The Catalytic Effect of Pt on Lignin Pyrolysis: A Reactive Molecular Dynamics Study
Abstract
:1. Introduction
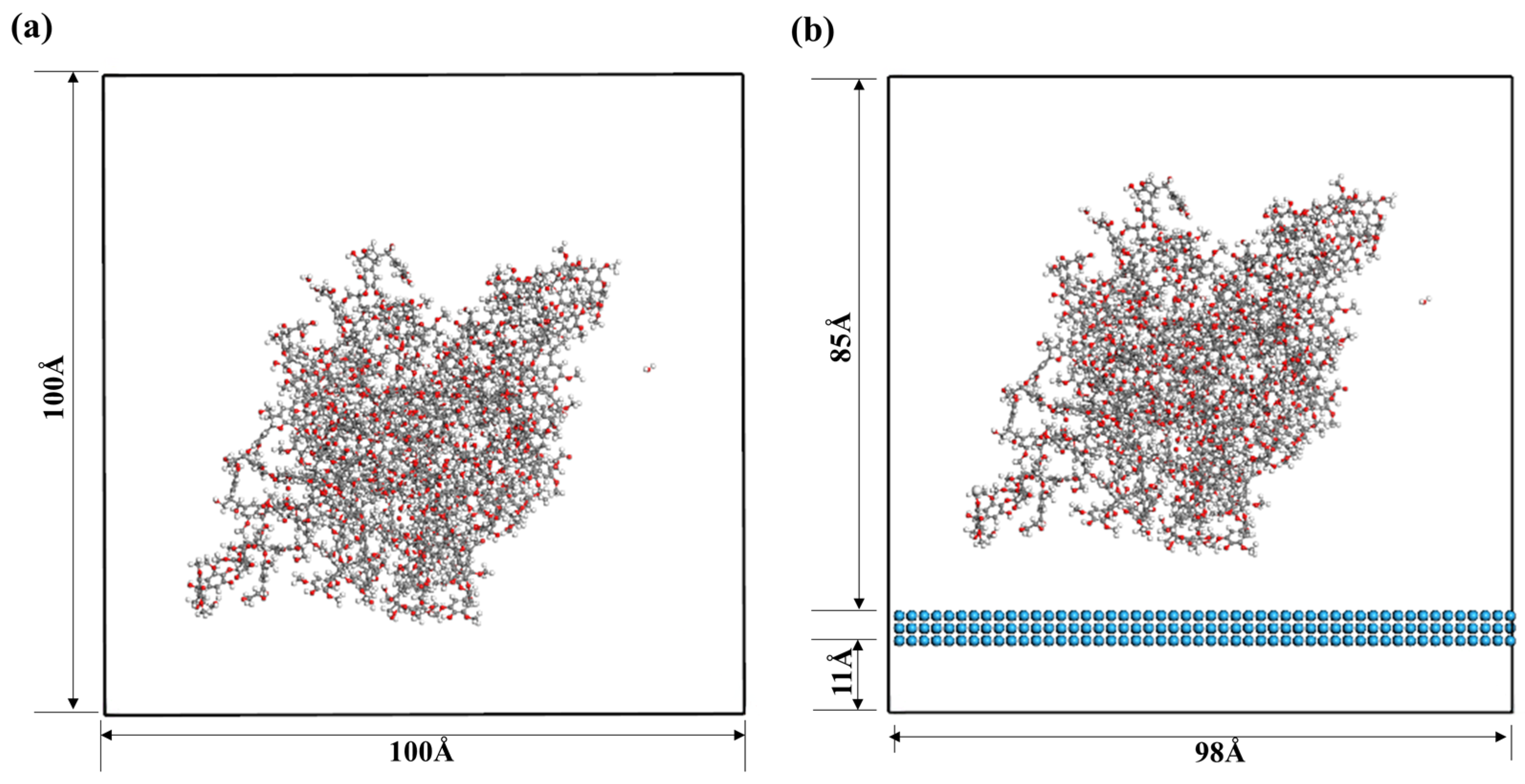
2. Materials and Methods
3. Results
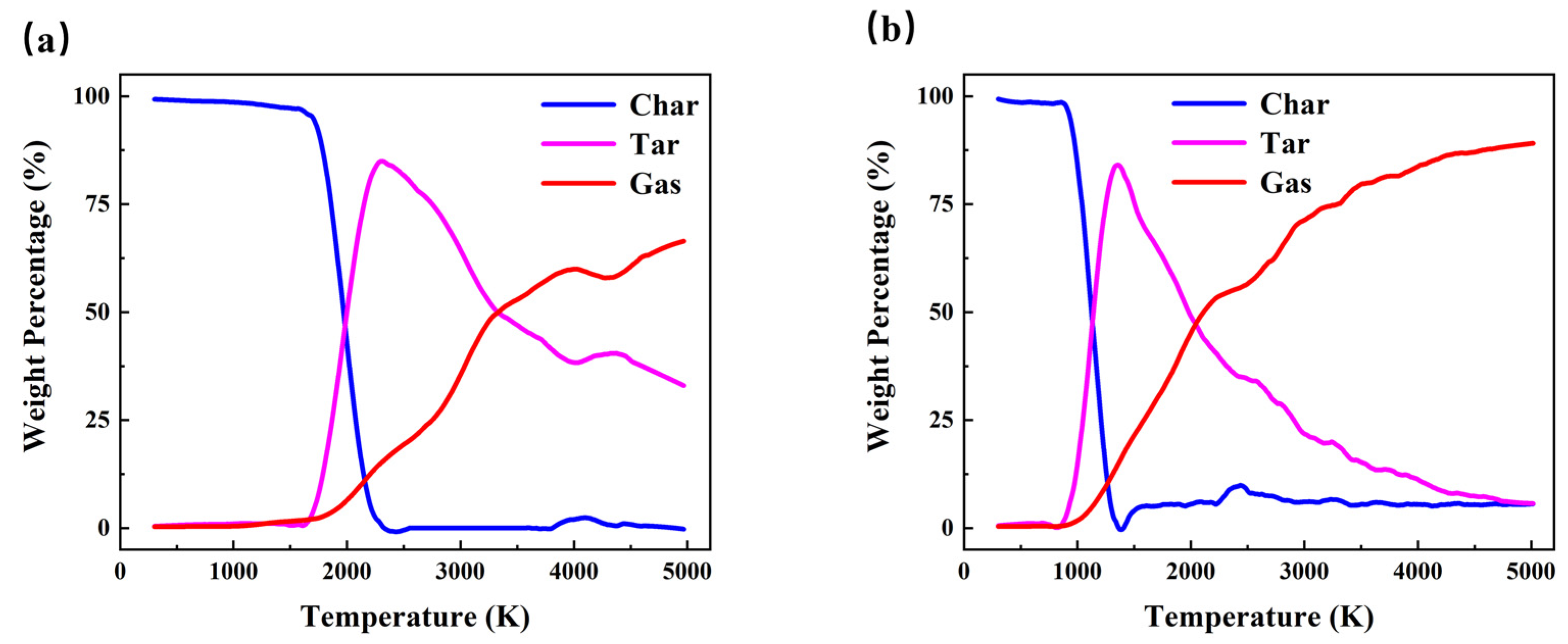
3.1. Evolution of Pyrolysis Products
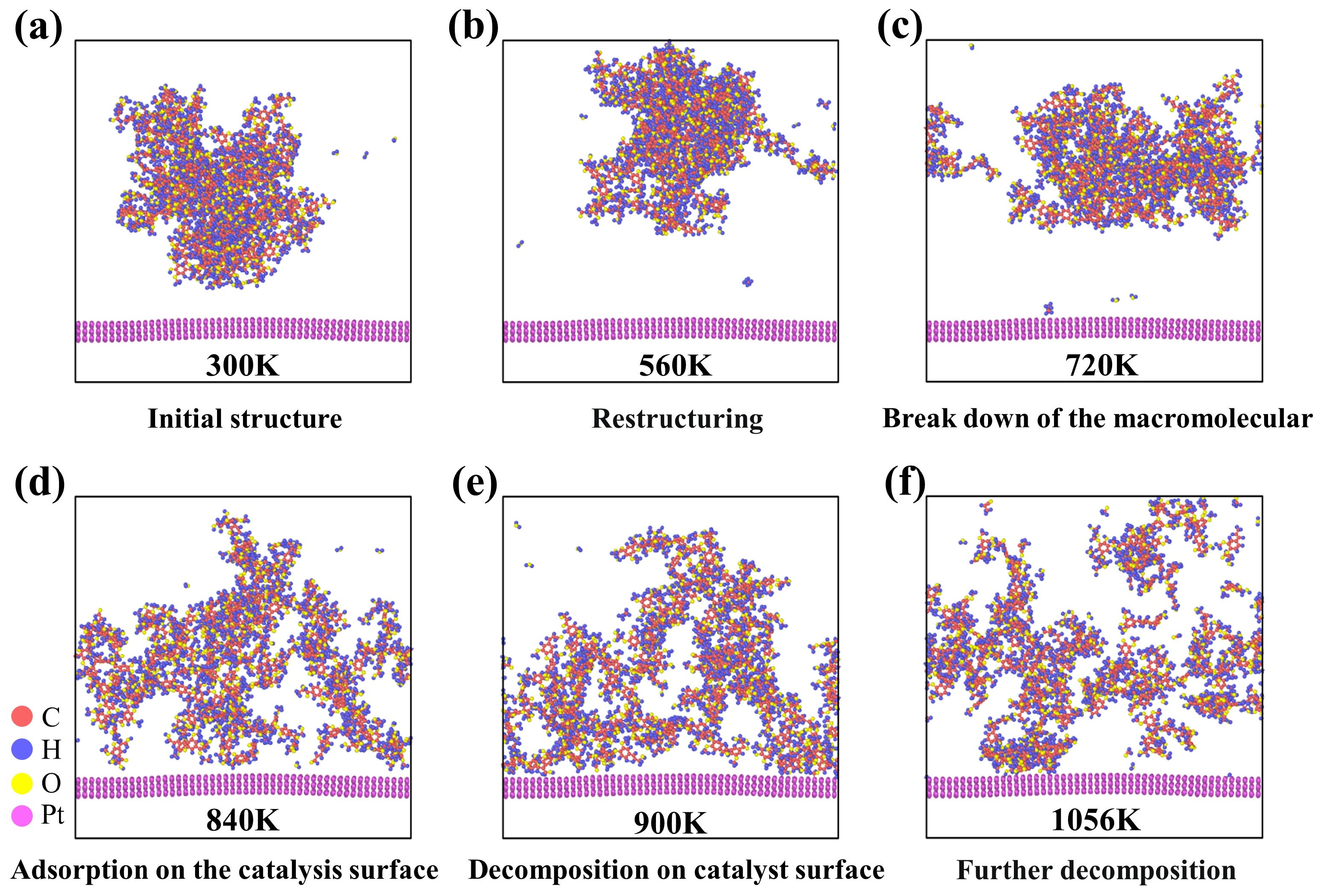
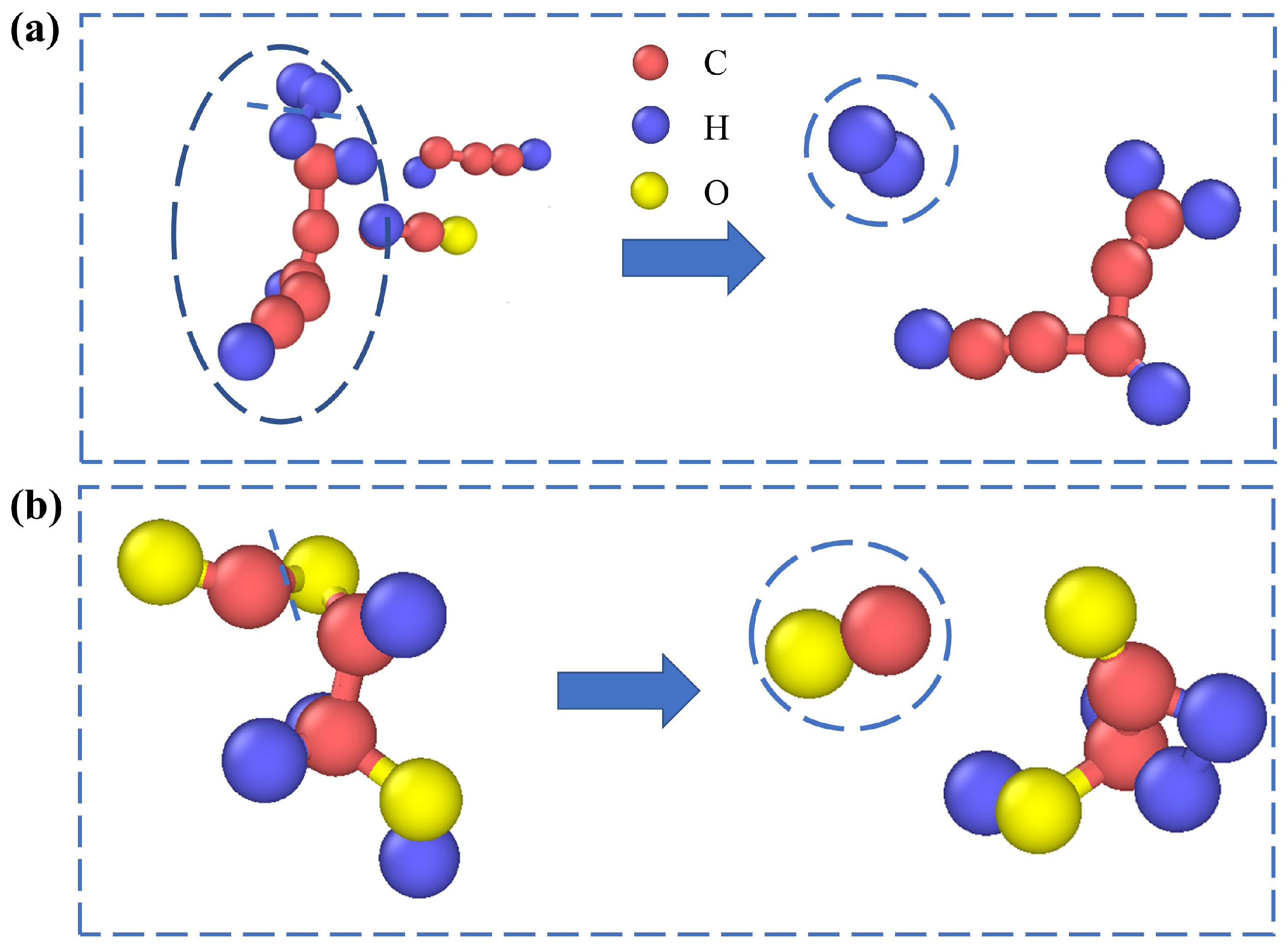
3.2. Structural Evolution of Lignin Molecules
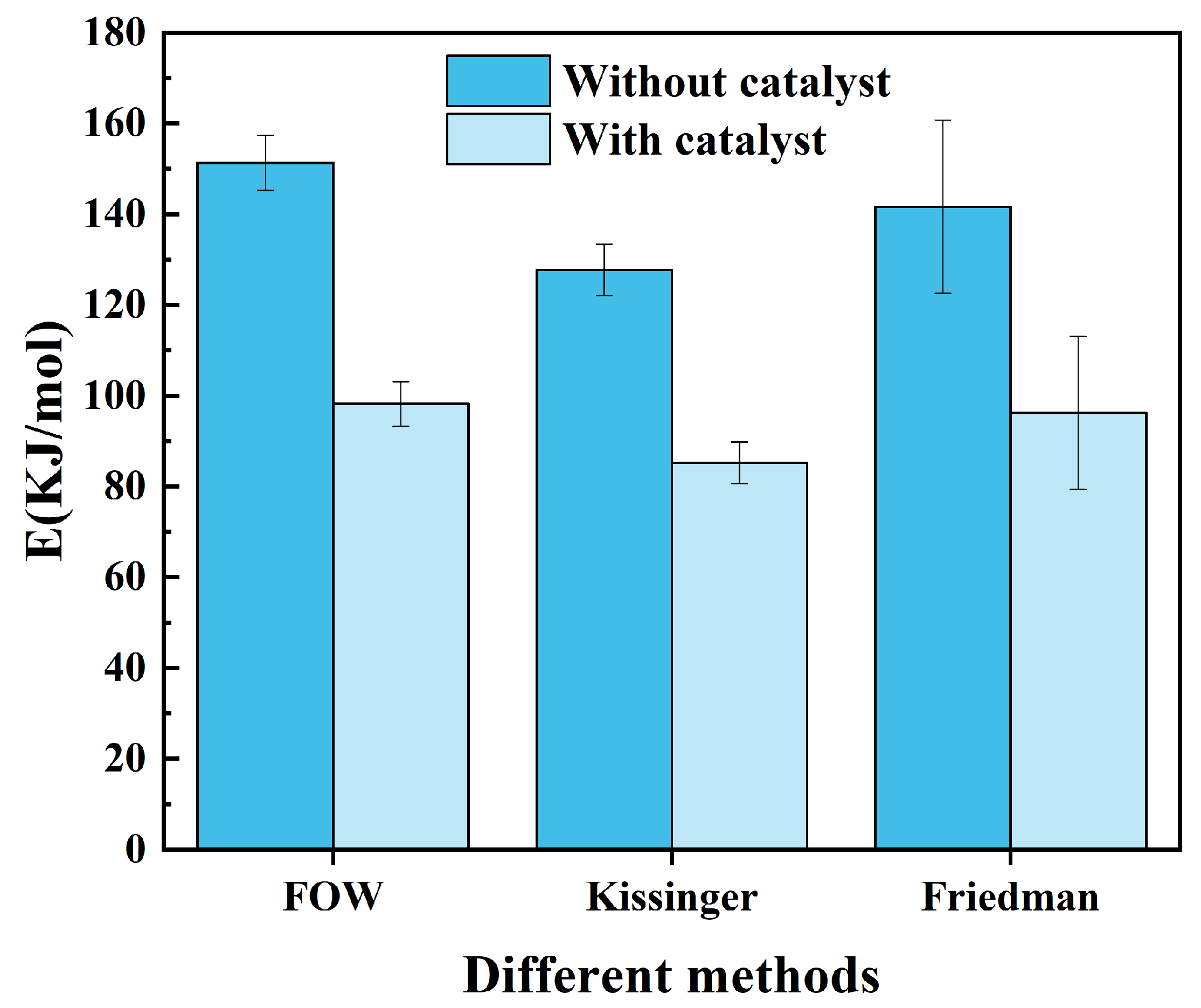
3.3. Kinetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huo, E.; Duan, D.; Lei, H.; Liu, C.; Zhang, Y.; Wu, J.; Zhao, Y.; Huang, Z.; Qian, M.; Zhang, Q.; et al. Phenols production form Douglas fir catalytic pyrolysis with MgO and biomass-derived activated carbon catalysts. Energy 2020, 199, 117459. [Google Scholar] [CrossRef]
- Kim, S.K.; Han, J.Y.; Lee, H.-s.; Yum, T.; Kim, Y.; Kim, J. Production of renewable diesel via catalytic deoxygenation of natural triglycerides: Comprehensive understanding of reaction intermediates and hydrocarbons. Appl. Energy 2014, 116, 199–205. [Google Scholar] [CrossRef]
- Atsonios, K.; Kougioumtzis, M.-A.; Panopoulos, K.D.; Kakaras, E. Alternative thermochemical routes for aviation biofuels via alcohols synthesis: Process modeling, techno-economic assessment and comparison. Appl. Energy 2015, 138, 346–366. [Google Scholar] [CrossRef]
