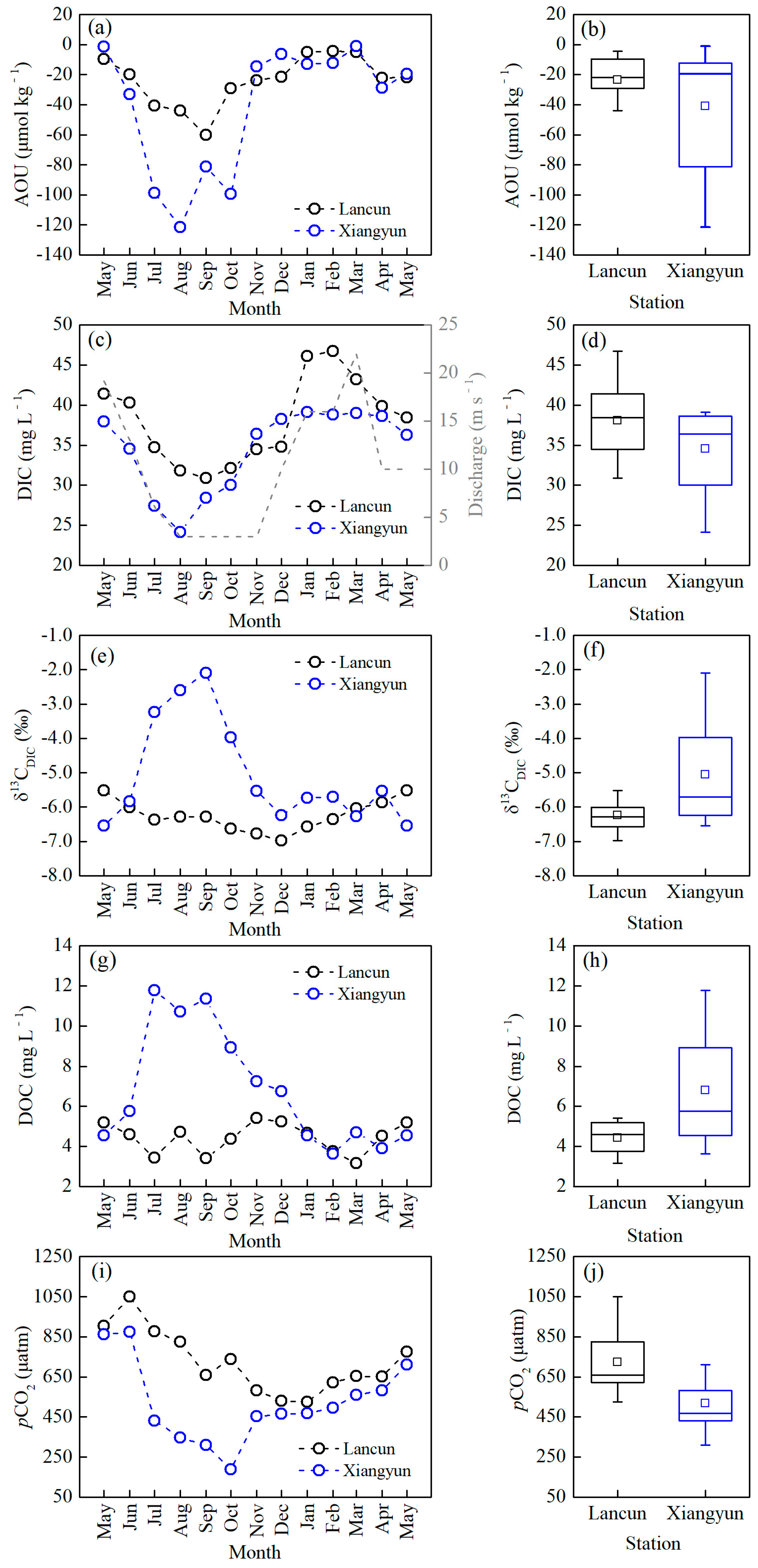
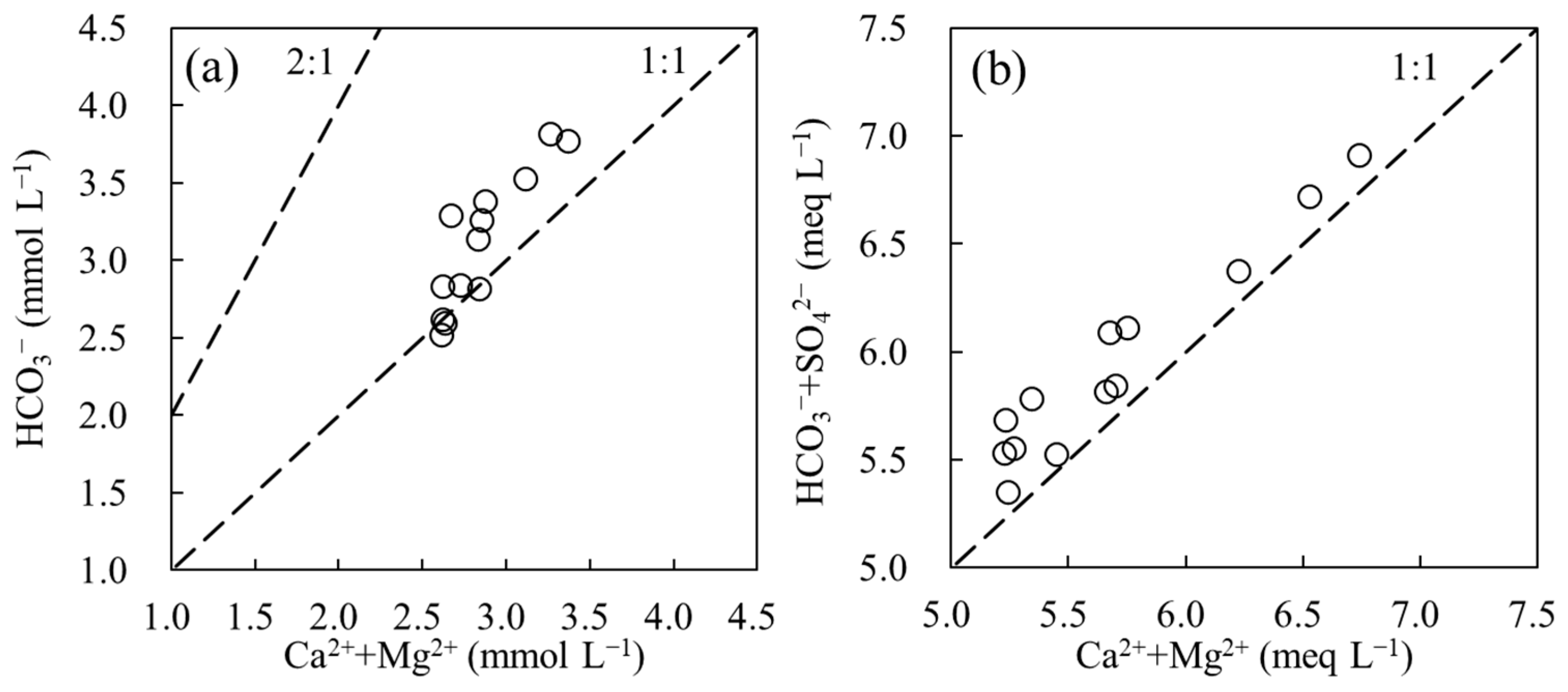
3.1. Variations of Water Chemistry
3.2. Variations of Riverine DIC, DOC, δ13CDIC and pCO2
4. Discussion
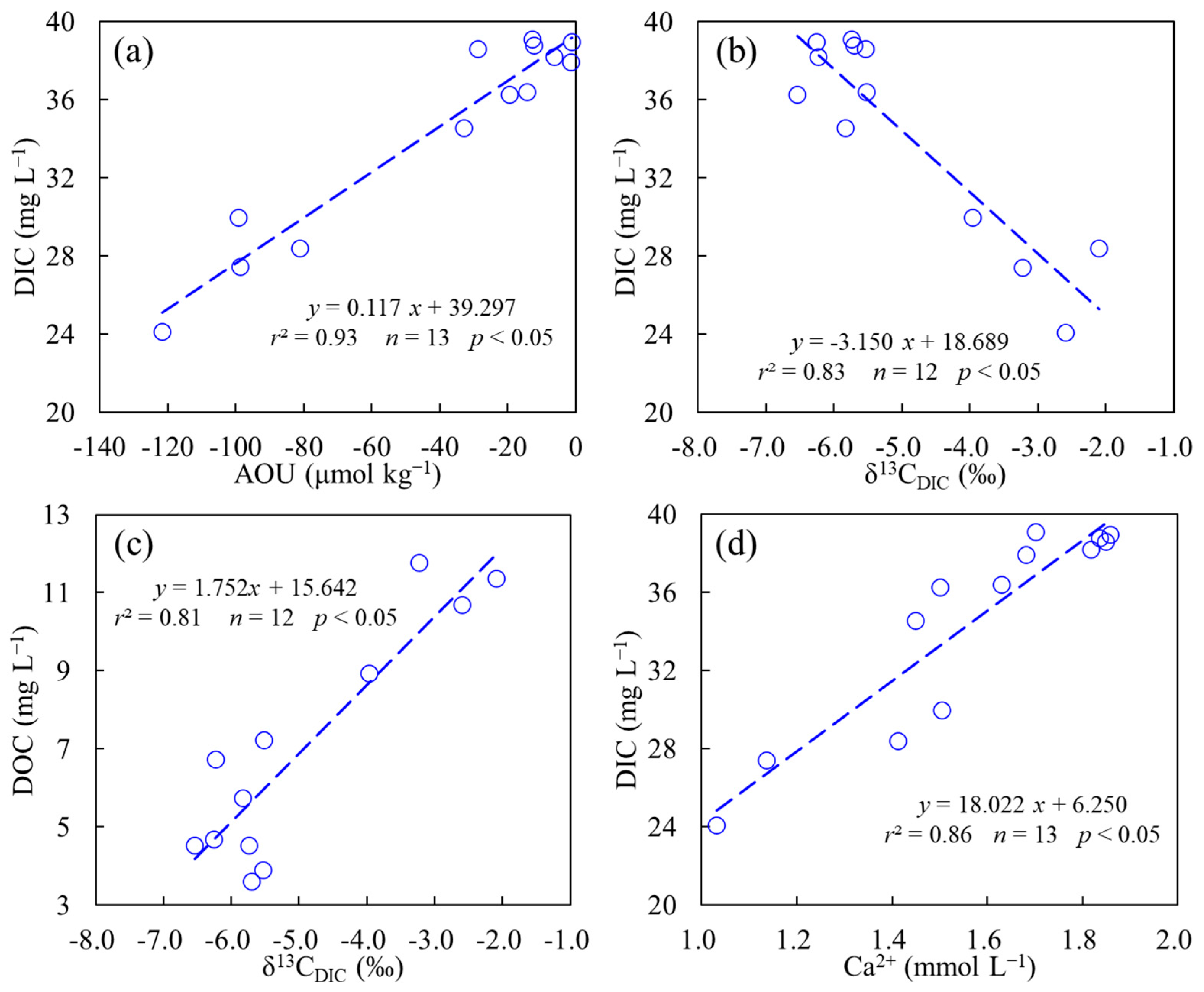
4.1. The Controls of DIC Variation at Background Station
4.2. The Effect of Urban Constructed Wetland on Riverine DIC
4.3. The Effect of the Urban Constructed Wetland on Riverine pCO2 Variability
4.4. Limitation and Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barnes, R.T.; Raymond, P.A. The contribution of agricultural and urban activities to inorganic carbon fluxes within temperate watersheds. Chem. Geol. 2009, 266, 318–327. [Google Scholar] [CrossRef]
- Chaplot, V.; Mutema, M. Sources and main controls of dissolved organic and inorganic carbon in river basins: A worldwide meta-analysis. J. Hydrol. 2021, 603, 126941. [Google Scholar] [CrossRef]
- Ran, L.; Butman, D.E.; Battin, T.J.; Yang, X.; Tian, M.; Duvert, C.; Hartmann, J.; Geeraert, N.; Liu, S. Substantial decrease in CO2 emissions from Chinese inland waters due to global change. Nat. Commun. 2021, 12, 1730. [Google Scholar] [CrossRef] [PubMed]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef]
- Wehrli, B. Biogeochemistry: Conduits of the carbon cycle. Nature 2013, 503, 346–347. [Google Scholar] [CrossRef]
- Lauerwald, R.; Hartmann, J.; Moosdorf, N.; Kempe, S.; Raymond, P.A. What controls the spatial patterns of the riverine carbonate system?—A case study for North America. Chem. Geol. 2013, 337–338, 114–127. [Google Scholar]
