Is Biomethane Production from Common Reed Biomass Influenced by the Hydraulic Parameters of Treatment Wetlands?
Abstract
:1. Introduction
2. Materials and Methods
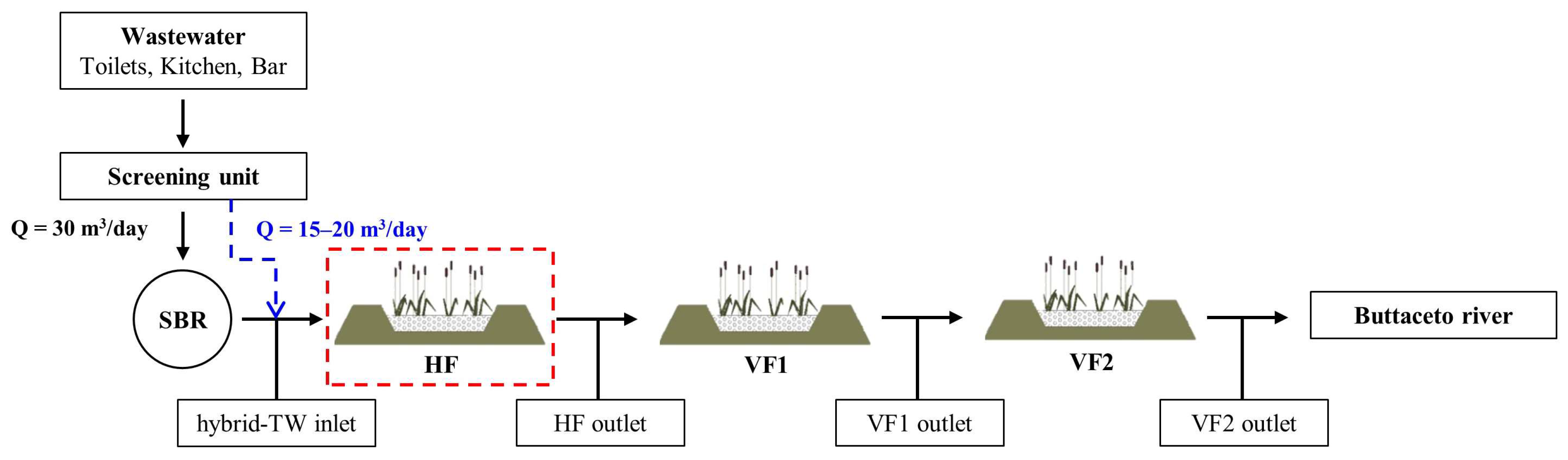
2.1. Study Area
2.2. Common Reed Biomass Composition
2.3. Biochemical Methane Potential (BMP) Assay
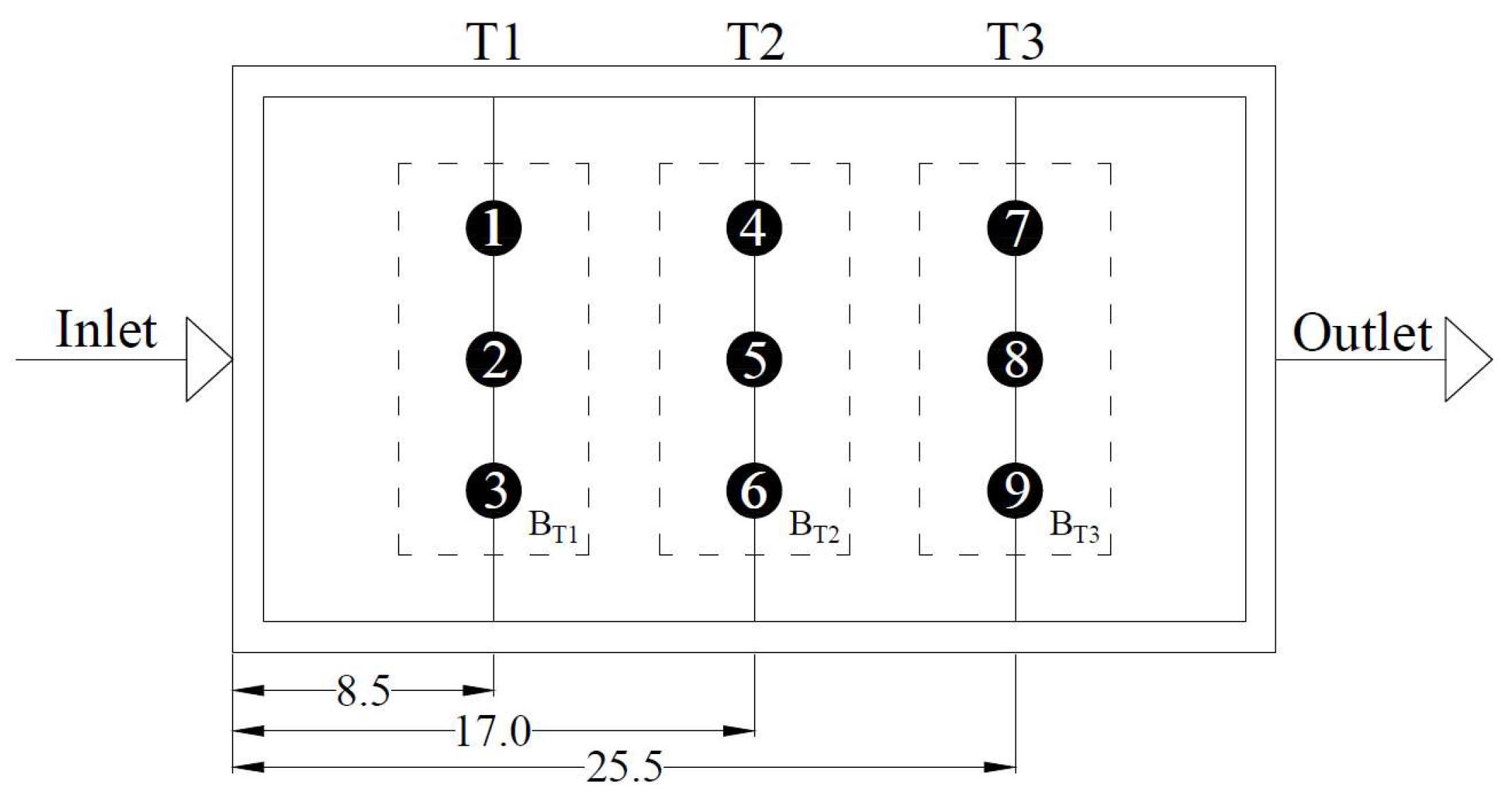
2.4. Ks Measurements
2.5. Data Analysis
3. Results
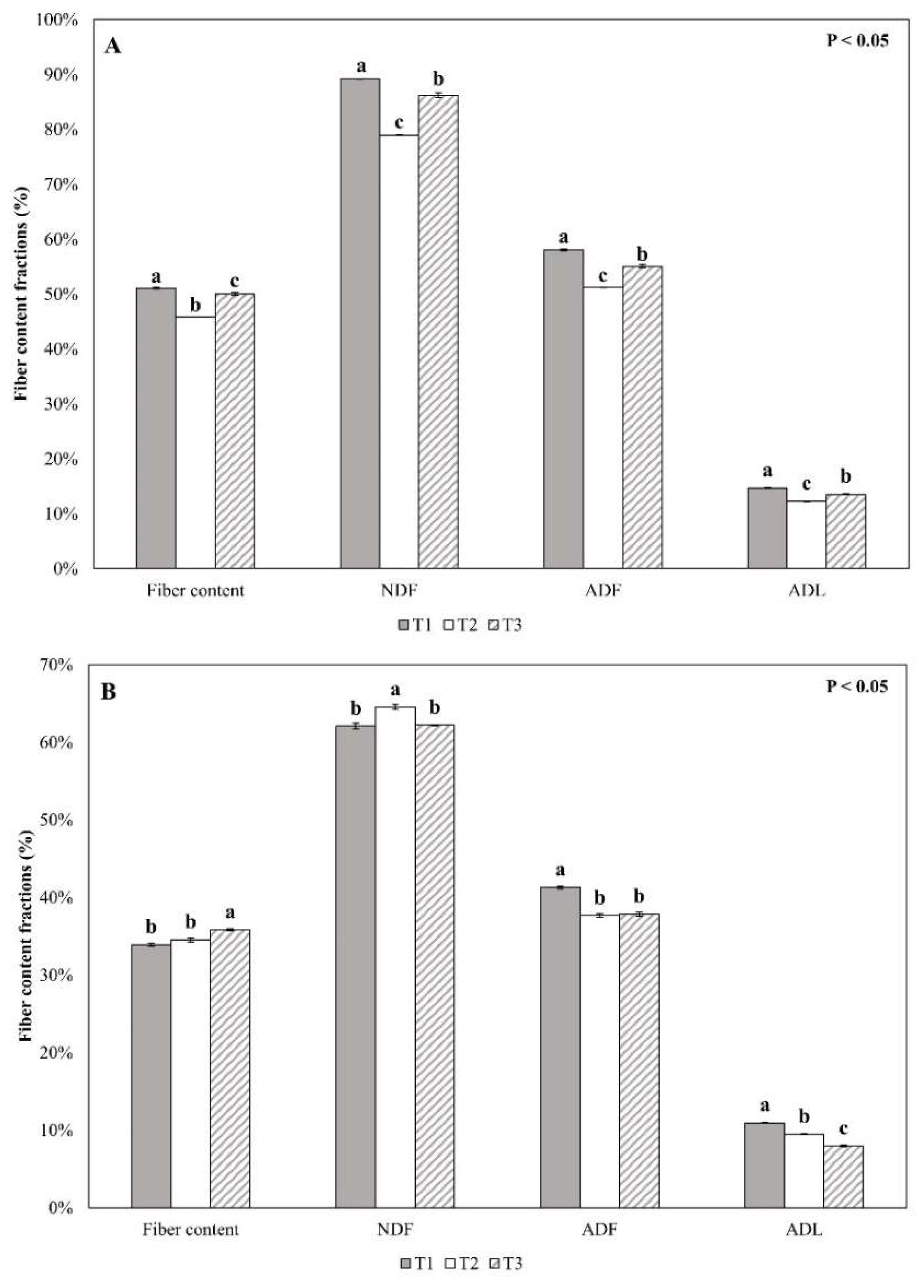
3.1. Common Reed Biomass Characteristics Variation at Different Harvest Times
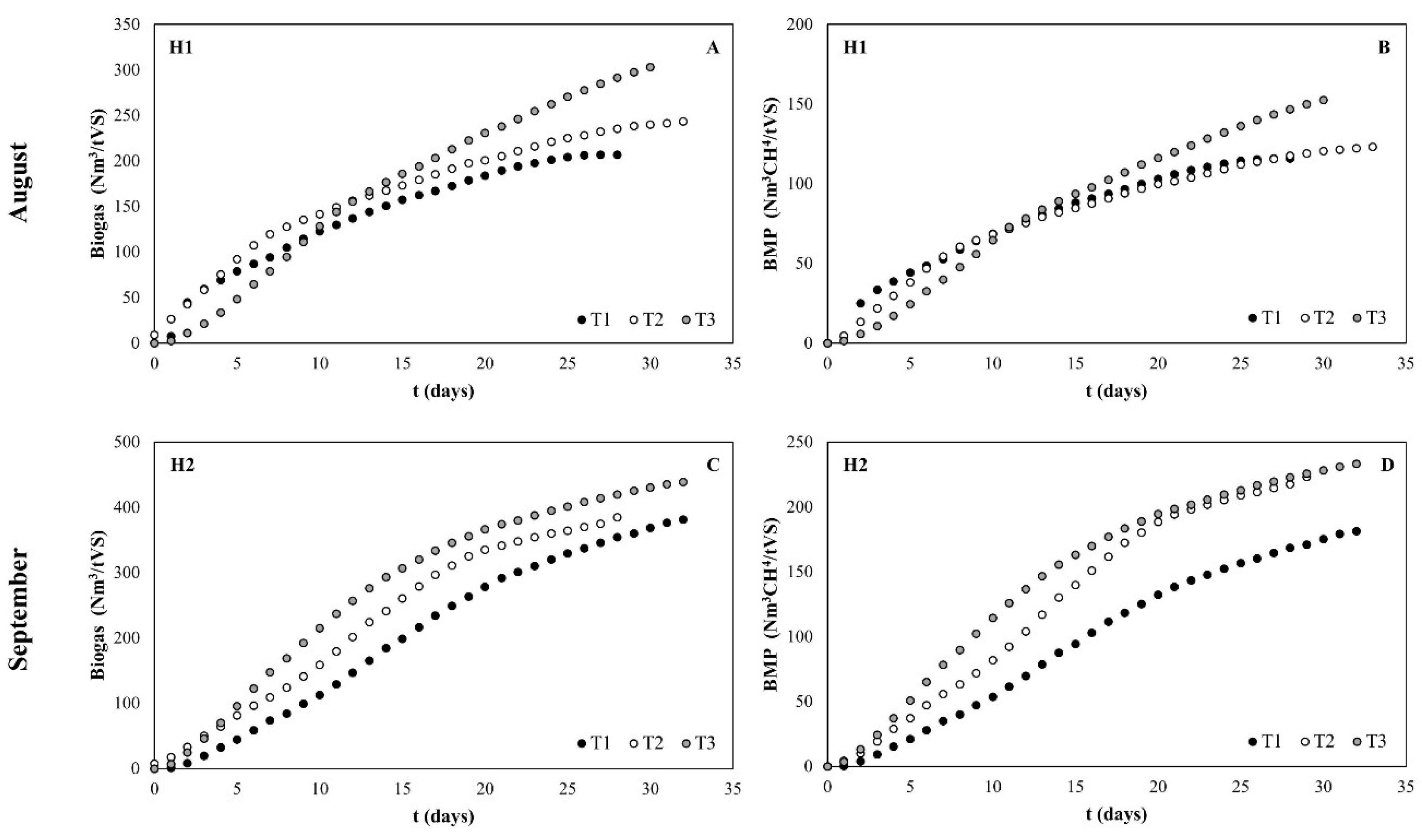
3.2. Common Reed Methane Potential Production
3.3. Ks Measurements within the HSTW
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vymazal, J. Emergent plants used in free water surface constructed wetlands: A review. Ecol. Eng. 2013, 61, 582–592. [Google Scholar] [CrossRef]
- Avellan, C.T.; Ardakanian, R.; Gremillion, P. The role of constructed wetlands for biomass production within the water-soil-waste nexus. Water Sci. Technol. 2017, 75, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Licata, M.; Gennaro, M.C.; Tuttolomondo, T.; Leto, C.; La Bella, S. Research focusing on plant performance in constructed wetlands and agronomic application of treated wastewater—A set of experimental studies in Sicily (Italy). PLoS ONE 2019, 14, e0219445. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Hu, W.; Pei, H.; Hou, Q.; Ji, Y. Effect of different plant species on nutrient removal and rhizospheric microorganisms distribution in horizontal-flow constructed wetlands. Environ. Technol. 2014, 35, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Jinadasa, K.; Gersberg, R.M.; Liu, Y.; Ng, W.J.; Tan, S.K. Application of constructed wetlands for wastewater treatment in developing countries—A review of recent developments (2000–2013). J. Environ. Manag. 2014, 141, 116–131. [Google Scholar] [CrossRef]
- Liu, D.; Wu, X.; Chang, J.; Gu, B.; Min, Y.; Ge, Y.; Shi, Y.; Xue, H.; Peng, C.; Wu, J. Constructed wetlands as biofuel production systems. Nat. Clim. Chang. 2012, 2, 190–194. [Google Scholar] [CrossRef]
- Köbbing, J.F.; Thevs, N.; Zerbe, S. The utilisation of reed (Phragmites australis): A review. Mires Peat 2013, 13, 1–14. [Google Scholar]
- Geurts, J.J.; Oehmke, C.; Lambertini, C.; Eller, F.; Sorrell, B.K.; Mandiola, S.R.; Grootjans, A.P.; Brix, H.; Wichtmann, W.; Lamers, L.P.; et al. Nutrient removal potential and biomass production by Phragmites australis and Typha latifolia on European rewetted peat and mineral soils. Sci. Total. Environ. 2020, 747, 141102. [Google Scholar] [CrossRef] [PubMed]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Drewes, J.E.; Koch, K. Correlation between Biogas Yield and Chemical Composition of Grassland Plant Species. Energy Fuels 2015, 29, 7221–7229. [Google Scholar] [CrossRef]
