Preliminary Tests of Tomato Plant Protection Method with Ozone Gas Fumigation Supported with Hydrogen Peroxide Solution and Its Effect on Some Fruit Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Tomato Growing Conditions
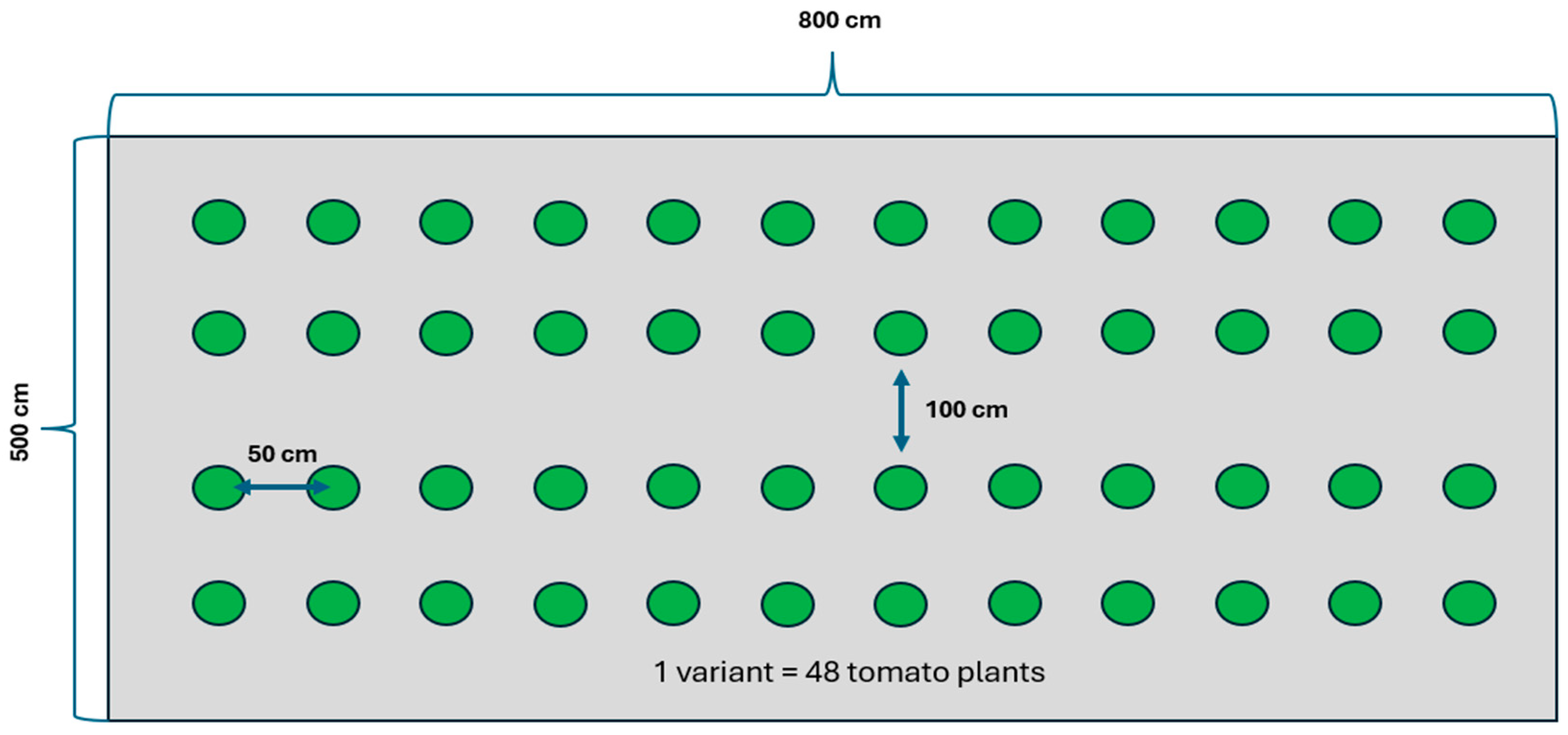
2.1.1. Plant Material in a Field Experiment under Cover
- -
- Control sample—no variable factors were used (control);
- -
- Variant of plants sprayed with hydrogen peroxide—only hydrogen peroxide sprays were used (W1—1% H2O2; W2—3% H2O2);
- -
- Variant of ozonated plants—fumigation with ozone gas was carried out (W3—2 ppm 1 min; W4—2 ppm 1.5 min; W5—2 ppm 3 min);
- -
- Variant of plants ozonated and sprayed with hydrogen peroxide—fumigation with ozone gas and hydrogen peroxide spraying were carried out (W6—2 ppm 1 min and 1% H2O2; W7—2 ppm 1.5 min and 1% H2O2; W8—2 ppm 3 min and 1% H2O2; W9—2 ppm 1 min and 3% H2O2; W10—2 ppm 1.5 min and 3% H2O2; W11—2 ppm 3 min and 3% H2O2). See Supplementary Materials: S4.