- Reiman, J.H.; Xu, Y.J. Dissolved carbon export and CO2 outgassing from the lower Mississippi River—Implications of future river carbon fluxes. J. Hydrol. 2019, 578, 124093. [Google Scholar] [CrossRef]
- Zhang, F.; Xiao, X.; Wang, L.; Zeng, C.; Yu, Z.; Wang, G.; Shi, X. Chemical weathering and CO2 consumption in the glaciated Karuxung River catchment, Tibetan Plateau. Hydrol. Process 2021, 35, e14330. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, Z.; Zhang, F.; Xiao, J. Seasonal variation in river water chemistry of the middle reaches of the Yellow River and its controlling factors. J. Geochem. Explor. 2015, 156, 101–113. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, J.; Li, S.; Ran, L.; Wang, W.; Xu, S.; Yan, Z.; Xu, S. Multiple controls on carbon dynamics in mixed karst and non-karst mountainous rivers, Southwest China, revealed by carbon isotopes (δ13C and Δ14C). Sci. Total Environ. 2021, 791, 148347. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liu, T.; Yuan, X.; Chen, H.; He, Y.; Wu, S.; Yuan, Z.; Li, H.; Que, Z.; et al. pCO2 and CO2 evasion from two small suburban rivers: Implications of the watershed urbanization process. Sci. Total Environ. 2021, 788, 147787. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Li, S.L.; Cai, H.M.; Yue, F.J.; Tao, F.X. The response of carbon geochemistry to hydrological events within an urban river, Southwestern China. Geochem. Int. 2018, 56, 462–473. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Wu, D.; Jun Xu, Y. Carbon and nutrients as indictors of daily fluctuations of pCO2 and CO2 flux in a river draining a rapidly urbanizing area. Ecol. Indic. 2020, 109, 105821. [Google Scholar] [CrossRef]
