The Influence of Biowaste Type on the Physicochemical and Sorptive Characteristics of Corresponding Biochar Used as Sustainable Sorbent
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Material Moisture, Ash Content and Biochar Yield
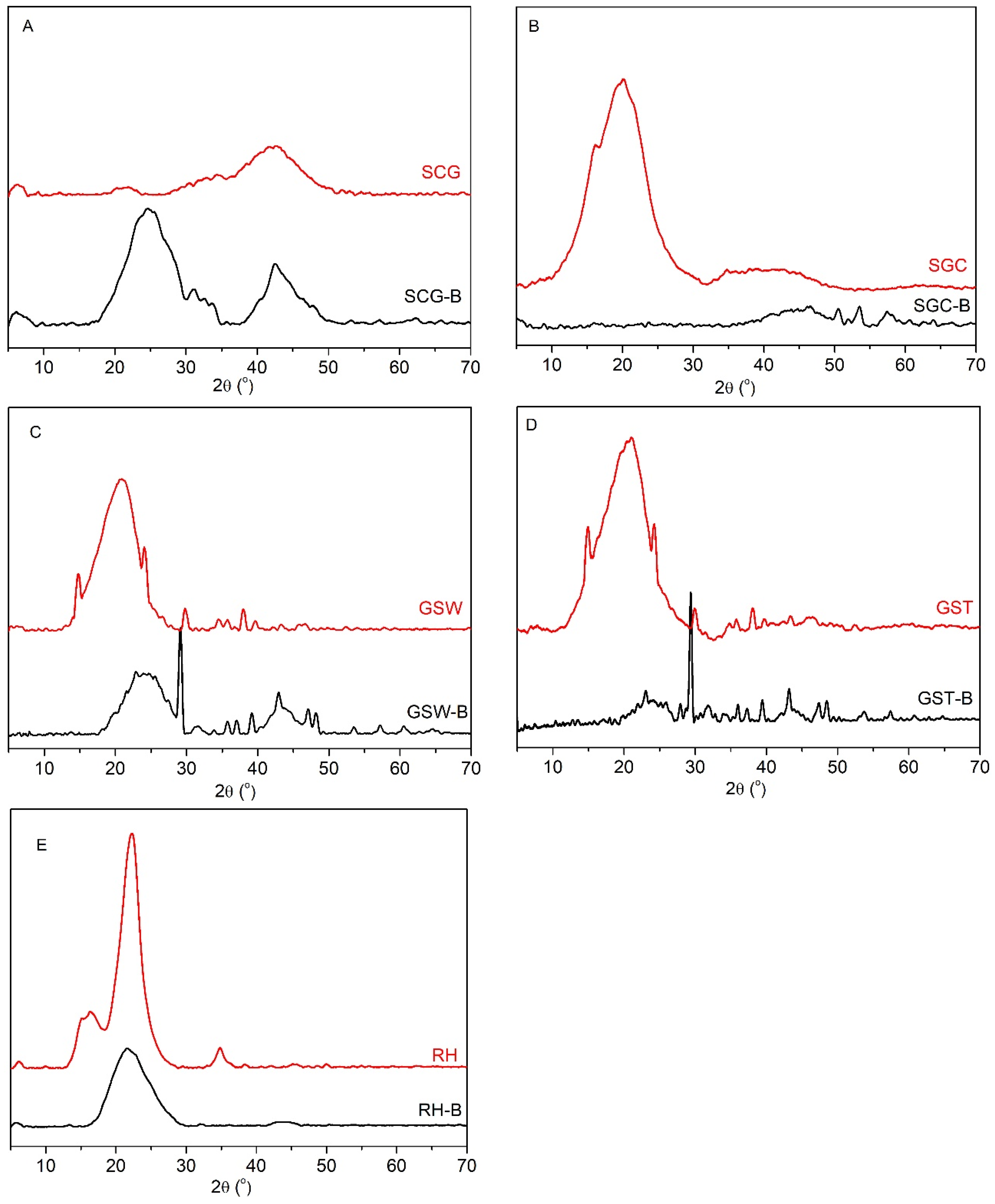
3.2. Material Structure
3.3. Raw Materials Pyrolysis and Thermal Stability of Biochar
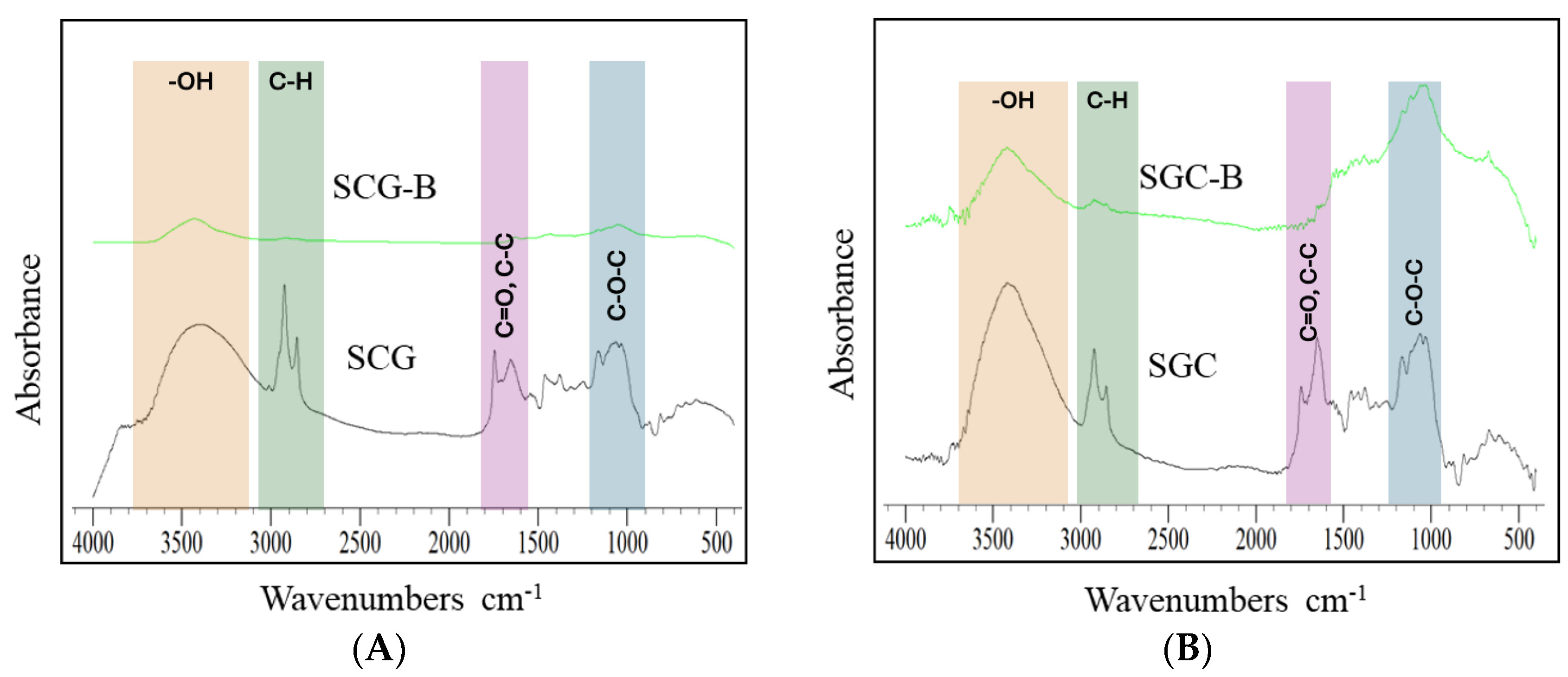
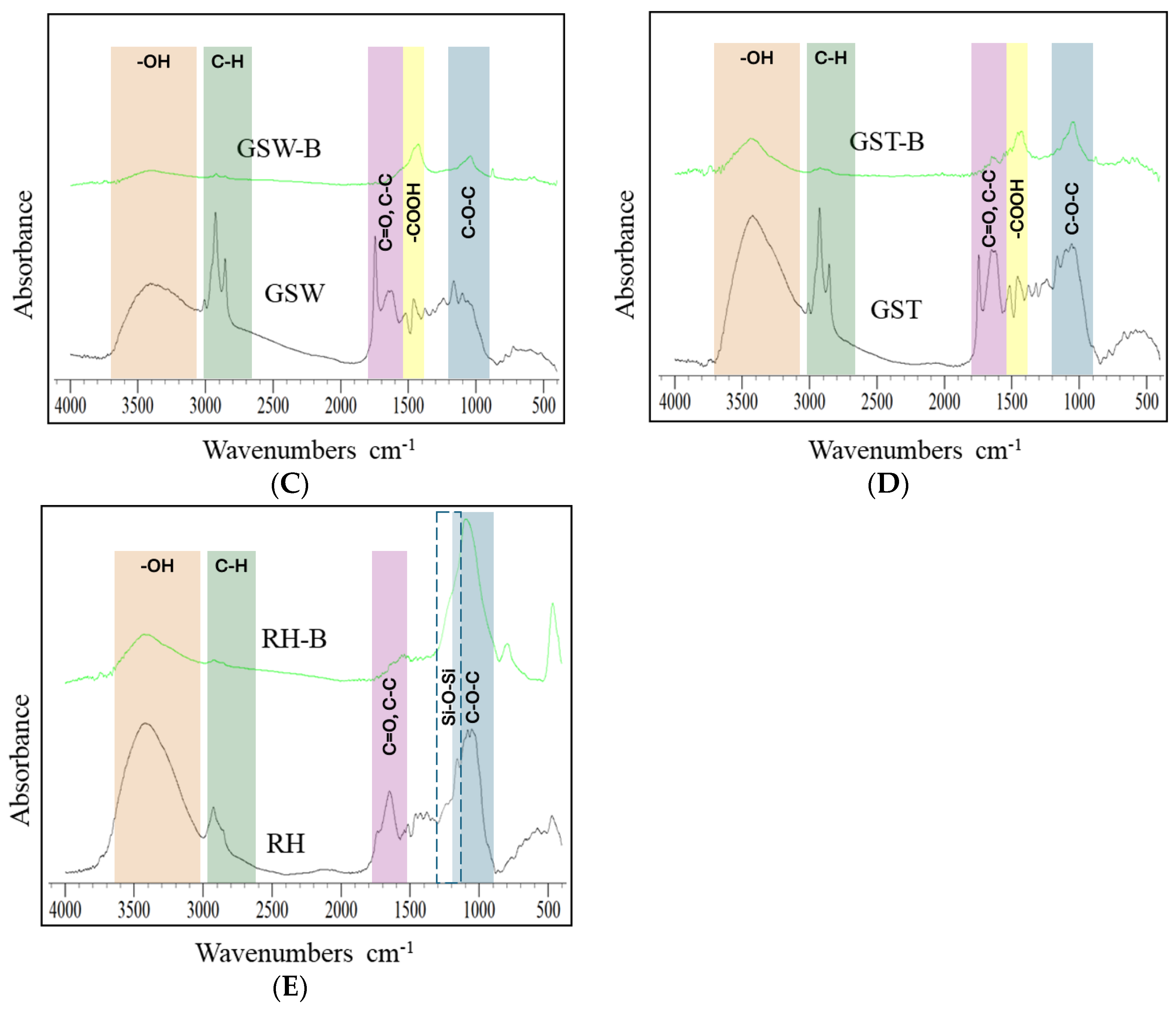
3.4. Chemical Characteristics
3.5. Morphology of the Samples
3.6. Biochar Surface Area and Porosity
3.7. Suspension pHeq
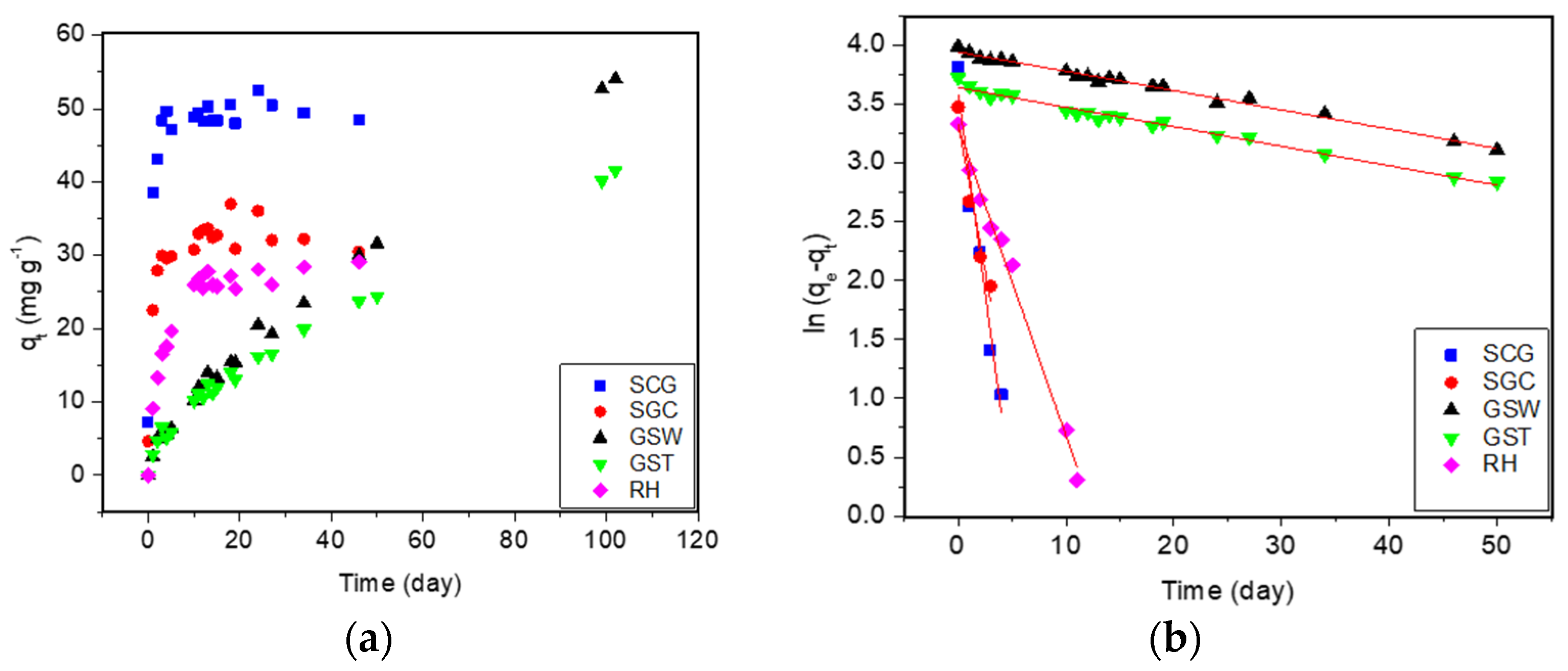
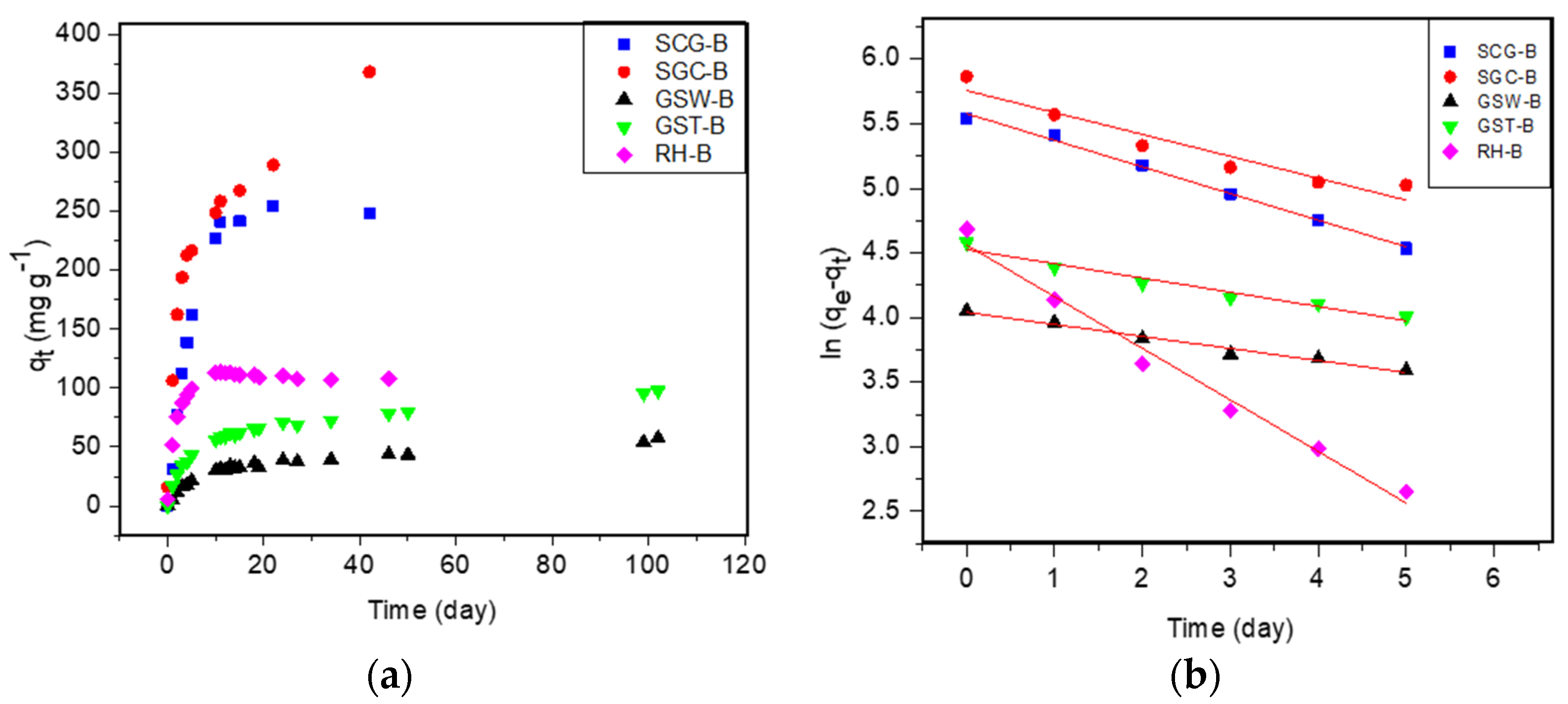