- Kandel, T.P.; Sutaryo, S.; Møller, H.B.; Jørgensen, U.; Lærke, P.E. Chemical composition and methane yield of reed canary grass as influenced by harvesting time and harvest frequency. Bioresour. Technol. 2013, 130, 659–666. [Google Scholar] [CrossRef]
- Ragaglini, G.; Dragoni, F.; Simone, M.; Bonari, E. Suitability of giant reed (Arundo donax L.) for anaerobic digestion: Effect of harvest time and frequency on the biomethane yield potential. Bioresour. Technol. 2014, 152, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, F.; Ragaglini, G.; Corneli, E.; o Di Nasso, N.N.; Tozzini, C.; Cattani, S.; Bonari, E. Giant reed (Arundo donax L.) for biogas production: Land use saving and nitrogen utilisation efficiency compared with arable crops. Ital. J. Agron. 2015, 10, 192–201. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Q.; Zhang, J.; Xie, H.; Feng, S. Effect of plant harvesting on the performance of constructed wetlands during summer. Water 2016, 8, 24. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, H.; Zhang, J.; Liang, S.; Ngo, H.H.; Guo, W.; Liu, C.; Zhao, C.; Li, H. Effect of plant harvesting on the performance of constructed wetlands during winter: Radial oxygen loss and microbial characteristics. Environ. Sci. Pollut. Res. 2015, 22, 7476–7484. [Google Scholar] [CrossRef] [PubMed]