- González-García, S.; Bacenetti, J. Exploring the production of bio-energy from wood biomass. Italian case study. Sci. Total Environ. 2019, 647, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Moset, V.; Hille, S.; Rubæk, G.H.; Møller, H.B.; Wahid, R.; Baattrup-Pedersen, A. Indicators of biomass and methane yields in vegetated buffer strips. J. Clean. Prod. 2019, 210, 907–915. [Google Scholar] [CrossRef]
- Nazimudheen, G.; Sekhar, N.C.; Sunny, A.; Kallingal, A.; Hasanath, B. Physiochemical characterization and thermal kinetics of lignin recovered from sustainable agrowaste for bioenergy applications. Int. J. Hydrog. Energy 2021, 46, 4798–4807. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Patel, M.; Zhang, X.; Kumar, A. Techno-economic and life cycle assessment on lignocellulosic biomass thermochemical conversion technologies: A review. Renew. Sustain. Energy Rev. 2016, 53, 1486–1499. [Google Scholar] [CrossRef]
- Tungal, R.; Shende, R.V. Hydrothermal liquefaction of pinewood (Pinus ponderosa) for H2, biocrude and bio-oil generation. Appl. Energy 2014, 134, 401–412. [Google Scholar] [CrossRef]
- Srirangan, K.; Akawi, L.; Moo-Young, M.; Chou, C.P. Towards sustainable production of clean energy carriers from biomass resources. Appl. Energy 2012, 100, 172–186. [Google Scholar] [CrossRef]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Fatehi, H.; Weng, W.; Li, Z.; Bai, X.S.; Aldén, M.J.E. Recent Development in Numerical Simulations and Experimental Studies of Biomass Thermochemical Conversion. Energy Fuels 2021, 35, 6940–6963. [Google Scholar] [CrossRef]