3.8. Methylene Blue Sorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2015; 976p. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Yang, B.; Li, D.; Yuan, S.; Jin, L. Role of biochar from corn straw in influencing crack propagation and evaporation in sodic soils. CATENA 2021, 204, 105457. [Google Scholar] [CrossRef]
- Manariotis, I.D.; Fotopoulou, K.N.; Karapanagioti, H.K. Preparation and characterization of biochar sorbents produced from malt spent rootlets. Ind. Eng. Chem. Res. 2015, 54, 9577–9584. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Rasa, K.; Viherä-Aarnio, A.; Rytkönen, P.; Hyväluoma, J.; Kaseva, J.; Suhonen, H.; Jyske, T. Quantitative analysis of feedstock structural properties can help to produce willow biochar with homogenous pore system. Ind. Crops Prod. 2021, 166, 113475. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Physicochemical and sorptive properties of biochars derived from woody and herbaceous biomass. Chemosphere 2015, 134, 257–262. [Google Scholar] [CrossRef]
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A review of biochar-based sorbents for separation of heavy metals from water. Int. J. Phytoremediation 2019, 22, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Zabaniotou, A.; Kamaterou, P. Food waste valorization advocating Circular Bioeconomy—A critical review of potentialities and perspectives of spent coffee grounds biorefinery. J. Clean. Prod. 2018, 211, 1553–1566. [Google Scholar] [CrossRef]
- ICO. Annual Review. 2021. Available online: https://www.ico.org/documents/cy2022-23/annual-review-2021-2022-e.pdf (accessed on 20 April 2023).
- Batista, M.J.P.A.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Polysaccharide-Rich Fraction of Spent Coffee Grounds as Promising Biomaterial for Films Fabrication. Carbohydr. Polym. 2020, 233, 115851. [Google Scholar] [CrossRef] [PubMed]
- Makri, E.; Tsimogiannis, D.; Dermesonluoglu, E.K.; Taoukisa, P.S. Modeling of Greek coffee aroma loss during storage at different temperatures and water activities. Procedia Food Sci. 2011, 1, 1111–1117. [Google Scholar] [CrossRef]
- Vieira, L.R.; Soares, A.M.V.M.; Freitas, R. Caffeine as a contaminant of concern: A review on concentrations and impacts in marine coastal systems. Chemosphere 2021, 286, 131675. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org (accessed on 20 April 2023).
- OIV. Statistical Report on World Vitiviniculture 2019. Available online: https://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 20 April 2023).