2.1.2. Tomato Growing Conditions
2.2. Experimental Treatments Used during Plant Vegetation
2.2.1. Ozonation of Tomatoes Plant
2.2.2. Spraying with Hydrogen Peroxide (H2O2)
2.3. Measurement of Mechanical Properties
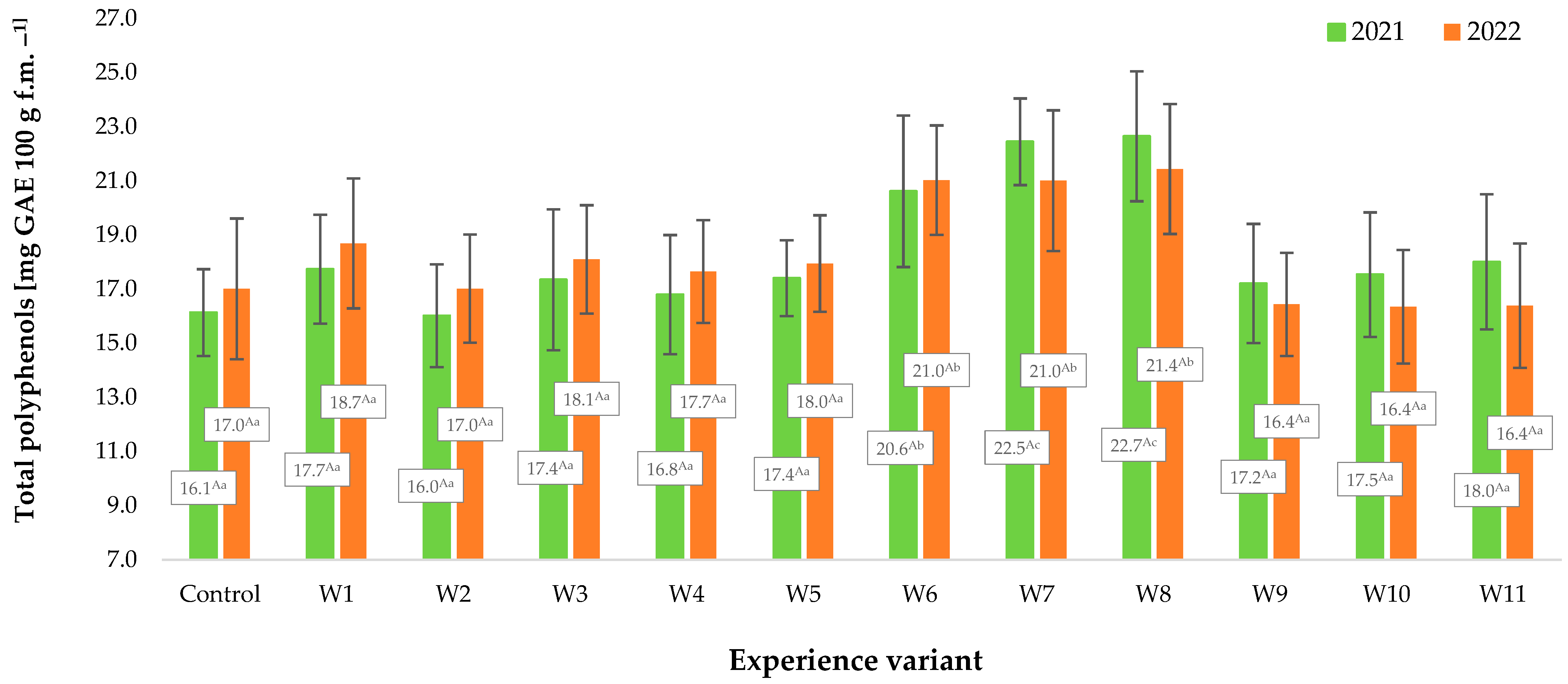