- Tang, W.; Jun Xu, Y.; Li, S. Rapid urbanization effects on partial pressure and emission of CO2 in three rivers with different urban intensities. Ecol. Indic. 2021, 125, 107515. [Google Scholar] [CrossRef]
- Yu, S.; He, S.; Sun, P.A.; Pu, J.; Huang, J.; Luo, H.; Li, Y.; Li, R.; Yuan, Y. Impacts of anthropogenic activities on weathering and carbon fluxes: A case study in the Xijiang River basin, southwest China. Environ. Earth Sci. 2016, 75, 589. [Google Scholar] [CrossRef]
- Park, J.; Jin, H.; Yoon, T.K.; Begum, M.S.; Eliyan, C.; Lee, E.; Lee, S.; Oh, N. Wastewater-boosted biodegradation amplifying seasonal variations of pCO2 in the Mekong-Tonle Sap river system. Biogeochemistry 2021, 155, 219–235. [Google Scholar] [CrossRef]
- Yoon, T.K.; Jin, H.; Begum, M.S.; Kang, N.; Park, J. CO2 outgassing from an urbanized river system fueled by wastewater treatment plant effluents. Environ. Sci. Technol. 2017, 51, 10459–10467. [Google Scholar] [CrossRef]
- Vymazal, J. Enhancing ecosystem services on the landscape with created, constructed and restored wetlands. Ecol. Eng. 2011, 37, 1–5. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, D.; He, F.; Zhang, Y.; Wu, Z. Application of constructed wetland for water pollution control in China during 1990–2010. Ecol. Eng. 2012, 47, 189–197. [Google Scholar] [CrossRef]