- Seo, M.W.; Lee, S.H.; Nam, H.; Lee, D.; Tokmurzin, D.; Wang, S.; Park, Y.-K. Recent advances of thermochemical conversion processes for biorefinery. Bioresour. Technol. 2022, 343, 126109. [Google Scholar] [CrossRef]
- Yang, M.-H.; Yeh, R.-H. The effects of composition ratios and pressure drops of R245fa/R236fa mixtures on the performance of an organic Rankine cycle system for waste heat recovery. Energy Convers. Manag. 2018, 175, 313–326. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, L.; Peng, Y.; Yang, Q.; Du, Z.; Dai, L.; Zhou, N.; Liu, Y.; Fu, G.; Ruan, R.; et al. Characteristics of the catalytic fast pyrolysis of vegetable oil soapstock for hydrocarbon-rich fuel. Energy Convers. Manag. 2020, 213, 112860. [Google Scholar] [CrossRef]
- Gosselink, R.J.A. Lignin as a Renewable Aromatic Resource for the Chemical Industry; Wageningen University and Research: Wageningen, The Netherlands, 2011. [Google Scholar]
- Li, Z.; Zhong, Z.; Yang, Q.; Ben, H.; Seufitelli, G.V.S.; Resende, F.L.P. Parametric study of catalytic hydropyrolysis of rice husk over a hierarchical micro-mesoporous composite catalyst for production of light alkanes, alkenes, and liquid aromatic hydrocarbons. Fuel 2022, 310, 122457. [Google Scholar] [CrossRef]
- Al-asadi, M.; Miskolczi, N.; Eller, Z. Pyrolysis-gasification of wastes plastics for syngas production using metal modified zeolite catalysts under different ratio of nitrogen/oxygen. J. Clean. Prod. 2020, 271, 122186. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, B.; Liu, S.; Zhu, D.; Huang, F.; Yang, H.; Guan, H.; Song, A.; Xu, D.; Sun, L.; et al. Catalytic pyrolysis of biomass with Ni/Fe-CaO-based catalysts for hydrogen-rich gas: DFT and experimental study. Energy Convers. Manag. 2022, 254, 115246. [Google Scholar] [CrossRef]
- van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Castro-Marcano, F.; van Duin, A.C.T. Comparison of thermal and catalytic cracking of 1-heptene from ReaxFF reactive molecular dynamics simulations. Combust. Flame 2013, 160, 766–775. [Google Scholar] [CrossRef]
- Gao, M.; Li, X.; Guo, L. Pyrolysis simulations of Fugu coal by large-scale ReaxFF molecular dynamics. Fuel Process. Technol. 2018, 178, 197–205. [Google Scholar] [CrossRef]
- Kwon, H.; Shabnam, S.; van Duin, A.C.T.; Xuan, Y. Numerical simulations of yield-based sooting tendencies of aromatic fuels using ReaxFF molecular dynamics. Fuel 2020, 262, 116545. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Qiao, X.; Zheng, M.; Guo, L.; Song, W.-l.; Lin, W.J.E. Initial Mechanisms for an Overall Behavior of Lignin Pyrolysis through Large-Scale ReaxFF Molecular Dynamics Simulations. Fuels 2016, 30, 3140–3150. [Google Scholar] [CrossRef]
- Li, H.; Xu, B.; Jin, H.; Luo, K.; Fan, J. Molecular dynamics investigation on the lignin gasification in supercritical water. Fuel Process. Technol. 2019, 192, 203–209. [Google Scholar] [CrossRef]