- Al-Isawi, R.; Scholz, M.; Wang, Y.; Sani, A. Clogging of vertical-flow constructed wetlands treating urban wastewater contaminated with a diesel spill. Environ. Sci. Pollut. Res. 2015, 22, 12779–12803. [Google Scholar] [CrossRef] [PubMed]
- Knowles, P.; Dotro, G.; Nivala, J.; García, J. Clogging in subsurface-flow treatment wetlands: Occurrence and contributing factors. Ecol. Eng. 2011, 37, 99–112. [Google Scholar] [CrossRef]
- Licciardello, F.; Sacco, A.; Barbagallo, S.; Ventura, D.; Cirelli, G.L. Evaluation of different methods to assess the hydraulic behavior in horizontal treatment wetlands. Water 2020, 12, 2286. [Google Scholar] [CrossRef]
- de Matos, M.P.; von Sperling, M.; de Matos, A.T. Clogging in horizontal subsurface flow constructed wetlands: Influencing factors, research methods and remediation techniques. Rev. Environ. Sci. Bio/Technol. 2018, 17, 87–107. [Google Scholar] [CrossRef]
- Tang, Y.; Yao, X.; Chen, Y.; Zhou, Y.; Zhu, D.Z.; Zhang, Y.; Zhang, T.; Peng, Y. Experiment research on physical clogging mechanism in the porousmedia and its impact on permeability. Granul. Matter 2020, 22, 37. [Google Scholar] [CrossRef]