- Scholz, C.; Jones, T.G.; West, M.; Ehbair, A.M.S.; Dunn, C.; Freeman, C. Constructed wetlands may lower inorganic nutrient inputs but enhance DOC loadings into a drinking water reservoir in North Wales. Environ. Sci. Pollut. Res. 2016, 23, 18192–18199. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, J.; Shang, D.; Zhang, Y.; Zhang, J.; Xie, H.; Kong, Q.; Wang, Q. Application of constructed wetlands in the PAH remediation of surface water: A review. Sci. Total Environ. 2021, 780, 146605. [Google Scholar] [CrossRef]
- Rizzo, A.; Tondera, K.; Pálfy, T.G.; Dittmer, U.; Meyer, D.; Schreiber, C.; Zacharias, N.; Ruppelt, J.P.; Esser, D.; Molle, D.; et al. Constructed wetlands for combined sewer over flow treatment: A state-of-the-art review. Sci. Total Environ. 2020, 727, 138618. [Google Scholar] [CrossRef]
- de Klein, J.J.M.; van der Werf, A.K. Balancing carbon sequestration and GHG emissions in a constructed wetland. Ecol. Eng. 2014, 66, 36–42. [Google Scholar] [CrossRef]
- Schäfer, K.V.R.; Tripathee, R.; Artigas, F.; Morin, T.H.; Bohrer, G. Carbon dioxide fluxes of an urban tidal marsh in the Hudson-Raritan estuary. J. Geophys. Res. Biogeosci. 2014, 119, 2065–2081. [Google Scholar] [CrossRef]
- Wu, Y.; Mao, X.; Zhang, Z.; Tang, W.; Cao, G.; Zhou, H.; Ma, J.; Yin, X. Temporal and spatial characteristics of CO2 flux in plateau urban wetlands and their influencing factors based on eddy covariance technique. Water 2021, 13, 1176. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, W.; Wang, W.; Yin, W.; Liu, H.; Ma, X.; Zhou, Y.; Lei, P.; Wei, D.; Zhang, L.; et al. A review on China’s constructed wetlands in recent three decades: Application and practice. J. Environ. Sci. 2021, 104, 53–68. [Google Scholar] [CrossRef]