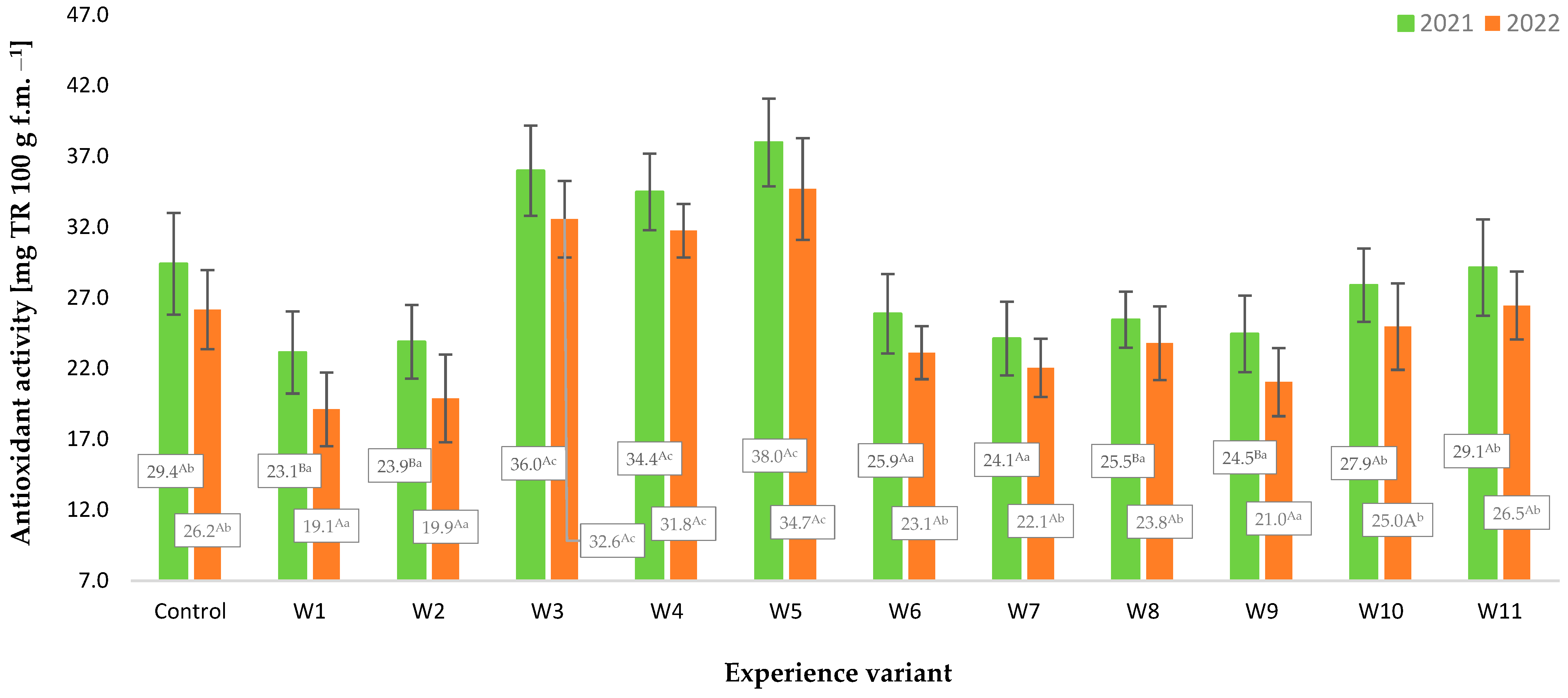
2.4. Antioxidant Potential, Contents of Polyphenols, and Vitamin C
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Crops and Livestock Products in 2020. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 16 March 2024).