- Vymazal, J. Does clogging affect long-term removal of organics and suspended solids in gravel-based horizontal subsurface flow constructed wetlands? Chem. Eng. J. 2018, 331, 663–674. [Google Scholar] [CrossRef]
- Sanford, W.E.; Steenhuis, T.S.; Parlange, J.-Y.; Surface, J.M.; Peverly, J.H. Hydraulic conductivity of gravel and sand as substrates in rock-reed filters. Ecol. Eng. 1995, 4, 321–336. [Google Scholar] [CrossRef]
- Suliman, F.; French, H.; Haugen, L.; Søvik, A. Change in flow and transport patterns in horizontal subsurface flow constructed wetlands as a result of biological growth. Ecol. Eng. 2006, 27, 124–133. [Google Scholar] [CrossRef]
- Pedescoll, A.; Uggetti, E.; Llorens, E.; Granés, F.; García, D.; García, J. Practical method based on saturated hydraulic conductivity used to assess clogging in subsurface flow constructed wetlands. Ecol. Eng. 2009, 35, 1216–1224. [Google Scholar] [CrossRef]
- Garcia-Artigas, R.; Himi, M.; Revil, A.; Urruela, A.; Lovera, R.; Sendrós, A.; Casas, A.; Rivero, L. Time-domain induced polarization as a tool to image clogging in treatment wetlands. Sci. Total Environ. 2020, 724, 138189. [Google Scholar] [CrossRef]
- UNI EN 12879:2002; Characterization of Sludges—Determination of the Loss on Ignition of Dry Mass. UNI: Rome, Italy, 2002.