- Cai, W.; Guo, X.; Chen, C.A.; Dai, M.; Zhang, L.; Zhai, W.; Lohrenz, S.E.; Yin, K.; Harrison, P.J.; Wang, Y. A comparative overview of weathering intensity and HCO3− flux in the world’s major rivers with emphasis on the Changjiang, Huanghe, Zhujiang (Pearl) and Mississippi Rivers. Cont. Shelf Res. 2008, 28, 1538–1549. [Google Scholar] [CrossRef]
- Wang, X.; Luo, C.; Ge, T.; Xu, C.; Xue, Y. Controls on the sources and cycling of dissolved inorganic carbon in the Changjiang and Huanghe River estuaries, China: 14C and 13C studies. Limnol. Oceanogr. 2016, 61, 1358–1374. [Google Scholar] [CrossRef]
- Shan, S.; Luo, C.; Qi, Y.; Cai, W.; Sun, S.; Fan, D.; Wang, X. Carbon isotopic and lithologic constraints on the sources and cycling of inorganic carbon in four large rivers in China: Yangtze, Yellow, Pearl, and Heilongjiang. J. Geophys. Res. Biogeo. 2021, 126, e2020JG005901. [Google Scholar] [CrossRef]
- Wu, W. Hydrochemistry of inland rivers in the north Tibetan Plateau: Constraints and weathering rate estimation. Sci. Total Environ. 2016, 541, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Department of Ecological Environment of Shanxi Province. Report on the Ecological Environment of Shanxi Province in 2021; Department of Ecological Environment of Shanxi Province: Taiyuan, China, 2021.