- Giuffrè, A.M.; Capocasale, M. Policosanol in Tomato (Solanum lycopersicum L.) Seed oil: The effect of cultivar. J. Oleo Sci. 2015, 64, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M.; Capocasale, M. Sterol composition of tomato (Solanum lycopersicum L.) seed oil: The effect of cultivar. Int. Food Res. J. 2016, 23, 116–122. [Google Scholar]
- Akubor, P.I.; Owuse, A.U. Chemical Composition, functional and biscuit making properties of tomato peel flour. S. Asian J. Food Technol. Environ. 2020, 6, 874–884. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2020, 343, 128396. [Google Scholar] [CrossRef]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-apoptotic effects of carotenoids in neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- Frankel, E.N.; Finley, J.W. How to standardize the multiplicity of methods to evaluate natural antioxidants. J. Agric. Food Chem. 2008, 56, 4901–4908. [Google Scholar] [CrossRef] [PubMed]
- Orlikowski, L.; Sas-Paszt, L.; Wojdyła, A.; Orlikowska, T. The Use of Hydrogen Peroxide and Silver Nanoparticles in Horticulture. J. Hortic. Res. 2023, 31, 1–22. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Özen, T.; Koyuncu, M.A.; Erbaş, D. Effect of ozone treatments on the removal of pesticide residues and postharvest quality in green pepper. J. Food Sci. Technol. 2021, 58, 2186–2196. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biological action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen peroxide: A key chemical for to-day’s sustainable development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G. The use of hydrogen peroxide for dis-infection and sterilization applications. In Patai’s Chemistry of Functional Groups; Rappoport, Z., Ed.; Wiley: Hoboken, NJ, USA, 2014; Volume 34. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.-J.; Zhang, B.; Shi, W.-W.; Li, H.-Y. Hydrogen peroxide in plants: A versatile mole-cule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Skrobacz, K.; Bobrecka-Jamro, D.; Balawejder, M. Response of Potato (Solanum Tuberosum L.) Plants to Spraying by Hydrogen Peroxide. Sustainability 2020, 12, 2469. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Lea, P.J. The role of peroxisomes in the integration of metabolism and evolutionary diversity of photosynthetic organisms. Phytochemistry 2002, 60, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Sandalio, L.M.; Romero-Puertas, M.C. Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Ann. Bot. 2015, 116, 475–485. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Heyno, E.; Klose, C.; Krieger-Liszkay, A. Origin of cadmium-induced reactive oxygen species production: Mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol. 2008, 179, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Karaca, H.; Velioğlu, Y.S. Effects of ozone treatments on microbial quality and some chemical properties of lettuce, spinach, and parsley. Postharvest Biol. Technol. 2014, 88, 46–53. [Google Scholar] [CrossRef]
- Whangchai, K.; Saengnil, K.; Uthaibutra, K. Effect of ozone in combination with some organic acids on the control of postharvest decay and pericarp browning of longan fruit. Crop Prot. 2006, 25, 821–825. [Google Scholar] [CrossRef]
- Kuşçu, A.; Pazır, F. Ozone application in food industry. J. Food 2004, 29, 123–129. [Google Scholar]
- Luo, A.; Bai, J.; Li, R.; Fang, Y.; Li, L.; Wang, D.; Zhang, L.; Liang, J.; Huang, T.; Kou, L. Effects of ozone treatment on the quality of kiwifruit during postharvest storage affected by Botrytis cinerea and Penicillium expansum. J. Phytopathol. 2019, 167, 470–478. [Google Scholar] [CrossRef]
- Bolel, H.; Koyuncu, M.A.; Erbaş, D. The effects of ozone and fungicide treatments on the fruit quality changes of pomegranate during cold storage. JIST 2019, 9, 1841–1850. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Z.; Yu, Z.; Gao, X. Preservation fresh-cut celery by treatment of ozonated water. Food Control 2005, 16, 279–283. [Google Scholar] [CrossRef]
- Wu, J.G.; Luan, T.G.; Lan, C.Y.; Lo, T.W.H.; Chan, G.Y.S. Removal of residual pesticides on vegetable using ozonated water. Food Cont. 2007, 18, 466–472. [Google Scholar] [CrossRef]