- Iuga, M.; Mironeasa, S. Potential of grape byproducts as functional ingredients in baked goods and pasta. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2473–2505. [Google Scholar] [CrossRef] [PubMed]
- Hellenic Statistical Authority (ELSTAT). Available online: https://www.statistics.gr/documents/20181/18166418/Annual+Agricultural+Statistical+Survey+%28Final+Results%29+%28+2020+%29.pdf/ba0e1942-afaf-12b2-1984-e065f6bd3d72?t=1693558783605 (accessed on 27 February 2024).
- Soufleros, E.H. Wine and Distillates; Papageorgiou Pub: Thessaloniki, Greece, 1987. [Google Scholar]
- Apostolopoulou, A.A.; Flouros, A.I.; Demertzis, P.G.; Akrida-Demertzi, K. Differences in concentration of principal volatile constituents in traditional Greek distillates. Food Controls 2005, 16, 157–164. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-Canul, M.I.; González, L.A. Review on the physicochemical treatments of rice husk for production of advanced materials. J. Chem. Eng. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Barana, D.; Salanti, A.; Orlandi, M.; Ali, D.S.; Zoia, L. Biorefinery process for the simultaneous recovery of lignin, hemicelluloses, cellulose nanocrystals and silica from rice husk and Arundo donax. Ind. Crops Prod. 2016, 86, 31–39. [Google Scholar] [CrossRef]
- Tala, W.; Chantara, S. Use of spent coffee ground biochar as ambient PAHs sorbent and novel extraction method for GC-MS analysis. Environ. Sci. Pollut. Res. 2019, 26, 13025–13040. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, P.; Ziegler, D.; Rovere, M.; Tulliani, J.M.; Tagliaferro, A. Waste Coffee Ground Biochar: A Material for Humidity Sensors. Sensors 2019, 19, 801. [Google Scholar] [CrossRef] [PubMed]
- Claoston, N.; Samsuri, A.; Husni, M.A.; Amran, M. Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag. Res. J. Sustain. Circ. Econ. 2014, 32, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzidis, S.; Karapanagioti, H.K.; Christanis, K.; Bouzinos, A.; Iliopoulou, E. Evaluation of peat and lignite phenanthrene sorption properties in relation to coal petrography: The impact of inertinite. Int. J. Coal Geol. 2006, 68, 30–38. [Google Scholar] [CrossRef]
- Karapanagioti, H.K.; Sabatini, D.A.; Kleineidam, S.; Grathwohl, P.; Ligouis, B. Phenanthrene sorption with heterogeneous organic matter in a landfill aquifer material. Phys. Chem. Earth Part B Hydrol. Oceans Atmos. 1999, 24, 535–541. [Google Scholar] [CrossRef]
- Sessa, F.; Veeyee, K.F.; Canu, P. Optimization of biochar quality and yield from tropical timber industry wastes. Waste Manag. 2021, 131, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, A.B.; Arbestain, M.C.; Sevilla, M.; Maciá-Agulló, J.A.; Fiol, S.; López, R.; Smernik, R.J.; Aitkenhead, W.P.; Arce, F.; Macías, F. Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Soil Res. 2010, 48, 618–626. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Z.; Zhang, Q.; Wang, J.; Ma, Q.; Yang, Y.; Luo, X.; Zhang, W. Comparison of the physicochemical characteristics of bio-char pyrolyzed from moso bamboo and rice husk with different pyrolysis temperatures. BioResources 2017, 12, 4652–4669. [Google Scholar] [CrossRef]
- Yargiloglou, E.N.; Sadasivam, B.Y.; Reddy, K.R.; Spokas, K. Physical and chemical characterization of waste wood derived biochars. Waste Manag. 2015, 36, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Zama, E.F.; Zhu, Y.-G.; Reid, B.J.; Sun, G.-X. The role of biochar properties in influencing the sorption and desorption of Pb(II), Cd(II) and As(III) in aqueous solution. J. Clean. Prod. 2017, 148, 127–136. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Sima, J.; Zhao, L.; Mašek, O.; Cao, X. Indispensable role of biochar-inherent mineral constituents in its environmental applications: A review. Bioresour. Technol. 2017, 241, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Dawei, L.; Xiaoxiao, Z.; Yu, W.; Peijie, Z.; Li, Z.; Zongbo, Z.; Xin, G.; Yingyun, Q.; Guixia, L.; Yuanyu, T. Adjusting ash content of char to enhance lithium storage performance of rice husk-based SiO2/C. J. Alloys Compd. 2020, 854, 156986. [Google Scholar] [CrossRef]