- Yang, Y.; Meng, Z.; Jiao, W. Hydrological and pollution processes in mining area of Fenhe River Basin in China. Environ. Pollut. 2018, 234, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis, 3rd ed.; Wiley: Weinheim, Germany, 1999. [Google Scholar]
- Liu, Z.; Zhang, L.; Cai, W.; Wang, L.; Xue, M.; Zhang, X. Removal of dissolved inorganic carbon in the Yellow River Estuary. Limnol. Oceanogr. 2014, 59, 413–426. [Google Scholar] [CrossRef]
- Xue, L.; Yang, X.; Li, Y.; Li, L.; Jiang, L.; Xin, M.; Wang, Z.; Sun, X.; Wei, Q. Processes controlling sea surface pH and aragonite saturation state in a large northern temperate bay: Contrasting temperature effects. J. Geophys. Res. Biogeo. 2020, 125, e2020JG005805. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Pu, J.; Yuan, D. Carbon dioxide exchanges and their controlling factors in Guijiang River, SW China. J. Hydrol. 2019, 578, 124073. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Dang, J.; Yang, X.; Xue, L.; Zhang, L. Seasonal variation of sea surface pH and its controls in the Jiaozhou Bay, China. Cont. Shelf Res. 2022, 232, 104613. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace, D.W. Program Developed for CO2 Systems Calculations; ORNL/CDIAC 105; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory US Department of Energy: Oak Ridge, TN, USA, 1998.
- Chen, B.; Cai, W.; Brodeur, J.R.; Hussain, N.; Testa, J.M.; Ni, W.; Li, Q. Seasonal and spatial variability in surface pCO2 and air-water CO2 flux in the Chesapeake Bay. Limnol. Oceanogr. 2020, 65, 3046–3065. [Google Scholar] [CrossRef]
- Millero, F.J. The thermodynamics of the carbonate system in seawater. Geochim. Cosmochim. Acta 1979, 43, 1651–1661. [Google Scholar] [CrossRef]
- Redfield, A.C. The influence of organisms on the composition of seawater. Sea 1963, 2, 26–77. [Google Scholar]
- Calabrese, S.; Parolari, A.J.; Porporato, A. Hydrologic transport of dissolved inorganic carbon and its control on chemical weathering. J. Geophys. Res. Earth 2017, 122, 2016–2032. [Google Scholar] [CrossRef]
- Sun, P.; He, S.; Yu, S.; Pu, J.; Yuan, Y.; Zhang, C. Dynamics in riverine inorganic and organic carbon based on carbonate weathering coupled with aquatic photosynthesis in a karst catchment, Southwest China. Water Res. 2021, 189, 116658. [Google Scholar] [CrossRef]
- Meybeck, M. Global chemical weathering of surficial rocks estimated from river dissolved loads. Am. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- Martin, J.B. Carbonate minerals in the global carbon cycle. Chem. Geol. 2017, 449, 58–72. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis Publishers: New York, NY, USA, 1997; pp. 111–169. [Google Scholar]
- Deuser, W.G.; Degens, E.T. Carbon isotope fractionation in the system CO2(gas)-CO2(aqueous)-HCO3−(aqueous). Nature 1967, 215, 1033–1035. [Google Scholar] [CrossRef]
- Telmer, K.; Veizer, J. Carbon fluxes, pCO2 and substrate weathering in a large northern river basin, Canada: Carbon isotope perspectives. Chem. Geol. 1999, 159, 61–86. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. China Statistical Yearbook 2021; National Bureau of statistics of China: Beijing, China, 2021.