- Kuşvuran, E.; Yıldırım, D.; Mavruk, F.; Ceyhan, M. Removal of chloropyrifos ethyl, tetradifon and chlorothalonil pesticide residues from citrus by using ozone. J. Hazard. Mater. 2012, 241–242, 287–300. [Google Scholar] [CrossRef]
- Guzzon, R.; Nardin, T.; Micheletti, O.; Nicolini, G.; Larcher, R. Antimicrobial activity of ozone. Effectiveness against the main wine spoilage microorganisms and evaluation of impact on simple phenols in wine. Aust. J. Grape Wine Res. 2013, 19, 180–188. [Google Scholar] [CrossRef]
- Río Segade, S.; Vilanova, M.; Pollon, M.; Giacosa, S.; Torchio, F.; Rolle, L. Grape VOCs response to postharvest short-term ozone treatments. Front. Plant Sci. 2018, 9, 1826. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Tahir, I.I.; Olsson, M.E. Effect of ozone application on bioactive compounds of apple fruit during short-term cold storage. Sci. Hortic. 2019, 253, 49–60. [Google Scholar] [CrossRef]
- Singh, A.A.; Fatima, A.; Mishra, A.K.; Chaudhary, N.; Mukherjee, A.; Agrawal, M.; Agrawal, S.B. Assessment of ozone toxicity among 14 Indian wheat cultivars under field conditions: Growth and productivity. Environ. Monit. Assess. 2018, 190, 190. [Google Scholar] [CrossRef]
- Booker, F.; Muntifering, R.; McGrath, M.; Burkey, K.; Decoteau, D.; Fiscus, E.; Manning, W.; Krupa, S.; Chappelka, A.; Grantz, D. The ozone component of global change: Potential effects on agricultural and horticultural plant yield, product quality and interactions with invasive species. J. Integr. Plant Biol. 2009, 51, 337–351. [Google Scholar] [CrossRef]
- Kangasjärvi, J.; Jaspers, P.; Kollist, H. Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 2005, 28, 1021–1036. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Morris, K. Senescence and cell death in Brassica napus and Arabidopsis. Program. Cell Death Anim. Plants 2000, 52, 163–174. [Google Scholar]
- Pell, E.J.; Schlagnhaufer, C.D.; Arteca, R.N. Ozone-induced oxidative stress: Mechanisms of action and reaction. Physiol. Plant. 1997, 100, 264–273. [Google Scholar] [CrossRef]
- Han, Y.J.; Gharibeshghi, A.; Mewis, I.; Förster, N.; Beck, W.; Ulrichs, C. Plant responses to ozone: Effects of different ozone exposure durations on plant growth and biochemical quality of Brassica campestris L. ssp. chinensis. Sci. Hortic. 2020, 262, 108921. [Google Scholar] [CrossRef]
- Pääkkönen, E.; Günthardt-Goerg, M.; Holopainen, T. Responses of Leaf Processes in a Sensitive Birch (Betula pendula Roth.) Clone to Ozone Combined with Drought. Ann. Bot. 1998, 82, 49–59. [Google Scholar] [CrossRef]
- Schenone, G.; Botteschi, G.; Fumagalli, I.; Montinaro, F. Effects of ambient air pollution in open-top chambers on bean (Phaseolus vulgaris L.) I. Effects on growth and yield. New Phytol. 1992, 122, 689–697. [Google Scholar] [CrossRef]
- Shahrtash, F. Photochemical reactions and surface ozone measurements in Tehran city center. Renew. Energy Environ. Sustain. 2022, 7, 22. [Google Scholar] [CrossRef]
- Available online: https://open.alberta.ca/dataset/5a1c43f9-ad11-4dcc-889b-48ffbfcbc6ba/resource/80369b84-eb10-47f2-8f72-e05d2127df80/download/aenv-ozone-protection-in-plants-the-potential-use-of-chemical-protectants-to-measure-6718.pdf (accessed on 9 April 2024).
- Matłok, N.; Piechowiak, T.; Zardzewiały, M.; Gorzelany, J.; Balawejder, M. Effects of Ozone Treatment on Microbial Status and the Contents of Selected Bioactive Compounds in Origanum majorana L. Plants. Plants 2020, 9, 1637. [Google Scholar] [CrossRef] [PubMed]
- Panich, S.; Amatatongchai, M. A non-toxic approach to assess total antioxidant capacity (TAC) of exotic tropical fruits from Thailand. J. Food Sci. Technol. 2019, 56, 3547–3552. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Shang, M.; Lv, H.; Liang, B.; Li, J.; Zhou, W. Hydrogen peroxide regulates the biosynthesis of phenolic compounds and antioxidant quality enhancement in lettuce under low nitrogen condition. Food Chem. X 2022, 16, 100481. [Google Scholar] [CrossRef]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef]
- Liu, H.; Xu, L.; Yu, F.; Tan, J.; Cao, L.; Xing, Y.; Xu, Q.; Yang, S.; Liu, X.; Yang, P.; et al. Effects of different ozone treatments on the storage quality and stability of fresh peeled garlic. RSC Adv. 2021, 11, 22530–22543. [Google Scholar] [CrossRef]