- UNI/TS 11703:2018; Method for the Measurement of the Potential Production of Methane from Wet Anaerobic Digestion—Matrices in Feed. UNI: Rome, Italy, 2018.
- Sciuto, L.; Licciardello, F.; Barbera, A.; Cirelli, G. Giant reed from wetlands as a potential resource for biomethane production. Ecol. Eng. 2023, 190, 106947. [Google Scholar] [CrossRef]
- Naval Facilities Engineering Command. Soil Mechanics. Design Manual 7.01; Nav. Facil. Eng. Command: Alexandria, VA, USA, 1986. [Google Scholar]
- Pedescoll, A.; Samsó, R.; Romero, E.; Puigagut, J.; García, J. Reliability, repeatability and accuracy of the falling head method for hydraulic conductivity measurements under laboratory conditions. Ecol. Eng. 2011, 37, 754–757. [Google Scholar] [CrossRef]
- Licciardello, F.; Aiello, R.; Alagna, V.; Iovino, M.; Ventura, D.; Cirelli, G.L. Assessment of clogging in constructed wetlands by saturated hydraulic conductivity measurements. Water Sci. Technol. 2019, 79, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Hansson, P.-A.; Fredriksson, H. Use of summer harvested common reed (Phragmites australis) as nutrient source for organic crop production in Sweden. Agric. Ecosyst. Environ. 2004, 102, 365–375. [Google Scholar] [CrossRef]
- Komulainen, M.; Simi, P.; Hagelberg, E.; Ikonen, I.; Lyytinen, S. Reed Energy—Possibilities of Using the Common Reed for Energy Generation in Southern Finland. Rep. Turku Univ. Appl. Sci. 2008, 67, 81. [Google Scholar]
- Dragoni, F.; Giannini, V.; Ragaglini, G.; Bonari, E.; Silvestri, N. Effect of Harvest Time and Frequency on Biomass Quality and Biomethane Potential of Common Reed (Phragmites australis) Under Paludiculture Conditions. BioEnergy Res. 2017, 10, 1066–1078. [Google Scholar] [CrossRef]
- Dinka, M.; Ágoston-Szabó, E.; Tóth, I. Changes in nutrient and fibre content of decomposing Phragmites australis litter. Int. Rev. Hydrobiol. 2004, 89, 519–535. [Google Scholar] [CrossRef]