- Zhang, Y.; Jiang, Y.; Yuan, D.; Cui, J.; Li, Y.; Yang, J.; Cao, M. Source and flux of anthropogenically enhanced dissolved inorganic carbon: A comparative study of urban and forest karst catchments in Southwest China. Sci. Total Environ. 2020, 725, 138255. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Zhong, J.; Li, C.; Yi, Y.; Chen, S.; Ren, Y. Understanding transport and transformation of dissolved inorganic carbon (DIC) in the reservoir system using δ13CDIC and water chemistry. J. Hydrol. 2019, 574, 193–201. [Google Scholar] [CrossRef]
- Jiang, D.; Li, Z.; Luo, Y.; Xia, Y. River damming and drought affect water cycle dynamics in an ephemeral river based on stable isotopes: The Dagu River of North China. Sci. Total Environ. 2021, 758, 143682. [Google Scholar] [CrossRef]
- Maavara, T.; Lauerwald, R.; Regnier, P.; Van Cappellen, P. Global perturbation of organic carbon cycling by river damming. Nat. Commun. 2017, 8, 15347. [Google Scholar] [CrossRef]
- Ran, L.; Li, L.; Tian, M.; Yang, X.; Yu, R.; Zhao, J.; Wang, L.; Lu, X.X. Riverine CO2 emissions in the Wuding River catchment on the Loess Plateau: Environmental controls and dam impoundment impact. J. Geophys. Res. Biogeo. 2017, 122, 1439–1455. [Google Scholar] [CrossRef]
- Cui, G.; Li, X.; Li, Q.; Huang, J.; Tao, Y.; Li, S.; Zhang, J. Damming effects on dissolved inorganic carbon in different kinds of reservoirs in Jialing River, Southwest China. Acta Geochim. 2017, 36, 581–597. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Zhong, J.; Maberly, S.C.; Li, C.; Wang, F.; Xiao, H.; Liu, C. Climatic and anthropogenic regulation of carbon transport and transformation in a karst river-reservoir system. Sci. Total Environ. 2020, 707, 135628. [Google Scholar] [CrossRef]
- Cicerone, D.S.; Stewart, A.J.; Roh, Y. Diel cycles in calcite production and dissolution in a eutrophic basin. Environ. Toxicol. Chem. 1999, 18, 2169–2177. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Liu, Z.; Macpherson, G.L.; Groves, C.; Martin, J.B.; Yuan, D.; Zeng, S. Large and active CO2 uptake by coupled carbonate weathering. Earth-Sci. Rev. 2018, 182, 42–49. [Google Scholar] [CrossRef]
- Rogerson, M.; Pedley, H.M.; Wadhawan, J.D.; Middleton, R. New insights into biological influence on the geochemistry of freshwater carbonate deposits. Geochim. Cosmochim. Acta 2008, 72, 4976–4987. [Google Scholar] [CrossRef]
- de Montety, V.; Martin, J.B.; Cohen, M.J.; Foster, C.; Kurz, M.J. Influence of diel biogeochemical cycles on carbonate equilibrium in a karst river. Chem. Geol. 2011, 283, 31–43. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Z.; Sun, H.; Yang, R.; Chen, B. Organic carbon source tracing and DIC fertilization effect in the Pearl River: Insights from lipid biomarker and geochemical analysis. Appl. Geochem. 2016, 73, 132–141. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.G. Observations on nutrient elements and sulphate in atmospheric wet depositions over the northwest Pacific coastal oceans—Yellow Sea. Mar. Chem. 1994, 47, 173–189. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Liu, S. Characterization of nutrients in the atmospheric wet and dry deposition observed at the two monitoring sites over Yellow Sea and East China Sea. J. Atmos. Chem. 2007, 57, 41–57. [Google Scholar] [CrossRef]