- Shi, S.-C.; Liu, G.-T. Cellulose nanocrystal extraction from rice straw using a chlorine-free bleaching process. Cellulose 2021, 28, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulos, I.; Kordouli, E.; Mourgkogiannis, N.; Karapanagioti, H.K.; Lycourghiotis, A.; Kordulis, C. Valorization of Pyrolyzed Biomass Residues for the Transformation of Waste Cooking Oil into Green Diesel. Catalysts 2023, 13, 1004. [Google Scholar] [CrossRef]
- Kalampaliki, D.; Jayasinghe, G.D.T.M.; Avramiotis, E.; Manariotis, I.D.; Venieri, D.; Poulopoulos, S.G.; Szpunar, J.; Vakros, J.; Mantzavinos, D. Application of a KOH-activated biochar for the activation of persulfate and the degradation of sulfamethoxazole. Chem. Eng. Res. Des. 2023, 194, 306–317. [Google Scholar] [CrossRef]
- Kyriakou, M.; Chatziiona, V.K.; Costa, C.N.; Kallis, M.; Koutsokeras, L.; Constantinides, G.; Koutinas, M. Biowaste-based biochar: A new strategy for fermentative bioethanol overproduction via whole-cell immobilization. Appl. Energy 2019, 242, 480–491. [Google Scholar] [CrossRef]
- Song, B.; Chen, M.; Zhao, L.; Qiu, H.; Cao, X. Physicochemical property and colloidal stability of micron- and nano-particle biochar derived from a variety of feedstock sources. Sci. Total. Environ. 2019, 661, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Das, O.; Sarmah, A.K.; Zujovic, Z.; Bhattacharyya, D. Characterisation of waste derived biochar added biocomposites: Chemical and thermal modifications. Sci. Total. Environ. 2016, 550, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Burachevskaya, M.; Minkina, T.; Bauer, T.; Lobzenko, I.; Fedorenko, A.; Mazarji, M.; Sushkova, S.; Mandzhieva, S.; Nazarenko, A.; Butova, V.; et al. Fabrication of biochar derived from different types of feedstocks as an efficient adsorbent for soil heavy metal removal. Sci. Rep. 2023, 13, 2020. [Google Scholar] [CrossRef] [PubMed]
- Mansaray, K.G.; Ghaly, A.E. Thermal degradation of rice husks in nitrogen atmosphere. Bioresour. Technol. 1998, 65, 13–20. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manage 2021, 278, 111501. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Anaya, N.M.; Schifman, L.A.; Oyanedel-Craver, V. Fourier transform infrared spectroscopy to assess molecular-level changes in microorganisms exposed to nanoparticles. Nanotechnol. Environ. Eng. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Usman, A.R.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Sun, L.; Xiang, J.; Yang, T.; Zhang, A.; Zhang, J. Structural evolution of maize stalk/char particles during pyrolysis. Bioresour. Technol. 2009, 100, 4877–4883. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Chen, B. Dual role of biochars as adsorbents for aluminum: The effects of oxygen-containing organic components and the scattering of silicate particles. Environ. Sci. Technol. 2013, 47, 8759–8768. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.N.; Wen, T.H.; Zakaria, Z.A. Activated carbon and biochar from pineapple waste biomass for the removal of methylene blue. Environ. Toxicol. Manag. 2021, 1, 30–36. [Google Scholar] [CrossRef]