- Wohler-Geske, A.; Moschner, C.R.; Gellerich, A.; Militz, H.; Greef, J.M.; Hartung, E. Provenances and properties of thatching reed (Phragmites australis). Landbauforsch 2016, 66, 1–10. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, S.; Huang, Z.; Cui, L.; Wang, X. Evaluation of organic matter removal efficiency and microbial enzyme activity in vertical-flow constructed wetland systems. Environments 2016, 3, 26. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of BOD in constructed wetlands with horizontal sub-surface flow: Czech experience. Water Sci. Technol. 1999, 40, 133–138. [Google Scholar] [CrossRef]
- Knowles, P.; Davies, P. A method for the in-situ determination of the hydraulic conductivity of gravels as used in constructed wetlands for wastewater treatment. Desalination Water Treat. 2009, 5, 257–266. [Google Scholar] [CrossRef]
- Pedescoll, A.; Knowles, P.R.; Davies, P.; García, J.; Puigagut, J. A comparison of in situ constant and falling head permeameter tests to assess the distribution of clogging within horizontal subsurface flow constructed wetlands. Water Air Soil Pollut. 2012, 223, 2263–2275. [Google Scholar] [CrossRef]
- Sacco, A.; Sciuto, L.; Licciardello, F.; Cirelli, G.L.; Milani, M.; Barbera, A.C. Effects of Solids Accumulation on Greenhouse Gas Emissions, Substrate, Plant Growth and Performance of a Mediterranean Horizontal Flow Treatment Wetland. Environments 2023, 10, 30. [Google Scholar] [CrossRef]











| Properties | H1 (Aug) | H2 (Sept) | H3 (Oct) |
|---|---|---|---|
| Plant height (m) a | 2.50 ± 0.05 | 0.78 ± 0.19 | 0.87 ± 0.30 |
| Culms number (-) a | 19 ± 5 | 25 ± 13 | 67 ± 26 |
| DM (%) b | 45.08 ± 0.92 | 22.62 ± 0.08 | 32.00 ± 6.38 |
| Dry weight (g DM per culm) b | 39.11 ± 8.28 | 4.03 ± 1.23 | 3.34 ± 1.10 |
| Fiber content (%) c | 48.98 ± 2.76 | 34.76 ± 0.97 | 35.21 ± 2.90 |
| Neutral Detergent Fiber—NDF (%) c | 84.75 ± 5.28 | 62.93 ± 1.40 | 63.98 ± 3.73 |