- Weiss, R.F. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Xia, M.; Craig, P.M.; Schaeffer, B.; Stoddard, A.; Liu, Z.; Peng, M.; Zhang, H.; Wallen, C.M.; Bailey, N.; Mandrup-Poulsen, J. Influence of physical forcing on bottom-water dissolved oxygen within Caloosahatchee River Estuary, Florida. J. Environ. Eng. 2010, 136, 1032–1044. [Google Scholar] [CrossRef]
- Liu, Y.; Weisberg, R.H.; Zheng, L.; Heil, C.A.; Hubbard, K.A. Termination of the 2018 Florida red tide event: A tracer model perspective. Estuar. Coast. Shelf Sci. 2022, 272, 107901. [Google Scholar] [CrossRef]
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef]
- Pacheco, F.S.; Roland, F.; Downing, J.A. Eutrophication reverses whole-lake carbon budgets. Inland Waters 2014, 4, 41–48. [Google Scholar] [CrossRef]
- Roberts, B.J.; Mulholland, P.J.; Hill, W.R. Multiple scales of temporal variability in ecosystem metabolism rates: Results from 2 years of continuous monitoring in a forested headwater stream. Ecosystems 2007, 10, 588–606. [Google Scholar] [CrossRef]
- Gratani, L.; Catoni, R.; Puglielli, G.; Varone, L.; Crescente, M.F.; Sangiorgio, S.; Lucchetta, F. Carbon dioxide (CO2) sequestration and air temperature amelioration provided by urban parks in Rome. Energy Procedia 2016, 101, 408–415. [Google Scholar] [CrossRef]
- Poulter, B.; Frank, D.; Ciais, P.; Myneni, R.B.; Andela, N.; Bi, J.; Broquet, G.; Canadell, J.G.; Chevallier, F.; Liu, Y.Y.; et al. Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 2014, 509, 600–603. [Google Scholar] [CrossRef]
- Ni, M.; Li, S. Dynamics and internal links of dissolved carbon in a karst river system: Implications for composition, origin and fate. Water Res. 2022, 226, 119289. [Google Scholar] [CrossRef]
- Bledsoe, R.B.; Bean, E.Z.; Austin, S.S.; Peralta, A.L. A microbial perspective on balancing trade-offs in ecosystem functions in a constructed stormwater wetland. Ecol. Eng. 2020, 158, 109000. [Google Scholar] [CrossRef]

| Location | Sample | Tw | pH | AOU | DIC | δ13CDIC | DOC | pCO2 | Ca2+ | Mg2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| °C | μmol kg−1 | mg L−1 | ‰ | mg L−1 | μatm | mmol L−1 | mmol L−1 | |||
| Lancun | Max | 22.4 | 8.55 | −60.1 | 46.7 | −5.51 | 5.40 | 1050 | 2.01 | 1.36 |
| Min | 0.3 | 8.29 | −4.2 | 30.9 | −6.98 | 3.16 | 524 | 1.46 | 1.06 | |
| Average | 11.4 | 8.39 | −23.6 | 38.1 | −6.31 | 4.37 | 722 | 1.68 | 1.16 | |
| SD | 7.8 | 0.08 | 16.1 | 5.2 | 0.39 | 0.73 | 151 | 0.17 | 0.08 | |
| Xiangyun | Max | 27.4 | 8.86 | −121.7 | 39.1 | −2.10 | 11.77 | 873 | 1.86 | 1.29 |
| Min | 2.2 | 8.33 | −1.2 | 24.1 | −6.54 | 3.62 | 188 | 1.03 | 0.74 | |
| Average | 13.9 | 8.52 | −40.9 | 34.5 | −4.94 | 6.99 | 519 | 1.57 | 1.16 | |
| SD | 9.1 | 0.13 | 41.3 | 5.0 | 1.48 | 2.88 | 193 | 0.26 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, J.; Zhang, M.; Li, Y. The Sustained Response of Dissolved Inorganic Carbon to Urban Constructed Wetland in the Fenhe River, China: A Case Study. Sustainability 2024, 16, 1930. https://doi.org/10.3390/su16051930
Dang J, Zhang M, Li Y. The Sustained Response of Dissolved Inorganic Carbon to Urban Constructed Wetland in the Fenhe River, China: A Case Study. Sustainability. 2024; 16(5):1930. https://doi.org/10.3390/su16051930
Chicago/Turabian StyleDang, Jiajia, Meifang Zhang, and Yunxiao Li. 2024. "The Sustained Response of Dissolved Inorganic Carbon to Urban Constructed Wetland in the Fenhe River, China: A Case Study" Sustainability 16, no. 5: 1930. https://doi.org/10.3390/su16051930