- Han, Y.; Chen, F.; Ma, T.; Gong, H.; Al-Shwafy, K.W.A.; Li, W.; Zhang, J.; Zhang, M.J.I.; Research, E.C. Size Effect of a Ni Nanocatalyst on Supercritical Water Gasification of Lignin by Reactive Molecular Dynamics Simulations. Ind. Eng. Chem. Res. 2019, 58, 23014–23024. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Shin, Y.K.; Gai, L.; Raman, S.; van Duin, A.C. Development of a ReaxFF Reactive Force Field for the Pt-Ni Alloy Catalyst. J. Phys. Chem. A 2016, 120, 8044–8055. [Google Scholar] [CrossRef]
- Nimz, H. Beech Lignin—Proposal of a Constitutional Scheme. Angew. Chem. Int. Ed. Engl. 1974, 13, 313–321. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Guo, L.; Guo, X.J.E. Reaction Mechanisms in Pyrolysis of Hardwood, Softwood, and Kraft Lignin Revealed by ReaxFF MD Simulations. Fuels 2019, 33, 11210–11225. [Google Scholar] [CrossRef]
- Li, K.; Khanna, R.; Zhang, H.; Conejo, A.; Ma, S.; Liang, Z.; Li, G.; Barati, M.; Zhang, J. Thermal behaviour, kinetics and mechanisms of CO2 interactions with graphene: An atomic scale reactive molecular dynamic study. Chem. Eng. J. 2021, 425, 131529. [Google Scholar] [CrossRef]
- Li, K.; Khanna, R.; Zhang, H.; Ma, S.; Liang, Z.; Li, G.; Barati, M.; Zhang, J. Thermal behaviour during initial stages of graphene oxidation: Implications for reaction kinetics and mechanisms. Chem. Eng. J. 2021, 421, 129742. [Google Scholar] [CrossRef]
- Liang, Z.; Khanna, R.; Li, K.; Guo, F.; Ma, Y.; Zhang, H.; Bu, Y.; Bi, Z.; Zhang, J. Impact of oxidants O2, H2O, and CO2 on graphene oxidation: A critical comparison of reaction kinetics and gasification behavior. Chem. Eng. J. 2022, 450, 138045. [Google Scholar] [CrossRef]