- Sun, K.; Wang, Y.; Zhang, L.; Shao, Y.; Li, C.; Zhang, S.; Hu, X. High yield of carbonaceous material from biomass via pyrolysis-condensation. Chem. Eng. J. 2024, 485, 149823. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, P.R.; Della Rocca, P.A.; Cerrella, E.G.; Cukierman, A.L. Effect of pyrolysis temperature on composition, surface properties and thermal degradation rates of Brazil Nut shells. Bioresour. Technol. 2001, 76, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.; Miller, A.Z.; Knicker, H.; Costa-Pereira, M.F.; Merino, A.; De la Rosa, J.M. Chemical, physical and morphological properties of biochars produced from agricultural residues: Implications for their use as soil amendment. Waste Manag. 2020, 105, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Orfanos, A.G.; Manariotis, I.D.; Karapanagioti, H.K. Removal of Methylene Blue from Water by Food Industry By-products and Biochars. Desalinat. Water Treat. 2018, 103, 113–121. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]


| Samples | pHeq | Moisture Content of Raw Materials (%) | Yield of Initial Material in Biochar (%) | Yield of Dry Raw Material in Biochar (%) | Ash Content (%) * | Yield of Dry Raw Material in Carbonaceous Material Fraction in Biochar (%) |
|---|---|---|---|---|---|---|
| SCG | 5.5 | 63 | - | - | 1.7 | - |
| SCG-Β | 10.6 | - | 3.6 | 9.8 | 13 (17) | 8.6 |
| SGC | 6.4 | 11 | - | - | 1.2 | - |
| SGC-Β | 10.3 | - | 6.0 | 6.8 | 12 (18) | 6.0 |
| GSW | 5.8 | 20 | - | - | 2.6 | - |
| GSW-B | 10.2 | - | 17 | 21 | 7 (10) | 20 |
| GST | 7.0 | 18 | - | - | 2.4 | - |
| GST-B | 10.7 | - | 16 | 20 | 9 (15) | 18 |
| RH | 6.0 | 4 | - | - | 18 | - |
| RH-B | 9.4 | - | 27 | 28 | 60 (64) | 11 |
| Biochar | SSA (m2·g−1) | SSAMicro (m2·g−1) | SSAExternal (m2·g−1) | SPV (cm3·g−1) | SPVMicro (cm3·g−1) | MPD (nm) |
|---|---|---|---|---|---|---|
| SCG-Β | 751 | 514 | 237 | 0.39 | 0.24 | 3.1 |
| SGC-Β | 870 | 587 | 283 | 0.44 | 0.27 | 3.1 |
| GSW-B | 529 | 420 | 109 | 0.27 | 0.19 | 3.8 |
| GST-B | 464 | 350 | 114 | 0.24 | 0.17 | 3.8 |
| RH-B | 367 | 230 | 137 | 0.23 | 0.11 | 4.3 |
| Experimental and Theoretical Sorption Parameters | Materials | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SCG | SCG-B | SGC | SGC-B | GSW | GSW-B | GST | GST-B | RH | RH-B | |
| k1 (day−1) | 0.68 | 0.21 | 0.50 | 0.17 | 0.02 | 0.09 | 0.02 | 0.11 | 0.26 | 0.39 |
| qe (mg·g−1) | 36 | 265 | 28 | 316 | 52 | 57 | 38 | 92 | 27 | 96 |
| qe exp (mg·g−1) | 49 | 208 | 31 | 249 | 10 | 30 | 10 | 56 | 20 | 100 |
| qe exp final (mg·g−1) | 53 | 280 | 35 | 370 | 54 | 58 | 42 | 99 | 29 | 110 |
| R2 | 0.952 | 0.995 | 0.914 | 0.905 | 0.989 | 0.976 | 0.982 | 0.952 | 0.990 | 0.980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourgkogiannis, N.; Nikolopoulos, I.; Kordouli, E.; Lycourghiotis, A.; Kordulis, C.; Karapanagioti, H.K. The Influence of Biowaste Type on the Physicochemical and Sorptive Characteristics of Corresponding Biochar Used as Sustainable Sorbent. Sustainability 2024, 16, 2889. https://doi.org/10.3390/su16072889
Mourgkogiannis N, Nikolopoulos I, Kordouli E, Lycourghiotis A, Kordulis C, Karapanagioti HK. The Influence of Biowaste Type on the Physicochemical and Sorptive Characteristics of Corresponding Biochar Used as Sustainable Sorbent. Sustainability. 2024; 16(7):2889. https://doi.org/10.3390/su16072889
Chicago/Turabian StyleMourgkogiannis, Nikolaos, Ioannis Nikolopoulos, Eleana Kordouli, Alexis Lycourghiotis, Christos Kordulis, and Hrissi K. Karapanagioti. 2024. "The Influence of Biowaste Type on the Physicochemical and Sorptive Characteristics of Corresponding Biochar Used as Sustainable Sorbent" Sustainability 16, no. 7: 2889. https://doi.org/10.3390/su16072889