| Acid Detergent Fiber—ADF (%) c | 54.76 ± 3.41 | 38.94 ± 2.05 | 38.04 ± 4.04 |
| Acid Detergent Lignin—ADL (%) c | 13.48 ± 1.22 | 9.47 ± 1.49 | 10.32 ± 1.71 |
| Properties | H1 (Aug) | H2 (Sept) |
|---|---|---|
| Biogas (Nm3/tVS) a | 251.16 ± 54.72 | 402.08 ± 89.38 |
| BMP (Nm3CH4/tVS) a | 130.57 ± 24.29 | 212.70 ± 50.62 |
| CH4 (%) a | 52.32 ± 3.18 | 52.88 ± 5.24 |
| CO2 (%) a | 16.88 ± 4.51 | 14.82 ± 3.04 |
| H2S (ppm) a | 20.5 ± 14.26 | 74.7 ± 9.42 |
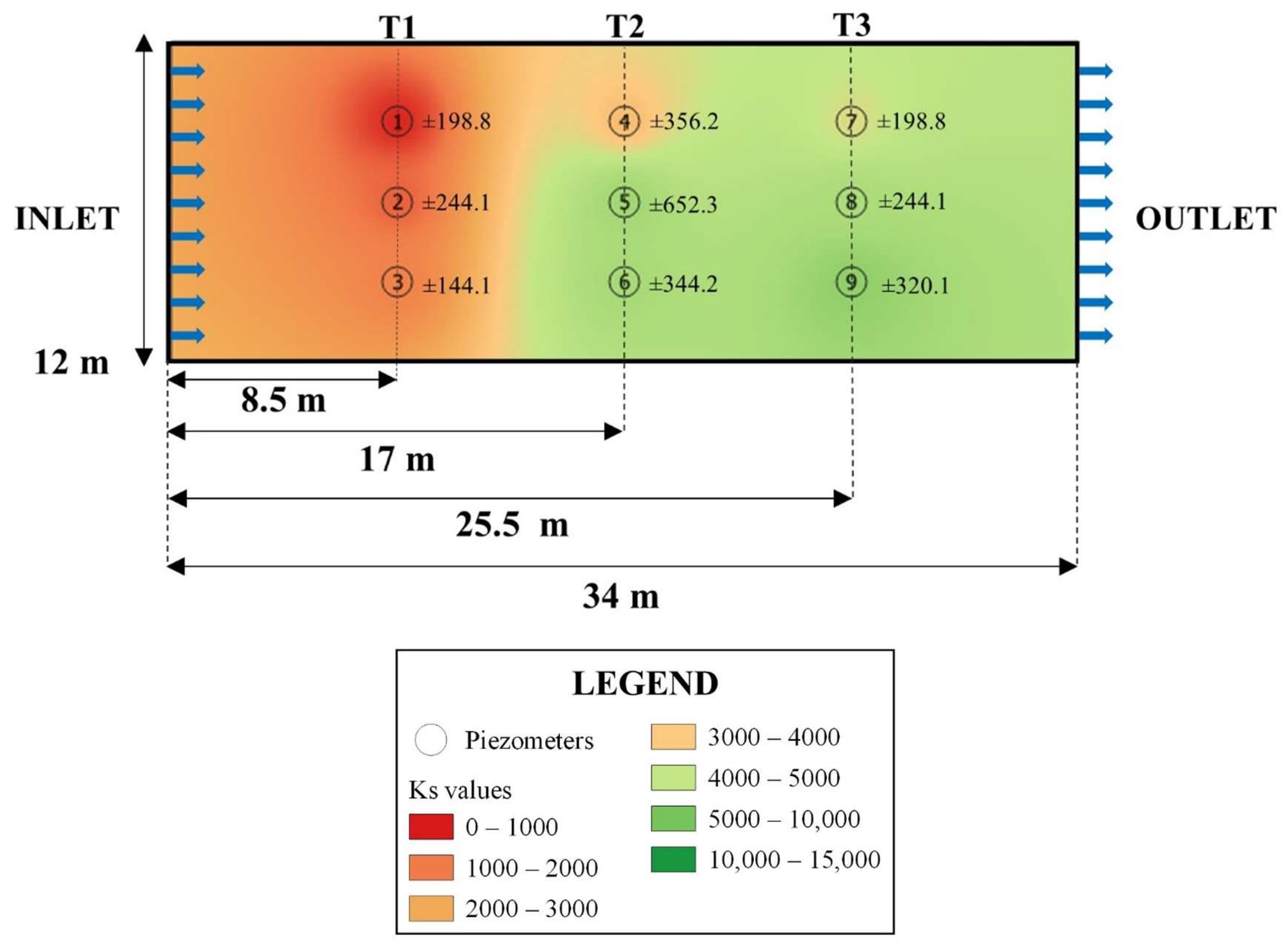
| September 2022 | ||||
|---|---|---|---|---|
| Piezometers | Distance from the Inlet | Ks | SD | Reductions of Ks (%) |
| (m) | (m day−1) | Relative to Clean Gravel 1 | ||
| 1 | 8.5 | 1135.02 | 198.8 | 94 |
| 2 | 17 | 1725.13 | 244.1 | 91 |
| 3 | 25.5 | 2135.68 | 144.1 | 89 |
| 4 | 8.5 | 3568.94 | 356.2 | 82 |
| 5 | 17 | 7288.32 | 652.3 | 63 |
| 6 | 25.5 | 6895.41 | 344.2 | 65 |
| 7 | 8.5 | 4597.07 | 198.8 | 76 |
| 8 | 17 | 6570.20 | 244.1 | 66 |
| 9 | 25.5 | 8432.20 | 320.1 | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciuto, L.; Licciardello, F.; Barbera, A.C.; Scavera, V.; Musumeci, S.; Severino, M.; Cirelli, G.L. Is Biomethane Production from Common Reed Biomass Influenced by the Hydraulic Parameters of Treatment Wetlands? Sustainability 2024, 16, 2751. https://doi.org/10.3390/su16072751
Sciuto L, Licciardello F, Barbera AC, Scavera V, Musumeci S, Severino M, Cirelli GL. Is Biomethane Production from Common Reed Biomass Influenced by the Hydraulic Parameters of Treatment Wetlands? Sustainability. 2024; 16(7):2751. https://doi.org/10.3390/su16072751
Chicago/Turabian StyleSciuto, Liviana, Feliciana Licciardello, Antonio Carlo Barbera, Vincenzo Scavera, Salvatore Musumeci, Massimiliano Severino, and Giuseppe Luigi Cirelli. 2024. "Is Biomethane Production from Common Reed Biomass Influenced by the Hydraulic Parameters of Treatment Wetlands?" Sustainability 16, no. 7: 2751. https://doi.org/10.3390/su16072751