- Sørensen, M.R.; Voter, A.F. Temperature-accelerated dynamics for simulation of infrequent events. J. Chem. Phys. 2000, 112, 9599–9606. [Google Scholar] [CrossRef]
- Shang, Z.; Li, H. Unraveling pyrolysis mechanisms of lignin dimer model compounds: Neural network-based molecular dynamics simulation investigations. Fuel 2024, 357, 129909. [Google Scholar] [CrossRef]
- Yu, J.; Dang, Q.; Wu, T.; Wu, Y.; Lei, T.; Qi, F. Catalytic hydropyrolysis of lignin: Insights into the effect of Ni catalyst and hydrogen using ReaxFF molecular dynamics simulation. J. Anal. Appl. Pyrolysis 2023, 175, 106212. [Google Scholar] [CrossRef]
- Jiang, C.; Liang, W.; Li, K.; Barati, M.; Conejo, A.; Guo, P.; Danaei, A.; Liang, Z.; Bu, Y.; Zhang, J. A reactive molecular dynamics study of thermal pyrolysis behavior and mechanisms of lignin during the hydrothermal process: The function of the water molecules. Bioresour. Technol. 2023, 368, 128338. [Google Scholar] [CrossRef]
- Rismiller, S.C.; Groves, M.M.; Meng, M.; Dong, Y.; Lin, J. Water assisted liquefaction of lignocellulose biomass by ReaxFF based molecular dynamic simulations. Fuel 2018, 215, 835–843. [Google Scholar] [CrossRef]
- Lele, A.; Kwon, H.; Ganeshan, K.; Xuan, Y.; van Duin, A.C.T. ReaxFF molecular dynamics study on pyrolysis of bicyclic compounds for aviation fuel. Fuel 2021, 297, 120724. [Google Scholar] [CrossRef]