- Rabie, M.A.; Soliman, A.Z.; Diaconeasa, Z.S.; Constantin, B. Effect of pasteurization and shelf life on the physicochemical properties of Physalis (Physalis peruviana L.) juice. J. Food Process. Preserv. 2015, 39, 1051–1060. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 29, 519. [Google Scholar] [CrossRef] [PubMed]
- Matłok, N.; Piechowiak, T.; Krempa, A.; Puchalski, C.; Balawejder, M. Cyclic Storage Chamber Ozonation as a Method to Inhibit Ethylene Generation during Plum Fruit Storage. Agriculture 2023, 13, 2274. [Google Scholar] [CrossRef]
- Zapałowska, A.; Matłok, N.; Zardzewiały, M.; Piechowiak, T.; Balawejder, M. Effect of Ozone Treatment on the Quality of Sea Buckthorn (Hippophae rhamnoides L.). Plants 2021, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Kuźniar, P.; Belcar, J.; Zardzewiały, M.; Basara, O.; Gorzelany, J. Effect of Ozonation on the Mechanical, Chemical, and Microbiological Properties of Organically Grown Red Currant (Ribes rubrum L.) Fruit. Molecules 2022, 27, 8231. [Google Scholar] [CrossRef] [PubMed]
- Panno, S.; Davino, S.; Caruso, A.; Bertacca, S.; Karacic, C.; Mandić, A.; Noris, E.; Matic, S. A Review of the Most Common and Economically Important Diseases That Undermine the Cultivation of Tomato Crop in the Mediterranean Basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Chiu, M.C.; Chen, C.L.; Chen, C.W.; Lin, H.J. Weather fluctuation can override the effects of integrated nutrient management on fungal disease incidence in the rice fields in Taiwan. Sci. Rep. 2022, 12, 4273. [Google Scholar] [CrossRef]
- Pogacean, M.; Gavrilescu, M. Plant protection products and their sustainable and environmentally friendly use. Environ. Eng. Manag. J. 2009, 8, 607–627. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Rosello, C.; Bélanger, R.; Ratti, C. Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing. Foods 2020, 9, 1468. [Google Scholar] [CrossRef]
- Available online: https://www.pspo.com.pl/publications/ae467296bb8fcbc928d418c62301f2b8656bc104.pdf (accessed on 16 March 2024).
- Czyżewska, K.; Trusek-Holownia, A.; Dabrowa, M.; Sarmiento, F.; Blamey, J. A catalytic membrane used for H2O2 decomposition. Catal. Today 2019, 331, 30–34. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Gusein-zade, N.; Burmistrov, D.E.; Kolik, L.V.; Dorokhov, A.S.; Izmailov, A.Y.; Shokri, B.; Gudkov, S.V. Advancements in Plasma Agriculture: A Review of Recent Studies. Int. J. Mol. Sci. 2023, 24, 15093. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Antos, P.; Kosowski, P.; Skrobacz, K.; Józefczyk, R.; Balawejder, M. Impact of Ozonation Process on the Microbiological and Antioxidant Status of Raspberries (Rubus Ideaeus L.) during Storage at Room Temperature. Agric. Food Sci. 2019, 28, 35–44. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free-Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Bonikowski, R.; Balawejder, M. Optimization of extraction process of antioxidant compounds from yellow onion skin and their use in functional bread production. LWT 2020, 117, 108614. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zardzewiały, M.; Matłok, N.; Piechowiak, T.; Saletnik, B.; Balawejder, M.; Gorzelany, J. Preliminary Tests of Tomato Plant Protection Method with Ozone Gas Fumigation Supported with Hydrogen Peroxide Solution and Its Effect on Some Fruit Parameters. Sustainability 2024, 16, 3481. https://doi.org/10.3390/su16083481
Zardzewiały M, Matłok N, Piechowiak T, Saletnik B, Balawejder M, Gorzelany J. Preliminary Tests of Tomato Plant Protection Method with Ozone Gas Fumigation Supported with Hydrogen Peroxide Solution and Its Effect on Some Fruit Parameters. Sustainability. 2024; 16(8):3481. https://doi.org/10.3390/su16083481
Chicago/Turabian StyleZardzewiały, Miłosz, Natalia Matłok, Tomasz Piechowiak, Bogdan Saletnik, Maciej Balawejder, and Józef Gorzelany. 2024. "Preliminary Tests of Tomato Plant Protection Method with Ozone Gas Fumigation Supported with Hydrogen Peroxide Solution and Its Effect on Some Fruit Parameters" Sustainability 16, no. 8: 3481. https://doi.org/10.3390/su16083481