- Castro-Marcano, F.; Russo, M.F.; van Duin, A.C.T.; Mathews, J.P. Pyrolysis of a large-scale molecular model for Illinois no. 6 coal using the ReaxFF reactive force field. J. Anal. Appl. Pyrolysis 2014, 109, 79–89. [Google Scholar] [CrossRef]
- Blasi, C.D.; Signorelli, G.; Russo, A.; Rea, G.J.I.; Research, E.C. Product Distribution from Pyrolysis of Wood and Agricultural Residues. Ind. Eng. Chem. Res. 1999, 38, 2216–2224. [Google Scholar] [CrossRef]
- Fletcher, T.H.; Kerstein, A.R.; Pugmire, R.J.; Solum, M.S.; Grant, D.M.J.E. Chemical percolation model for devolatilization. 3. Direct use of carbon-13 NMR data to predict effects of coal type. Fuels 1992, 6, 414–431. [Google Scholar] [CrossRef]
- Song, Z.; Bai, M.; Yang, Z.; Lei, H.; Qian, M.; Zhao, Y.; Zou, R.; Wang, C.; Huo, E. Gasification of α-O-4 linkage lignin dimer in supercritical water into hydrogen and carbon monoxide: Reactive molecular dynamic simulation study. Fuel 2022, 329, 125387. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Liu, Z.; Ku, X.; Jin, H. Pyrolysis Mechanism of Wheat Straw Based on ReaxFF Molecular Dynamics Simulations. ACS Omega 2022, 7, 21075–21085. [Google Scholar] [CrossRef] [PubMed]
- Oyedun, A.O.; Tee, C.Z.; Hanson, S.; Hui, C.W. Thermogravimetric analysis of the pyrolysis characteristics and kinetics of plastics and biomass blends. Fuel Process. Technol. 2014, 128, 471–481. [Google Scholar] [CrossRef]
- Sørum, L.; Grønli, M.G.; Hustad, J.E. Pyrolysis characteristics and kinetics of municipal solid wastes. Fuel 2001, 80, 1217–1227. [Google Scholar] [CrossRef]
- Woo Park, J.; Cheon Oh, S.; Pyeong Lee, H.; Taik Kim, H.; Ok Yoo, K. A kinetic analysis of thermal degradation of polymers using a dynamic method. Polym. Degrad. Stab. 2000, 67, 535–540. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General Treatment of the Thermogravimetry of Polymers. Sect. A Phys. Chem. 1966, 70A, 487–523. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Method of determining activation deterioration constant of electrical insulating materials. Res. Rep. Chiba Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. J. Polym. Sci. Part C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, W.; Li, K.; Khanna, R.; Konyukhov, Y.; Liang, Z.; Bu, Y.; Sun, Z.; Jiang, C.; Zhang, J. The Catalytic Effect of Pt on Lignin Pyrolysis: A Reactive Molecular Dynamics Study. Sustainability 2024, 16, 3419. https://doi.org/10.3390/su16083419
Zhan W, Li K, Khanna R, Konyukhov Y, Liang Z, Bu Y, Sun Z, Jiang C, Zhang J. The Catalytic Effect of Pt on Lignin Pyrolysis: A Reactive Molecular Dynamics Study. Sustainability. 2024; 16(8):3419. https://doi.org/10.3390/su16083419
Chicago/Turabian StyleZhan, Weiming, Kejiang Li, Rita Khanna, Yuri Konyukhov, Zeng Liang, Yushan Bu, Zhen Sun, Chunhe Jiang, and Jianliang Zhang. 2024. "The Catalytic Effect of Pt on Lignin Pyrolysis: A Reactive Molecular Dynamics Study" Sustainability 16, no. 8: 3419. https://doi.org/10.3390/su16083419




